Abstract
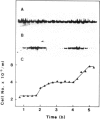
Two independent DNA sequences, of 5.6 and 2.4 kb in size, were isolated from Schizosaccharomyces pombe gene libraries on the basis of their ability to rescue the temperature-conditional lethality conferred by a cdc22 mutation. Integration of these sequences into the S.pombe genome by homologous recombination, followed by genetic mapping, demonstrated that the site of integration of the 5.6 kb fragment is tightly linked to the cdc22 locus, while that of the 2.4 kb fragment is unlinked. This shows that the 5.6 kb fragment carries the authentic cdc22+ gene while the 2.4 kb fragment carries and extragenic suppressor sequence. The cdc22 transcript was identified by Northern blot analysis and shown to be 3.3 kb in size. The level of the transcript during the cell cycle was investigated in synchronous cultures prepared by elutriation. The cdc22 transcript is cell cycle regulated, reaching a maximum level during late G1/S phase, at least 12-fold higher than the minimum level observed in mid G2.
Keywords: cell cycle, cell cycle mutant, periodicity, S. pombe, transcript
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aves S. J., Durkacz B. W., Carr A., Nurse P. Cloning, sequencing and transcriptional control of the Schizosaccharomyces pombe cdc10 'start' gene. EMBO J. 1985 Feb;4(2):457–463. doi: 10.1002/j.1460-2075.1985.tb03651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach D., Durkacz B., Nurse P. Functionally homologous cell cycle control genes in budding and fission yeast. Nature. 1982 Dec 23;300(5894):706–709. doi: 10.1038/300706a0. [DOI] [PubMed] [Google Scholar]
- Beach D., Piper M., Nurse P. Construction of a Schizosaccharomyces pombe gene bank in a yeast bacterial shuttle vector and its use to isolate genes by complementation. Mol Gen Genet. 1982;187(2):326–329. doi: 10.1007/BF00331138. [DOI] [PubMed] [Google Scholar]
- Creanor J., Elliott S. G., Bisset Y. C., Mitchison J. M. Absence of step changes in activity of certain enzymes during the cell cycle of budding and fission yeasts in synchronous cultures. J Cell Sci. 1983 May;61:339–349. doi: 10.1242/jcs.61.1.339. [DOI] [PubMed] [Google Scholar]
- Creanor J., Mitchison J. M. Patterns of protein synthesis during the cell cycle of the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1982 Dec;58:263–285. doi: 10.1242/jcs.58.1.263. [DOI] [PubMed] [Google Scholar]
- Dickinson J. R. The cdc 22 mutation by Schizosaccharomyces pombe is a temperature-sensitive defect in nucleoside diphosphokinase. Eur J Biochem. 1981 Oct;119(2):341–345. doi: 10.1111/j.1432-1033.1981.tb05613.x. [DOI] [PubMed] [Google Scholar]
- Elliott S. G., McLaughlin C. S. Rate of macromolecular synthesis through the cell cycle of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4384–4388. doi: 10.1073/pnas.75.9.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco S. C., Rose M., Botstein D. Homologous Recombination between Episomal Plasmids and Chromosomes in Yeast. Genetics. 1983 Dec;105(4):843–856. doi: 10.1093/genetics/105.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Game J. C. Yeast cell-cycle mutant cdc21 is a temperature-sensitive thymidylate auxotroph. Mol Gen Genet. 1976 Aug 2;146(3):313–315. doi: 10.1007/BF00701257. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Sequential function of gene products relative to DNA synthesis in the yeast cell cycle. J Mol Biol. 1976 Jul 15;104(4):803–817. doi: 10.1016/0022-2836(76)90183-2. [DOI] [PubMed] [Google Scholar]
- Hereford L. M., Osley M. A., Ludwig T. R., 2nd, McLaughlin C. S. Cell-cycle regulation of yeast histone mRNA. Cell. 1981 May;24(2):367–375. doi: 10.1016/0092-8674(81)90326-3. [DOI] [PubMed] [Google Scholar]
- Ludwig J. R., 2nd, Foy J. J., Elliott S. G., McLaughlin C. S. Synthesis of specific identified, phosphorylated, heat shock, and heat stroke proteins through the cell cycle of Saccharomyces cerevisiae. Mol Cell Biol. 1982 Feb;2(2):117–126. doi: 10.1128/mcb.2.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lörincz A. T., Miller M. J., Xuong N. H., Geiduschek E. P. Identification of proteins whose synthesis is modulated during the cell cycle of Saccharomyces cerevisiae. Mol Cell Biol. 1982 Dec;2(12):1532–1549. doi: 10.1128/mcb.2.12.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison J. M., Creanor J. Induction synchrony in the fission yeast. Schizosaccharomyces pombe. Exp Cell Res. 1971 Aug;67(2):368–374. doi: 10.1016/0014-4827(71)90421-6. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. Molecular analysis of a cell lineage. Nature. 1983 Apr 21;302(5910):670–676. doi: 10.1038/302670a0. [DOI] [PubMed] [Google Scholar]
- Nasmyth K., Nurse P. Cell division cycle mutants altered in DNA replication and mitosis in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1981;182(1):119–124. doi: 10.1007/BF00422777. [DOI] [PubMed] [Google Scholar]
- Palmieri R., Yue R. H., Jacobs H. K., Maland L., Wu L., Kuby S. A. Nucleoside triphosphate-nucleoside diphosphate transphosphorylase (nucleoside diphosphokinase). 3. Subunit structure of the crystalline enzyme from brewers' yeast. J Biol Chem. 1973 Jun 25;248(12):4486–4499. [PubMed] [Google Scholar]
- Peterson T. A., Prakash L., Prakash S., Osley M. A., Reed S. I. Regulation of CDC9, the Saccharomyces cerevisiae gene that encodes DNA ligase. Mol Cell Biol. 1985 Jan;5(1):226–235. doi: 10.1128/mcb.5.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sclafani R. A., Fangman W. L. Yeast gene CDC8 encodes thymidylate kinase and is complemented by herpes thymidine kinase gene TK. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5821–5825. doi: 10.1073/pnas.81.18.5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storms R. K., Ord R. W., Greenwood M. T., Mirdamadi B., Chu F. K., Belfort M. Cell cycle-dependent expression of thymidylate synthase in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Dec;4(12):2858–2864. doi: 10.1128/mcb.4.12.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss W. A., Friedberg E. C. Molecular cloning and characterization of the yeast RAD10 gene and expression of RAD10 protein in E. coli. EMBO J. 1985 Jun;4(6):1575–1582. doi: 10.1002/j.1460-2075.1985.tb03819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]