Abstract
Background:
Thymoma-associated myasthenia gravis (TAMG) is a subtype of myasthenia gravis (MG) that is associated with more severe symptoms and a relatively poor prognosis. Eculizumab, an inhibitor to target human C5 component of the complement cascade, is considered a treatment option for refractory generalized MG (gMG).
Objectives:
To explore the safety and efficacy of eculizumab in patients with TAMG.
Design:
This is an observational multicenter real-world cohort study to assess TAMG who were treated with eculizumab from June 2023 to June 2024.
Data sources and methods:
Clinical features associated with thymoma-associated multi-organ autoimmunity (TAMA), Myasthenia Gravis Activities of Daily Living (MG-ADL) score, and the incidence of treatment-emergent adverse events (TEAEs) were prospectively collected.
Results:
Overall, 42 patients with gMG were treated with eculizumab at 5 research centers, of whom 22 patients with TAMG were finally included. This cohort had a mean age of 51.5 ± 12.1 years and an average disease duration of 4.0 ± 4.3 years. Regarding thymomas, the World Health Organization (WHO) histological classification was primarily B2 and B3 (63.7%), and Masaoka staging was predominantly IV (45.5%). Nine participants (40.9%) switched from efgartigimod to eculizumab aiming at a better clinical improvement and reducing steroid use. By week 12, the MG-ADL score decreased to 4.8 ± 4.7 (baseline: 11.7 ± 6.0), and the corticosteroid dose reduced to 23.2 ± 26.5 mg (baseline: 41.8 ± 63.9 mg). Two patients with TAMA showed significant improvement in skin lesions and thrombocytopenia. Two TEAEs were recorded including COVID-19 and herpes labialis infection. Four patients (18.2%) died of respiratory or circulatory failure owing to thymoma metastasis.
Conclusion:
This real-world study demonstrates the efficacy of eculizumab in achieving symptom control and corticosteroid reduction for TAMG. It may also be a therapeutic option for refractory TAMG and TAMA.
Trial registration:
Keywords: corticosteroid, eculizumab, refractory, TAMA, TAMG
Plain language summary
Eculizumab in thymoma-associated myasthenia gravis
Patients with unresectable thymomas tend to have more severe symptoms at a younger age, a higher frequency of myasthenic crisis (MC), longer respiratory support duration, and increased mortality compared to those without thymomas. Only a few case reports have explored the therapeutic efficacy of eculizumab in thymoma-associated myasthenia gravis (TAMG), which is a subtype of myasthenia gravis (MG) that is associated with anti-acetylcholine receptor antibodies. Using a multicenter, observational cohort of patients with generalized myasthenia gravis from China, we enrolled patients with TAMG who were refractory to immunotherapies and then switched to eculizumab. Our study revealed a significant improvement in the Myasthenia Gravis Activities of Daily Living (MG-ADL) score in patients with TAMG (7.4 points) by week 12. Moreover, this study demonstrated the efficacy and safety of eculizumab in the TAMG cohort, with most patients experiencing clinical improvement within 2 weeks and an average reduction in steroid dosage of approximately one-third in the first month. Further, we believe that this article will be of interest to the readership of your journal because it provides evidence-based findings that indicate that eculizumab has emerged as a viable treatment option not only for TAMG but also for thymoma-associated multiorgan autoimmunity (TAMA).
Introduction
Myasthenia gravis (MG) is a rare neurological disorder mediated by autoantibodies, such as anti-acetylcholine receptor (AChR) antibody, muscle-specific kinase (MuSK) antibody, and low-density lipoprotein receptor-related protein 4 antibody, resulting in fatigable muscle weakness and potentially life-threatening complications. 1 Approximately 10%–20% of MG are associated with thymomas, a condition known as thymoma-associated myasthenia gravis (TAMG).1,2 Patients with unresectable thymomas tend to have more severe symptoms at a younger age, a higher frequency of myasthenic crisis (MC), longer respiratory support duration, and increased mortality compared to those without thymoma.3,4 Furthermore, the 5-year and 10-year survival rates of patients with TAMG were lower than those of patients without TAMG (83.4% vs 91.6% and 72.3% vs 87.2%, respectively). This discrepancy may be attributed to the severity of MG, Masaoka staging, and World Health Organization (WHO) classification.5,6
Currently, recommended therapies for generalized MG (gMG) include symptomatic treatment (acetylcholinesterase inhibitors), corticosteroids, nonsteroidal immunosuppressants, and rescue treatments, including intravenous immunoglobulin (IVIg) and plasma exchange (PE).7,8 Biologics, including CD20 monoclonal antibodies and neonatal Fc receptor (FcRn) antagonists, have also shown promising therapeutic potential in managing gMG. 8 In addition, thymectomy offers prominent benefits and is recommended for treating TAMG and early-onset MG.9–12 However, due to thymoma recurrence, metastasis, or autoreactive T cells, some patients may experience acute worsening and even develop thymoma-associated multiorgan autoimmunity (TAMA).4,13,14 TAMA is defined as an inflammatory disorder affecting the skin, liver, and intestines that histopathologically resembles graft-versus-host disease (GVHD) but is associated with thymoma rather than allogeneic hematopoietic cell transplantation. 15 Even with sufficient immunotherapies including steroids and IVIg, the mortality rate of patients with TAMA remains greater than 50%.14,16
Eculizumab is a humanized monoclonal antibody that binds to the C5 component of the complement cascade and inhibits pro-inflammatory activation. 17 The REGAIN study and its extension demonstrated that eculizumab significantly improved clinical scores and disease severity in refractory gMG, with 57.3% of the patients achieving clinically meaningful improvements (CMI).18–21 This has led to its approval for the treatment of refractory gMG in multiple countries. Although patients with a history of thymoma or thymic neoplasms were excluded from the REGAIN study, the interim analysis of postmarketing surveillance in Japan revealed equal clinical improvements with eculizumab in treating patients with TAMG and without TAMG. 22
To date, only a few case reports have explored the therapeutic efficacy of eculizumab in TAMG.23–29 Using a multicenter, observational cohort of patients with gMG from China, we enrolled patients with TAMG who were refractory to immunotherapies and subsequently switched to eculizumab. We hypothesized that eculizumab could improve respiratory and limb muscle strength in patients with TAMG and potentially treat TAMA.
Methods
Patients and methods
This is a retrospective analysis in a prospective multicenter cohort of gMG treated with eculizumab at five hospitals in East, South, and West China from June 2023 to June 2024. The enrollment criteria were as follows: (1) Age ⩾18 years; (2) presence or history of thymoma; (3) seropositivity for anti-AChR antibody, with neuromuscular transmission abnormalities on repetitive nerve stimulations, or improvement of symptoms with oral acetylcholinesterase inhibitors; (4) received at least four infusions of eculizumab to complete the induction phase (900 mg once weekly for the first four administrations, followed by 1200 mg every 2 weeks starting at week 5 30 ; (5) vaccinated against Neisseria meningitidis before starting eculizumab or received antibiotic prophylaxis until 2 weeks after vaccination. Exclusion criteria included: (1) Non-TAMG patients; (2) fewer than four doses of eculizumab administered; (3) incomplete medical history data.
The following baseline data upon eculizumab initiation were recorded: demographic information, disease duration, onset symptoms, antibody subtypes, thymectomy, WHO classification of thymoma, Masaoka staging, chemoradiotherapies, previous treatment, and status at eculizumab initiation. Additionally, some data were derived from a registry study (NCT04535843) that involved prospective data collection within the cohort. The Myasthenia Gravis Activities of Daily Living (MG-ADL) score was collected each week via telephone questionnaires, and steroid dosage was collected every 4 weeks for 12 weeks. The incidence of treatment-emergent adverse events (TEAEs) and postintervention MG status (MG-PIS) at the latest follow-up (up to the current date) were documented during the observation period.
Refractory gMG was defined by meeting at least one of the following criteria: (1) Use of ⩾2 immunosuppressants for over 12 months without achieving symptom control (Myasthenia Gravis Foundation of America (MGFA) IIb or beyond); (2) Use of one immunosuppressant for over 12 months with repeated IVIg or PE treatments yearly, without achieving symptom control; (3) Frequent MC (⩾2 times per year). The definition of long-term sustained IVIg/PE aligned with the second criterion for refractory MG, particularly in the portion related to IVIg/PE.
The efficacy of eculizumab was mainly assessed based on changes in MG-ADL from baseline and steroid dosage at week 12. Additional evaluation criteria included the time to reach CMI, the proportion of ADL responders, and MG-PIS. CMI was defined as an improvement of 2 points or more in MG-ADL. ADL responders were defined as those with a decrease of at least three points in MG-ADL lasting for at least 4 weeks.
In our study, “MM” refers to an MG-ADL score of 0, but the patient still requires cholinesterase inhibitors and immunosuppressive therapy. “Improved” status in MG-PIS was defined as a sustained improvement of ⩾2 points in the MG-ADL scores for at least 2 weeks before the last follow-up. “Unchanged” was defined as a maximum improvement in the MG-ADL scores of no more than 2 points or an improvement duration of no more than 2 weeks. “Worse” was defined as a sustained increase of ⩾2 points in the MG-ADL scores for at least 2 weeks before the last follow-up.
Statistical analysis
Continuous variables are presented as mean ± SD, and categorical variables are expressed as n (%). All statistical analyses were performed using R version 4.4.0 (R Foundation for Statistical Computing, Vienna, Austria). Figures were generated using GraphPad Prism version 9.5.1 (GraphPad Software Inc., San Diego, CA, USA).
Results
Baseline clinical features
From June 2023 to June 2024, 42 patients with gMG received eculizumab at 5 neuromuscular diagnostic centers, with TAMG accounting for 78.6% (33/42) of the cases (Figure 1). Nine patients (five with early-onset MG and four with late-onset MG) were excluded from the study. Among the 33 TAMG cases, 11 patients were excluded due to financial constraints that they discontinued the sequential infusions. Ultimately, 22 patients with TAMG who received at least 4 eculizumab infusions and completed 12 weeks of follow-up were included in the final analysis.
Figure 1.
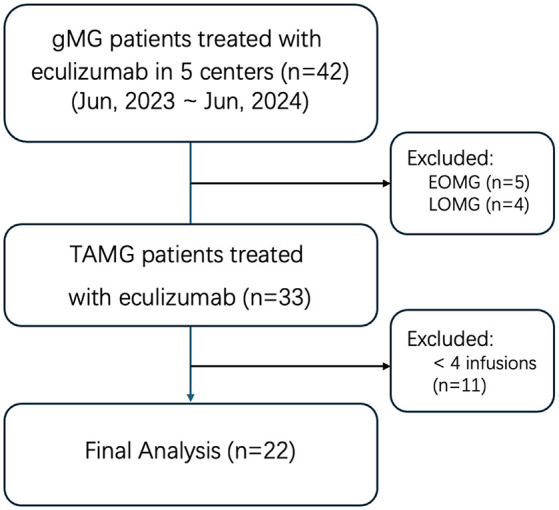
Patients inclusion and exclusion flowchart.
The baseline characteristics are shown in Table 1. All patients were seropositive for AChR-antibodies (15.3 ± 19.3 nmol/L). The mean age was 51.5 ± 12.1 years old while 12 patients were female (54.5%). The mean disease duration before eculizumab treatment was 4.0 ± 4.3 years. Thymectomies were performed in 19 patients (86.4%), 12 of whom (54.5%) received postoperative radiotherapies or chemotherapies. Thymoma pathology was mainly WHO histology, B2 or B3 (63.7%), with Masaoka stage IV predominance (45.6%). One patient was pathologically diagnosed with a thymic carcinoma.
Table 1.
Baseline clinical features for TAMG who were treated with eculizumab (n = 22).
| Clinical variables | Mean ± SD (range) or no. (%) |
|---|---|
| Age (years old) | 51.5 ± 12.1 |
| Sex (female%) | 12 (54.5%) |
| Disease duration (years) | 4.0 ± 4.3 |
| AChR-Ab positive | 22 (100%) |
| Thymectomy (n, %) | 19 (86.4%) |
| Chemoradiotherapies (n, %) | 12 (54.5%) |
| WHO histology | |
| AB | 0 |
| B1 | 4 (18.2%) |
| B2 | 8 (36.4%) |
| B3 | 6 (27.3%) |
| C | 1 (4.5%) |
| Unknown | 3 (13.6%) |
| Masaoka stage | |
| I | 2 (9.1%) |
| II | 2 (9.1%) |
| III | 3 (13.6%) |
| IV | 10 (45.5%) |
| Unknown | 5 (22.7%) |
| Refractory | 17 (77.3%) |
| Previous treatment | |
| Pyridostigmine | 21 (95.5%) |
| Prednisone | 17 (77.3%) |
| Tacrolimus | 12 (54.5%) |
| Azathioprine | 2 (9.1%) |
| Mycophenolate mofetil | 2 (9.1%) |
| Cyclophosphamide | 0 |
| Rituximab | 2 (9.1%) |
| Long-term sustained IVIg/PE | 9 (40.9%) |
| Efgartigimod | 9 (40.9%) |
| MGFA classification with eculizumab initiation | |
| I | 0 |
| II | 6 (27.3%) |
| III | 5 (22.7%) |
| IV | 5 (22.7%) |
| V | 6 (27.3%) |
| Initiation status | |
| MGAE | 3 (13.6%) |
| Impending MC | 5 (22.7%) |
| MC | 6 (27.3%) |
| Mild/moderate | 8 (36.4%) |
| Prednisone dosage at baseline (mg/d) | 41.8 ± 63.9 |
| MG-ADL score at baseline | 11.7 ± 6.0 |
Baseline refers to the first infusion of eculizumab. Disease duration was defined as the time between onset symptom and the baseline entry.
AChR, acetylcholine receptor; IVIg, intravenous immunoglobulin; MC, myasthenic crisis; MG-ADL, Myasthenia Gravis Activities of Daily Living; MGAE, MG acute exacerbation; MGFA, Myasthenia Gravis Foundation of America; PE, plasma exchange; TAMG, thymoma-associated MG; WHO, World Health Organization.
Of all TAMG participants, 77.3% (17/22) were classified as refractory, with 6 patients meeting criteria (1), 9 meeting criteria (2), and 2 meeting criteria (3). Overall, 40.9% (9/22) of patients received long-term sustained IVIg/PE. Two patients previously received rituximab treatment, while nine patients switched from efgartigimod to eculizumab. When initiating eculizumab, 10 patients were using tacrolimus with steroids (including 2 on efgartigimod), 3 combined steroids with IVIg/PE. The rest of the refractory TAMG used steroids with mycophenolate mofetil (n = 1), steroids alone (n = 1), rituximab (n = 1), and efgartigimod (n = 1).
All patients were classified as II–V according to the MGFA. The baseline MG-ADL score was 11.7 ± 6.0, and the baseline steroid dosage was 41.8 ± 63.9 mg. The reasons for initiating eculizumab treatment, as detailed in Table 1, included MG acute exacerbation (MGAE) (13.6%, 3/22), impending crisis (22.7%, 5/22), and crisis (27.3%, 6/22). Upon eculizumab treatment, most patients showed no evidence of active infection. The white blood cell count was 9.9 × 109/L (within the normal range of 3.5–9.5 × 109/L), and the C-reactive protein level was 5.4 mg/L (normal range: <10 mg/L) on average.
Among them, 63.6% (14/22) participants had normal levels of immunoglobulin G (IgG) (14.3 ± 7.8 g/L), as well as normal levels of absolute counts of B lymphocytes (100.6 ± 96.6/μL), CD4+ T lymphocytes (470.8 ± 328.8/μL), and CD8+ T lymphocytes (487.8 ± 253.0/μL). Two patients met the diagnostic criteria for Good’s syndrome, exhibiting significant reduction in immunoglobulins (5.1 and 8.6 g/L, respectively, normal range: 8.7–17 g/L), B lymphocytes (86.2/μL and 0.63/μL, respectively, normal range: 91.53–498/μL), and CD4+ T cells (232.6/μL and 94.8/μL, respectively, normal range: 395.36–1264.17/μL). For the molecules associated with the complement pathway, we identified a decrease in serum C1q levels (0.14 g/L, normal range: 0.159–0.233 g/L). Conversely, serum C3 (0.912 g/L, normal range: 0.9–1.8 g/L) and C4 (0.197 g/L, normal range: 0.1–0.4 g/L) were normal (n = 13).
The efficacy and safety of eculizumab in TAMG
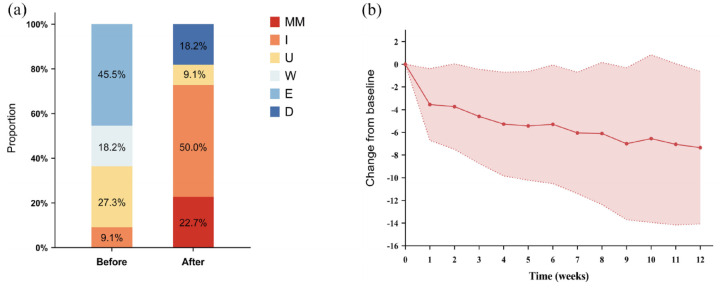
In our cohort, the average follow-up time was 155 ± 96.2 days, and the average duration of eculizumab treatment was 127 ± 85.6 days. Before eculizumab treatment, none achieving minimal manifestation (MM) and 9.1% achieving improvement before eculizumab initiation. At last follow-up, 72.7% of patients with TAMG had achieved a status of MM or improved (Figure 2(a) and (b)). CMI was achieved in 81.8% (18/22) of the patients within 1.7 ± 1.4 weeks. By weeks 4, 8, and 12, the mean changes in MG-ADL score were −5.3, −6.1, and −7.4, respectively. Seventeen (77.3%) patients were classified as ADL responders. By week 12, oral corticosteroid dosages decreased to 23.2 ± 26.5 mg, and 3 patients were steroid-free while taking one immunosuppressant (all tacrolimus).
Figure 2.
The proportions of different status in MG-PIS before and after eculizumab treatment (a) and the changes of MG-ADL score in the cohort of TAMG (n = 22) (b).
D, died; E, exacerbation; I, improved; MG-ADL, Myasthenia Gravis Activities of Daily Living; MG-PIS, Myasthenia Gravis Post Intervention Status; MM, minimal manifestation; TAMG, thymoma-associated myasthenia gravis; U, unchanged; W, worse.
Two patients (9.1%) experienced TEAEs, including one who tested positive for COVID-19 and another who developed rashes and herpes labialis. None of the patients experienced meningococcal infections, and no adverse events led to discontinuation. Four patients (18.2%) died of respiratory or circulatory failure secondary to metastasis. Among them, two patients had initially shown a period of symptom improvement following eculizumab treatment.
Patients switched from efgartigimod to eculizumab
Nine patients (40.9%) with TAMG had received efgartigimod treatment before eculizumab initiation. These included those in crisis or impending crisis (n = 5) who required highly effective therapy to wean off ventilation or stop disease progression, and those who were stable but had a high burden of corticosteroids (31.3 mg) and a strong desire for tapering. The baseline characteristics of the patients are presented in Table 2. This cohort had an average age of 51.6 ± 10.6 years, a male-to-female ratio of 3:6, and an average disease duration of 4.1 ± 4.1 years. The WHO histopathological classification was mainly B1, with no significant preference for Masaoka staging. After the initiation of eculizumab, 88.9% of the patients reached MM (n = 2) or improved status (n = 6), according to the MG-PIS. The MG-ADL score reduced to 3.4 ± 3.2 from baseline value (11.6 ± 5.3). The steroid dosage in the cohort gradually decreased, and by week 12, the average dose of corticosteroid reduced to 11.3 ± 7.4 (baseline: 31.7 ± 28.3) mg.
Table 2.
Characteristics of TAMG patients who have switched from efgartigimod to eculizumab (n = 9).
| Clinical variables | Mean ± SD (range) or No. (%) |
|---|---|
| Age (years old) | 51.6 ± 10.6 |
| Sex (female) | 6 (66.7%) |
| Disease duration (years) | 4.1 ± 4.1 |
| Thymectomy | 7 (77.8%) |
| Chemoradiotherapy | 3 (33.3%) |
| WHO histology | |
| AB | 0 |
| B1 | 4 (44.4%) |
| B2 | 2 (22.2%) |
| B3 | 1 (11.1%) |
| Unknown | 2 (22.2%) |
| Masaoka stage | |
| I | 2 (22.2%) |
| II | 2 (22.2%) |
| III | 2 (22.2%) |
| IV | 1 (11.1%) |
| Unknown | 2 (22.2%) |
| MGFA classification with eculizumab initiation | |
| I | 0 |
| II | 3 (33.3%) |
| III | 2 (22.2%) |
| IV | 1 (11.1%) |
| V | 3 (33.3%) |
| Duration of eculizumab (days) | 83 ± 47.0 |
| Regularly eculizumab | 4 (44.4%) |
| Time to CMI (weeks) | 1.5 ± 0.7 |
| Cumulative MSE | 4 (44.4%) |
| MG-ADL responder | 7 (77.8%) |
| MG-ADL score at baseline | 11.6 ± 5.3 |
| Change in MG-ADL | |
| W4 | −6.1 ± 4.0 |
| W8 | −5.8 ± 6.4 |
| W12 | −7.4 ± 4.7 |
| Treatments after eculizumab | |
| Prednisone | 7 (77.7%) |
| Tacrolimus | 5 (55.6%) |
| Tofacitinib | 2 (22.2%) |
| Dosage of prednisone (mg/d) | |
| Baseline | 31.7 ± 28.3 |
| W4 | 18.9 ± 11.7 |
| W8 | 15.0 ± 8.4 |
| W12 | 11.3 ± 7.4 |
| MG-PIS | |
| Minimal manifestation | 2 (22.2%) |
| Improved | 6 (66.7%) |
| Unchanged | 1 (11.1%) |
| Worse | 0 |
| Exacerbation | 0 |
| Died | 0 |
| TEAEs | 1 (1.11%) |
| COVID-19 positive | 1 (11.1%) |
Disease duration was defined as the time between the onset symptom and the baseline entry.
CMI, clinically meaningful improvement; MG-ADL, Myasthenia Gravis Activities of Daily Living; MGFA, Myasthenia Gravis Foundation of America; MG-PIS, Myasthenia Gravis Postintervention Status; MSE, minimal symptom expression; TAMG, thymoma-associated MG; TEAEs, treatment-emergent adverse events; WHO, World Health Organization.
Clinical profiles of patients with TAMA
Case 1: The first case was a 49-year-old male with extensive thymoma metastasis who had previously underwent thymectomy and radiotherapies. The patient presented mild myasthenic symptoms, paraneoplastic dermatosis, aplastic anemia, severe thrombocytopenia, and significant bone pain. After initiating eculizumab, the rash and thrombocytopenia improved significantly. He received eculizumab for 197 days but subsequently died from respiratory and circulatory failure related to metastasis.
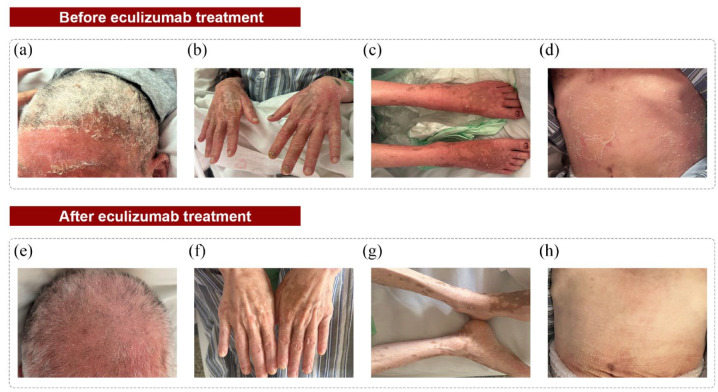
Case 2: The second case was a 40-year-old female previously reported by Chen et al., 31 who presented with GVHD-like erythroderma, neuromyelitis optica spectrum disorder, and gMG. After initiating eculizumab, her skin condition (confluent erythema reduced and the scales almost completely disappeared; Figure 3) and myasthenic symptoms improved; however, she still required oxygen inhalation. Thereafter, regular eculizumab treatment was continued.
Figure 3.
Changes in skin manifestations before (a–d) and after (e–h) receiving eculizumab in a patient with TAMA. Before eculizumab initiation, the patient had diffused erythema with desquamation, flushing, swelling, and erosions (a–d). After treatment, the swelling, erosion, and desquamation were significantly improved, leaving multiple scattered dark macules (e–h). The scalp: (a) and (e); the hands: (b) and (f); the lower extremities: (c) and (g); the abdomen: (d) and (h).
TAMA, thymoma associated multiorgan autoimmunity.
Discussion
The emergence of new biologics offers opportunities to shift from conventional treatments to target-specific immunotherapies for MG, promising fewer side effects and faster action. 32 This study analyzed the indications for eculizumab in adult Chinese patients in a real-world setting. The cohort mainly comprised patients with TAMG with high disease activity, including MGAE, impending MC, and MC, who were not adequately involved in the REGAIN and its extension.17–19,21,33 Since thymectomy or thymoma recurrence can precipitate respiratory failure in severely affected patients, prompt and effective treatment is crucial to achieve stable control of MG symptoms. This study provides a preliminary assessment of the safety and efficacy of eculizumab in patients with TAMG.
We observed a significant improvement in MG-ADL score for patients with TAMG (7.4 points) by week 12. This improvement appears promising compared with that observed in the REGAIN study (4 points) 17 and in the Japanese cohort (4.5 points). 34 This difference may be attributed to the severe disease status of most patients at the time of entry to our study. Additionally, the proportion of MG-ADL responders in the TAMG cohort was higher than that from the REGAIN study (77.3% vs 60%) and achieved this response in a shorter duration (12 vs 26 weeks). 17 Besides improvements in MG-ADL scores, we observed a rapid reduction in corticosteroid dosage within the first month. In a 130-week extension study, 88.0% of patients achieved improved status, 57.3% achieved MM status, and the MG-ADL response rate was as high as 84.7%.18,19 In real-world data, several cases have reported the efficacy of eculizumab in the TAMG subtype. A Japanese study reported that 4/5 TAMG patients achieved MM, while an American study found that all four TAMG patients showed improvement after 6 months. Given the sustained improvement in MG-ADL scores and the reduction in steroid dosage, there is strong support for the long-term use of eculizumab in patients with TAMG.
In the REGAIN study, headache and nasopharyngitis were the most common side effects, occurring in approximately 44.4% and 38.5% of cases, respectively.17,18 In our study, we observed two TEAEs, which may be related to the short follow-up period and small sample size. One patient tested positive for COVID-19, possibly due to the inhibition of complement function by eculizumab, which affects immune status. Despite this, eculizumab is considered safe in this small cohort, supported by case reports.35–37 Additionally, Ruggenenti et al. 38 found that eculizumab could safely improve respiratory function and reduce mortality in severe COVID-19 pneumonia. In our real-world cohort, four patients died of respiratory or circulatory failure unrelated to myasthenia symptoms. This mortality may be associated with thymoma recurrence and metastasis (Masaoka stage IV). 39 Other probable causes may be the existence of anticardiac and antiskeletal muscle antibodies, autonomic dysfunction, and thymic changes.40–42
Rituximab, a B-cell-depleting monoclonal antibody, has been proven to be effective in newly diagnosed or MuSK-antibody-positive gMG.43,44 Meta-analyses have also supported its efficacy for refractory gMG. 45 Moreover, several case reports suggest that rituximab may be beneficial for treating TAMG.46–49 Efgartigimod, a recently approved FcRn antagonist for adult AChR-antibody positive gMG, enhances the catabolism of IgG, selectively lowering serum IgG levels and effectively controlling gMG symptoms.50,51 Recent real-world data indicate that eculizumab had a stronger effect than efgartigimod in improving Quantitative MG scores and reducing steroid burden. 52 In our study, 10 patients (45.5%) did not achieve satisfactory response from anti-CD20 monoclonal antibodies or efgartigimod, suggesting that eculizumab may be beneficial for TAMG when other treatments are ineffective. A retrospective analysis from Germany comparing rituximab and eculizumab in gMG further supports this hypothesis, showing a more significant decrease in Quantitative MG scores and a higher proportion of patients achieving MM status at 12 and 24 months in the eculizumab group compared to the rituximab group. 53
TAMA is a recently proposed concept in paraneoplastic syndromes associated with thymoma, with the pathology similar to GVHD, often manifesting as damage to the skin, intestines, and liver, potentially related to T-cell mediation. 15 These disorders can cause profound disability but usually respond to immunotherapies and often improve with thymoma resection. 54 Previous therapies for TAMA include corticosteroids and IVIg. 14 Although corticosteroids may potentially enhance thymoma remission, they cannot significantly improve mucocutaneous lesions. Our study demonstrates the use of eculizumab for ameliorating skin and myasthenia symptoms in two patients with TAMA, suggesting its potential effectiveness in this context. However, there is currently no direct evidence of complement pathway abnormalities in the samples from patients with TAMA. A sensitive assay for the early detection of AChR autoantibody-associated membrane attack complex formation in patient samples is anticipated to identify those who might benefit from complement inhibitor therapy.
Limitations of this study include its retrospective nature, small sample size, and short follow-up duration. The placebo effect or prolonged effect of previous treatment regimens cannot be excluded. Regarding the limitations of a single-arm, retrospective study design, this research did not perform sample size calculation and lacks a control group for statistical comparison. Further, we used patient-reported outcomes as the primary outcome, rather than other objective outcome measures. Therefore, the conclusions should be interpreted with caution.
Large randomized controlled trials are required to confirm the efficacy of eculizumab in TAMG.
Conclusion
Our study demonstrated the efficacy and safety of eculizumab in the TAMG cohort, with most patients experiencing clinical improvement within 2 weeks and an average reduction in steroid dosage of approximately one-third in the first month. Overall, eculizumab has emerged as a viable treatment option not only for TAMG but also for TAMA.
Acknowledgments
We thank all participants recruited in this study.
Appendix
Abbreviations
AChR acetylcholine receptor
CMI clinically meaningful improvement
EOMG early-onset MG
FcRn neonatal Fc receptor
gMG generalized myasthenia gravis
GVHD graft-versus-host disease
IgG immunoglobulin G
IVIg intravenous immunoglobulin
LOMG late-onset MG
MAC membrane attack complex
MC myasthenic crisis
MG myasthenia gravis
MG-ADL Myasthenia Gravis Activities of Daily Living score
MGAE MG acute exacerbation
MGFA Myasthenia Gravis Foundation of America
MG-PIS postintervention MG status
MM minimal manifestation
MuSK muscle-specific kinase
NMOSD neuromyelitis optica spectrum disorder
NSISTs nonsteroidal immunosuppressants
PE plasma exchange
TAMA thymoma-associated multiorgan autoimmunity
TAMG thymoma-associated MG
TEAEs treatment-emergent adverse events
WHO the World Health Organization
Footnotes
ORCID iDs: Lei Jin  https://orcid.org/0000-0002-9052-4590
https://orcid.org/0000-0002-9052-4590
Dingxian He  https://orcid.org/0009-0005-9955-7179
https://orcid.org/0009-0005-9955-7179
Jianquan Shi  https://orcid.org/0000-0003-0536-1131
https://orcid.org/0000-0003-0536-1131
Jie Song  https://orcid.org/0000-0002-4572-5721
https://orcid.org/0000-0002-4572-5721
Xiao Huan  https://orcid.org/0000-0002-6122-4804
https://orcid.org/0000-0002-6122-4804
Chongbo Zhao  https://orcid.org/0000-0001-9481-1418
https://orcid.org/0000-0001-9481-1418
Sushan Luo  https://orcid.org/0000-0002-9033-7568
https://orcid.org/0000-0002-9033-7568
Contributor Information
Lei Jin, Huashan Rare Disease Center and Department of Neurology, Huashan Hospital, Shanghai Medical College, National Center for Neurological Disorders, Fudan University, Shanghai, China.
Dingxian He, Huashan Rare Disease Center and Department of Neurology, Huashan Hospital, Shanghai Medical College, National Center for Neurological Disorders, Fudan University, Shanghai, China.
Quantao Zeng, Department of Neurology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Song Tan, Department of Neurology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Jianquan Shi, Department of Neurology, Nanjing First Hospital, Nanjing Medical University, Nanjing, China.
Ying Liu, Department of Neurology, The Second Hospital of Dalian Medical University, Dalian, China.
Zhangyu Zou, Department of Neurology, Fujian Medical University Union Hospital, Fuzhou, China.
Jie Song, Huashan Rare Disease Center and Department of Neurology, Huashan Hospital, Shanghai Medical College, National Center for Neurological Disorders, Fudan University, Shanghai, China.
Chong Yan, Huashan Rare Disease Center and Department of Neurology, Huashan Hospital, Shanghai Medical College, National Center for Neurological Disorders, Fudan University, Shanghai, China.
Xiao Huan, Huashan Rare Disease Center and Department of Neurology, Huashan Hospital, Shanghai Medical College, National Center for Neurological Disorders, Fudan University, Shanghai, China.
Yuan Wang, Department of Blood Transfusion, Huashan Hospital, Fudan University, Shanghai, China.
Lei Yang, Department of Neurosurgery and Neurocritical Care, Huashan Hospital Fudan University, Shanghai, China.
Jianying Xi, Huashan Rare Disease Center and Department of Neurology, Huashan Hospital, Shanghai Medical College, National Center for Neurological Disorders, Fudan University, Shanghai, China.
Zongtai Wu, Faculty of Biology, University of Cambridge, Cambridge, UK.
Ziqi Liu, Department of Dermatology, Huashan Hospital, Fudan University, Shanghai, China.
Jianming Zheng, Department of Infectious Diseases, Huashan Hospital, National Medical Center for Infectious Diseases, Fudan University, Shanghai, China.
Chongbo Zhao, Huashan Rare Disease Center and Department of Neurology, Huashan Hospital, Shanghai Medical College, National Center for Neurological Disorders, Fudan University, Shanghai, China.
Xianglin Chu, Department of Thoracic Surgery, Huashan Hospital, Fudan University, Shanghai 200040, China.
Sushan Luo, Huashan Rare Disease Center and Department of Neurology, Huashan Hospital, Shanghai Medical College, National Center for Neurological Disorders, Fudan University, No.12 Urumqi Middle Road, Jing ‘an District, Shanghai 200040, China.
Declarations
Ethics approval and consent to participate: All procedures involving human subjects followed the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study protocol was approved by the Ethics Committees of Huashan Hospital (KY2020-999). All enrolled patients provided written informed consent to participate.
Consent for publication: Consent for publication was obtained from all participants.
Author contributions: Lei Jin: Conceptualization; Data curation; Writing – original draft.
Dingxian He: Conceptualization; Writing – original draft.
Quantao Zeng: Conceptualization; Data curation; Investigation; Writing – original draft.
Song Tan: Data curation; Investigation; Writing – original draft.
Jianquan Shi: Data curation; Investigation; Writing – original draft.
Ying Liu: Data curation; Investigation; Writing – original draft.
Zhangyu Zou: Investigation; Writing – original draft.
Jie Song: Data curation; Investigation; Writing – original draft.
Chong Yan: Investigation; Writing – original draft.
Xiao Huan: Data curation; Investigation; Writing – original draft.
Yuan Wang: Data curation; Investigation; Writing – original draft.
Lei Yang: Investigation; Writing – original draft.
Jianying Xi: Investigation; Writing – original draft.
Zongtai Wu: Investigation; Writing – original draft.
Ziqi Liu: Investigation; Writing – original draft.
Jianming Zheng: Data curation; Investigation; Writing – original draft.
Chongbo Zhao: Funding acquisition; Project administration; Writing – review & editing.
Xianglin Chu: Conceptualization; Data curation; Writing – review & editing.
Sushan Luo: Conceptualization; Data curation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by financial grants from the National Key research and development plan (2022YFC3501303, 2022YFC3501305), and the National Natural Science Foundation of China (82071410, 82001335).
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: Anonymized data will be made available upon reasonable request.
References
- 1. Gilhus NE. Myasthenia gravis. N Engl J Med 2016; 375: 2570–2581. [DOI] [PubMed] [Google Scholar]
- 2. Murai H, Utsugisawa K, Motomura M, et al. The Japanese clinical guidelines 2022 for myasthenia gravis and Lambert–Eaton myasthenic syndrome. Clin Exp Neuroimmunol 2023; 14: 19–27. [Google Scholar]
- 3. Wang Y, Huan X, Zhu X, et al. Independent risk factors for in-hospital outcome of myasthenic crisis: a prospective cohort study. Ther Adv Neurol Disord 2024; 17: 17562864241226745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Álvarez-Velasco R, Gutiérrez-Gutiérrez G, Trujillo JC, et al. Clinical characteristics and outcomes of thymoma-associated myasthenia gravis. Eur J Neurol 2021; 28: 2083–2091. [DOI] [PubMed] [Google Scholar]
- 5. Kharnas SS, Ippolitov LI, Fat’ianova AS. [Long-term prognostic analysis of surgical treatment of the thymoma-associated myasthenia gravis]. Khirurgiia (Mosk) 2009; (7): 47–54. [PubMed] [Google Scholar]
- 6. Wang F, Pang L, Fu J, et al. Postoperative survival for patients with thymoma complicating myasthenia gravis-preliminary retrospective results of the ChART database. J Thorac Dis 2016; 8: 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wiendl H, Abicht A, Chan A, et al. Guideline for the management of myasthenic syndromes. Ther Adv Neurol Disord 2023; 16: 17562864231213240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dalakas MC. Immunotherapy in myasthenia gravis in the era of biologics. Nat Rev Neurol 2019; 15: 113–124. [DOI] [PubMed] [Google Scholar]
- 9. Narayanaswami P, Sanders DB, Wolfe G, et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology 2021; 96: 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaminski HJ, Kusner LL, Cutter GR, et al. Does surgical removal of the thymus have deleterious consequences? Neurology 2024; 102: e209482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolfe GI, Kaminski HJ, Aban IB, et al. Long-term effect of thymectomy plus prednisone versus prednisone alone in patients with non-thymomatous myasthenia gravis: 2-year extension of the MGTX randomised trial. Lancet Neurol 2019; 18: 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med 2016; 375: 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shelly S, Agmon-Levin N, Altman A, et al. Thymoma and autoimmunity. Cell Mol Immunol 2011; 8: 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao J, Bhatnagar V, Ding L, et al. A systematic review of paraneoplastic syndromes associated with thymoma: treatment modalities, recurrence, and outcomes in resected cases. J Thorac Cardiovasc Surg 2020; 160: 306–314.e14. [DOI] [PubMed] [Google Scholar]
- 15. Wadhera A, Maverakis E, Mitsiades N, et al. Thymoma-associated multiorgan autoimmunity: a graft-versus-host-like disease. J Am Acad Dermatol 2007; 57: 683–689. [DOI] [PubMed] [Google Scholar]
- 16. Cara-Gualda MJ, Sánchez-Díaz M, Sánchez-Díaz M, et al. Cutaneous manifestations of thymoma-associated multiorgan autoimmunity: a systematic review. J Eur Acad Dermatol Venereol 2024; 38(9): e810–e812. [DOI] [PubMed] [Google Scholar]
- 17. Howard JF, Jr, Utsugisawa K, Benatar M, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol 2017; 16: 976–986. [DOI] [PubMed] [Google Scholar]
- 18. Mantegazza R, Wolfe GI, Muppidi S, et al. Post-intervention status in patients with refractory myasthenia gravis treated with eculizumab during REGAIN and its open-label extension. Neurology 2021; 96: e610–e618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Howard JF, Jr, Karam C, Yountz M, et al. Long-term efficacy of eculizumab in refractory generalized myasthenia gravis: responder analyses. Ann Clin Transl Neurol 2021; 8: 1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andersen H, Mantegazza R, Wang JJ, et al. Eculizumab improves fatigue in refractory generalized myasthenia gravis. Qual Life Res 2019; 28: 2247–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mantegazza R, O’Brien FL, Yountz M, et al. Consistent improvement with eculizumab across muscle groups in myasthenia gravis. Ann Clin Transl Neurol 2020; 7: 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murai H, Suzuki S, Hasebe M, et al. Safety and effectiveness of eculizumab in Japanese patients with generalized myasthenia gravis: interim analysis of post-marketing surveillance. Ther Adv Neurol Disord 2021; 14: 17562864211001995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vélez-Santamaría V, Nedkova V, Díez L, et al. Eculizumab as a promising treatment in thymoma-associated myasthenia gravis. Ther Adv Neurol Disord 2020; 13: 1756286420932035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takeuchi E, Kajiyama Y, Ando K, et al. [The efficacy of eculizumab against post-thymectomy exacerbations in thymoma associated myasthenia gravis (MG)]. Rinsho Shinkeigaku 2022; 62: 277–280. [DOI] [PubMed] [Google Scholar]
- 25. Amano E, Otsu S, Suzuki S, et al. Eculizumab improved weakness and taste disorder in thymoma-associated generalized myasthenia gravis with anti-striational antibodies: a case report. eNeurologicalSci 2019; 14: 72–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Furuta C, Yano M, Numanami H, et al. A case of thymoma-associated multiorgan autoimmunity including polymyositis and myocarditis. Surg Case Rep 2021; 7: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoshizumi K, Kimura T, Ukon S, et al. [Eclizumab in the treatment of myasthenia gravis crisis complicating invasive thymoma: a case study of efficacy]. Rinsho Shinkeigaku 2020; 60: 865–868. [DOI] [PubMed] [Google Scholar]
- 28. Suh J, Clarke V, Amato AA, et al. Safety and outcomes of eculizumab for acetylcholine receptor-positive generalized myasthenia gravis in clinical practice. Muscle Nerve 2022; 66: 348–353. [DOI] [PubMed] [Google Scholar]
- 29. Oyama M, Okada K, Masuda M, et al. Suitable indications of eculizumab for patients with refractory generalized myasthenia gravis. Ther Adv Neurol Disord 2020; 13: 1756286420904207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Monteleone JPR, Gao X, Kleijn HJ, et al. Eculizumab pharmacokinetics and pharmacodynamics in patients with generalized myasthenia gravis. Front Neurol 2021; 12: 696385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen JQ, Li W, Cai SQ. Erythroderma in a patient with thymoma-associated myasthenia gravis. JAMA Dermatol 2024; 160: 224–225. [DOI] [PubMed] [Google Scholar]
- 32. Pyzik M, Kozicky LK, Gandhi AK, et al. The therapeutic age of the neonatal Fc receptor. Nat Rev Immunol 2023; 23: 415–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vissing J, Jacob S, Fujita KP, et al. “Minimal symptom expression” in patients with acetylcholine receptor antibody-positive refractory generalized myasthenia gravis treated with eculizumab. J Neurol 2020; 267: 1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murai H, Uzawa A, Suzuki Y, et al. Long-term efficacy and safety of eculizumab in Japanese patients with generalized myasthenia gravis: a subgroup analysis of the REGAIN open-label extension study. J Neurol Sci 2019; 407: 116419. [DOI] [PubMed] [Google Scholar]
- 35. Kuroda Y, Watanabe G, Satou K, et al. [Eculizumab led to beneficial clinical course in a patient with generalized myasthenia gravis who developed COVID 19-associated pneumonia]. Rinsho Shinkeigaku 2024; 64: 109–112. [DOI] [PubMed] [Google Scholar]
- 36. Alis C, Emre DH, Karaali Savrun F, et al. A mild course of COVID-19 infection in a generalized myasthenia gravis patient under eculizumab treatment. Neurol Sci 2022; 43: 5185–5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mimori M, Komatsu T, Maku T, et al. Generalized myasthenia gravis patients infected with COVID-19 should continue eculizumab. Neurol Sci 2022; 43: 4081–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruggenenti P, Di Marco F, Cortinovis M, et al. Eculizumab in patients with severe coronavirus disease 2019 (COVID-19) requiring continuous positive airway pressure ventilator support: retrospective cohort study. PLoS One 2021; 16: e0261113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Du X, Cui J, Yu XT, et al. Risk factor analysis of thymoma resection and its value in guiding clinical treatment. Cancer Med 2023; 12: 13408–13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fenioux C, Abbar B, Boussouar S, et al. Thymus alterations and susceptibility to immune checkpoint inhibitor myocarditis. Nat Med 2023; 29: 3100–3110. [DOI] [PubMed] [Google Scholar]
- 41. Peric S, Rakocevic-Stojanovic V, Nisic T, et al. Cardiac autonomic control in patients with myasthenia gravis and thymoma. J Neurol Sci 2011; 307: 30–33. [DOI] [PubMed] [Google Scholar]
- 42. Kufukihara K, Watanabe Y, Inagaki T, et al. Cytometric cell-based assays for anti-striational antibodies in myasthenia gravis with myositis and/or myocarditis. Sci Rep 2019; 9: 5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brauner S, Eriksson-Dufva A, Hietala MA, et al. Comparison between rituximab treatment for new-onset generalized myasthenia gravis and refractory generalized myasthenia gravis. JAMA Neurol 2020; 77: 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hehir MK, Hobson-Webb LD, Benatar M, et al. Rituximab as treatment for anti-MuSK myasthenia gravis: multicenter blinded prospective review. Neurology 2017; 89: 1069–1077. [DOI] [PubMed] [Google Scholar]
- 45. Iorio R, Damato V, Alboini PE, et al. Efficacy and safety of rituximab for myasthenia gravis: a systematic review and meta-analysis. J Neurol 2015; 262: 1115–1119. [DOI] [PubMed] [Google Scholar]
- 46. Liu H, Dong Z, Zhang M, et al. Case report: complex paraneoplastic syndromes in thymoma with nephrotic syndrome, cutaneous amyloidosis, myasthenia gravis, and Morvan’s syndrome. Front Oncol 2022; 12: 1002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Antar AI, Otrock ZK, Kharfan-Dabaja MA, et al. Thymoma with concomitant pure red cell aplasia, Good’s syndrome and myasthenia gravis responding to rituximab. Indian J Hematol Blood Transfus 2016; 32: 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nelson RP, Jr, Pascuzzi RM, Kessler K, et al. Rituximab for the treatment of thymoma-associated and de novo myasthenia gravis: 3 cases and review. J Clin Neuromuscul Dis 2009; 10: 170–177. [DOI] [PubMed] [Google Scholar]
- 49. Hayashi R, Tahara M, Oeda T, et al. [A case of refractory generalized myasthenia gravis with anti-acetylcholine receptor antibodies treated with rituximab]. Rinsho Shinkeigaku 2015; 55: 227–232. [DOI] [PubMed] [Google Scholar]
- 50. Howard JF, Jr, Bril V, Burns TM, et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology 2019; 92: e2661–e2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Howard JF, Jr, Bril V, Vu T, et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2021; 20: 526–536. [DOI] [PubMed] [Google Scholar]
- 52. Pane C, Di Stefano V, Cuomo N, et al. A real-life experience with eculizumab and efgartigimod in generalized myasthenia gravis patients. J Neurol 2024; 271(9): 6209–6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nelke C, Schroeter CB, Stascheit F, et al. Eculizumab versus rituximab in generalised myasthenia gravis. J Neurol Neurosurg Psychiatry 2022; 93: 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Evoli A, Lancaster E. Paraneoplastic disorders in thymoma patients. J Thorac Oncol 2014; 9: S143–S147. [DOI] [PMC free article] [PubMed] [Google Scholar]