Abstract
Although several antithrombotic strategies have been investigated for the management of cryptogenic strokes, ie, ischemic strokes without known etiologies, an optimal antithrombotic strategy for cryptogenic strokes is unknown. We aim to assess oral antithrombotic agents’ comparative efficacy and safety after cryptogenic stroke to identify an optimal treatment.
A systematic review and meta-analysis synthesizing evidence from randomized controlled trials (RCTs) obtained from PubMed, Embase Cochrane, Scopus, and Web of Science until February 2024. We used the random-effects model to report dichotomous outcomes using a risk ratio (RR) with a 95% confidence interval (CI). Frequentist network meta-analysis was conducted using R, version 4.3.1.
Seven RCTs with 15,240 patients were included. None of the OACs showed a significant efficacy in preventing all-cause mortality, stroke recurrence, cardiovascular mortality, and major adverse cardiac events compared to aspirin. Also, safety measures were similar between different OACs and aspirin regarding safety measures, including major bleeding, intracranial hemorrhage, and gastrointestinal bleeding. However, only rivaroxaban significantly increased the incidence of major bleeding (RR: 2.69, CI [1.67, 4.33]).
There was no difference between various OACs and aspirin regarding efficacy and safety outcomes. There is a greater risk of major bleeding with rivaroxaban. Further research is still warranted to define a personalized strategy for selecting antithrombotic strategies after cryptogenic stroke on a case-by-case basis.
Keywords: brain infarct, ESUS, idiopathic, aspirin, warfarin, apixaban, rivaroxaban, dabigatran
Introduction
Cerebrovascular accidents (CVAs), commonly known as strokes, constitute a significant cause of morbidity and mortality worldwide, with more than 795,000 cases in the United States annually. 1 Strokes can be ischemic, hemorrhagic, or subarachnoid, with the ischemic strokes comprising most of (>60%) stroke cases. 2 Ischemic strokes exhibit marked heterogeneity with more than 100 implicated pathologies. 3 Yet, no definite etiology is identified in 30%–40% of ischemic stroke cases, to which the term cryptogenic stroke is used to refer.4,5 The Trial of Org 10172 defined cryptogenic stroke, in the acute stroke treatment criteria, as the brain infarct not attributed to a definite source of large-vessel atherosclerosis, cardio-embolism, or small-vessel disease in the presence of extensive cardiac, serologic, hematologic, and vascular evaluation, incomplete evaluation, or evidence of more than one competing cause. 6 However, it has been shown that some cryptogenic strokes are caused by a source of cardio-embolism, such as atrial cardiopathy and patent foramen ovale (PFO).7–9 For instance, PFO is detected in and likely the cause of 50% of cryptogenic stroke cases. 10
Comprising a significant cause of disability and mortality, research on the management of ischemic stroke is escalating.11,12 The combination of antiplatelet therapy and stroke risk factor modification, as the mainstay of stroke prevention strategies in patients with stroke of identified cause, has been applied to cryptogenic stroke cases.13,14 Interestingly, some studies showed a potential benefit from warfarin over aspirin in specific subgroups of cryptogenic stroke patients, which opened the investigations of the superiority of antiplatelets compared to anticoagulants.14,15 Additionally, percutaneous closure of PFO, in cases with PFO, showed successful results in preventing stroke recurrence in such cases. 16 Although it is hypothesized that anticoagulants can be beneficial in stroke cases with potential sources of emboli, most studies focused on surgical or percutaneous closure of PFO (source of emboli) and antiplatelets instead of comparing antiplatelets to anticoagulants. 17
Previous systematic reviews and meta-analyses, including five or six randomized clinical trials (RCTs) per each, have shown controversial results concerning the benefit of anticoagulants over antiplatelets in reducing the risk of stroke recurrence and safety issues such as significant bleeding.18–20 The most recent review of 2282 patients concluded that oral anticoagulants (OACs) were associated with a lower risk of recurrent ischemic stroke compared to antiplatelets and a non-significantly higher risk of significant bleeding. 19 However, two large RCTs were published recently, comprising 1367 patients, and apixaban was compared to aspirin for patients with cryptogenic stroke.15,21 We, therefore, conducted the systematic review and network meta-analysis of RCTs to provide comprehensive and up-to-date evidence of OACs efficacy and safety compared to antiplatelet therapy in patients with cryptogenic stroke with potential cardiac emboli.
Methodology
Protocol Registration
We submitted this systematic review and meta-analysis protocol to PROSPERO with ID: CRD42024519285. Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) extension statement for network meta-analyses22,23 and the Cochrane Handbook of Systematic Reviews and Meta-Analysis 24 were strictly followed to conduct this review.
Data Sources & Search Strategy
A comprehensive search was conducted using PubMed (MEDLINE), Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science Core Collection, and EMBASE up to Feb 20, 2024. The search involved customizing terms and keywords in each database with Mesh terms related to cryptogenic and embolic strokes of undetermined etiology and various antithrombotic medications. This search strategy details are highlighted in (Table S1).
Eligibility Criteria
We included randomized controlled trials (RCTs) that followed the following PICO criteria: population (P): patients with cryptogenic stroke or embolic stroke of undetermined source (ESUS); intervention (I): oral anticoagulant either vitamin K antagonists such as warfarin, or direct oral anticoagulant (DOACs); control (C) another oral anticoagulant or antiplatelets; and outcomes (O): efficacy outcomes including all-cause mortality, cardiovascular mortality, stroke recurrence, major adverse cardiac events (MACE), ischemic stroke, myocardial infarction, and systemic thromboembolism. While the safety outcomes included major bleeding, gastrointestinal bleeding, intracranial hemorrhage, and the incidence of any serious adverse event.
Observational studies, review articles, letters, single-arm clinical trials, animal studies, case series, case reports, comments, and consensus documents were excluded from this study.
Study Selection
The review process utilized the Covidence web tool to manage records. After removing duplicates, four investigators (A.N., M.Ay., S.R., and M.T.) independently assessed the records. These investigators also performed full-text reviews for records meeting the initial criteria, with any discrepancies resolved through discussion and consensus with M.Ab.
Data Extraction
Four independent reviewers (A.N., M.Ay., S.R., and M.T.) extracted the following data using a pilot-tested Excel sheet: summary characteristics (first author name, year of publication, country, study design, total participants, intervention and control details, main inclusion criteria, primary outcome, and follow-up duration); baseline characteristics (age, gender, number of patients in each arm, baseline scores, and comorbidities); and outcomes data as previously outlined. Any disagreements among the reviewers were resolved through a consensus conference.
Risk of Bias
Four reviewers (A.N., M.Ay., S.R., and M.T.) assessed the risk of bias using the Cochrane RoB 2 tool for RCTs. 25 The RoB 2 tool assessed five areas of potential bias in RCTs: randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of reported results. Any disagreements among the reviewers were resolved through a consensus conference.
Statistical Analysis
We used R version 4.3.1. to perform a network meta-analysis using a frequentist framework and a random effects model. We pooled dichotomous outcomes using risk ratio (RR) and the corresponding 95% confidence interval (CI). Statistical inconsistency between network arms was evaluated by calculating I2. We interpreted the I-square test as follows: not significant for 0%–40%, moderate heterogeneity for 30%–60%, and substantial heterogeneity for 50%–90%, following the Cochrane Handbook (chapter nine). Treatment ranking was determined by calculating the SUCRA (Surface Under the Cumulative Ranking Curve) to assess the probability of each treatment being at each possible rank. 26
Results
Search Results and Study Selection
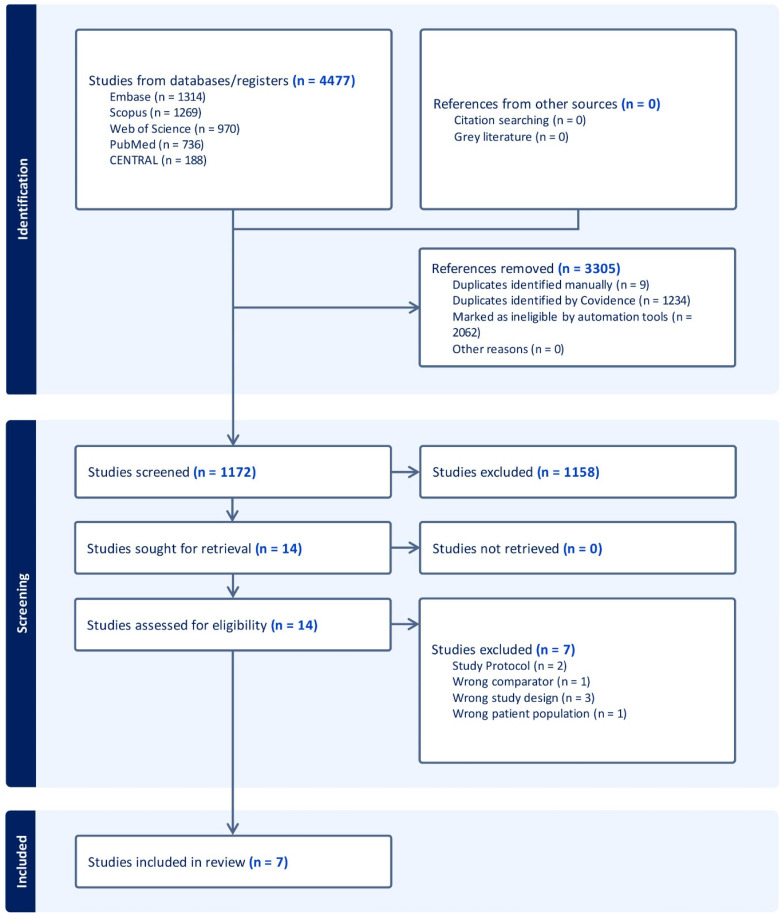
We identified a total of 4477 records after searching the databases. Three thousand and five duplicates and automatically identified irrelevant records were removed using Covidence, which left 1172 records for title and abstract screening. We excluded 1158 records and screened 14 full-text articles to include seven RCTs (Figure 1).
Figure 1.
PRISMA flow chart of the screening process.
Characteristics of Included Studies
This study involves seven RCTs15,16,21,27–30 with 15,240 patients. Three trials investigated warfarin as an intervention,16,28,29 while four trials investigated DOACs, either dabigatran, rivaroxaban, or apixaban.15,21,27,30 All the included trials used aspirin as a control. Further details are highlighted in (Table 1) about the included trials’ characteristics and in (Table 2) about the included patients’ baseline data.
Table 1.
Summary Characteristics of the Included RCTs.
| Study ID | Study Design | Country | Total Participants | Intervention (OACs) | Control | Primary Outcome | Follow-up duration | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Dose/Times of administration | Treatment Duration | Drug | Dose/Times of administration | Treatment Duration | ||||||
| Diener et al 2019 (RE-SPECT ESUS) 27 | Multicenter, double-blinded RCT | International | 5390 | dabigatran | 150 mg or 110 mg twice daily | A minimum of 6 months and a maximum of 3.5 years | Aspirin | 100 mg once daily |
A minimum of 6 months and a maximum of 3.5 years | Recurrent stroke of any type | Median of 19 months |
| Geisler et al 2023 (ATTICUS) 21 | Multicenter, open-label, blinded-outcome RCT | Germany | 352 | apixaban | 5 mg twice daily | 12 months | Aspirin | 100 mg once daily | N/A | New ischemic lesion on diffusion-weighted or fluid-attenuated inversion recovery brain MRI | 12 months |
| Hart et al 2018 (NAVIGATE ESUS) 30 | Multicenter, double-blinded RCT | International | 7213 | rivaroxaban | 15 mg once daily | N/A | Aspirin | 100 mg once daily | N/A | Recurrent stroke of any type | Median of 11 months |
| Homma et al 2002 (PICSS) 29 | Multicenter, double-blinded RCT | USA | 630 | warfarin | 2 mg daily | N/A | Aspirin | 325 mg daily | N/A | Recurrent ischemic stroke | 24 months |
| Kamel et al 2024 (ARCADIA) 15 | Multicenter, double-blinded RCT | USA | 1015 | apixaban | 5 mg or 2.5 mg, twice daily | N/A | Aspirin | 81 mg once daily | N/A | Recurrent stroke of any type | 1.8 ± 1.3 years |
| Mas et al 2017 (CLOSE) 16 | Multicenter, open-label, RCT | Germany and France | 596 | warfarin | Goal to achieve INR 2-3 | N/A | Aspirin | 75 mg | N/A | Fatal or nonfatal stroke | 5.2 ± 2.1 years |
| Shariat et al 2012 28 | Single-center, single-blinded RCT | Iran | 44 | warfarin | Started at 2.5 mg orally once daily and then adjusted to achieve an INR target of 2 to 3 |
18 months | Aspirin | 80 mg orally 3 times daily | N/A | Recurrence of ischemic event or death due to any cause | 14.6 ± 3.7 months |
Abbreviations: RCT, randomized controlled trial; INR, international normalized ratio; MRI, magnetic resonance imaging; N/A, not available; OACs, oral anticoagulants.
Table 2.
Baseline Characteristics of the Participants.
| Study ID | ARM | Number of patients in each group | Age (Years) Mean (SD) | Gender (Male) N. (%) | BMI, Mean (SD) | NIHSS, mean (SD) | Comorbidities N. (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AF | HTN | HF | DM | Renal disease | CAD or IHD | TIA | Smoking | DVT or PE | |||||||
| Diener et al 2019 (RE-SPECT ESUS) 27 | dabigatran | 2695 | 64.5 ± 11.4 | 1694 (62.9) | 27.2 ± 5.0 | 1 ± 1.48 | N/A | 1996 (74.1) | 117 (4.3) | 585 (21.7) | N/A | 301 (11.2) | 475 (17.6) | 458 (17.0) | N/A |
| aspirin | 2695 | 63.9 ± 11.4 | 1709 (63.4) | 27.3 ± 5.0 | 1 ± 1.48 | N/A | 1985 (73.7) | 124 (4.6) | 639 (23.7) | N/A | 276 (10.2) | 500 (18.6) | 433 (16.1) | N/A | |
| Geisler et al 2023 (ATTICUS) 21 | apixaban | 178 | 68.6 ± 11.1 | 92 (51.6) | 27.7 ± 5.2 | 1.33 ± 2.24 | N/A | 153 (86.0) | 3 (1.7) | 52 (29.2) | 10 (5.6) | 12 (6.7) | 24 (13.5) | 27 (15.2) | 5 (2.8) |
| aspirin | 174 | 68.3 ± 9.8 | 89 (51.1) | 27.7 ± 4.9 | 1.33 ± 2.24 | N/A | 150 (86.2) | 4 (2.3) | 48 (27.6) | 11 (6.3) | 17 (9.8) | 30 (17.2) | 26 (14.9) | 4 (2.3) | |
| Hart et al 2018 (NAVIGATE ESUS) 30 | rivaroxaban | 3609 | N/A | 2232 | 27.1 ± 4.9 | 1 | N/A | 2782 | N/A | 889 | N/A | N/A | 620 | 756 | N/A |
| aspirin | 3604 | N/A | 2204 | 27.3 ± 5.1 | 1 | N/A | 2803 | N/A | 917 | N/A | N/A | 643 | 728 | N/A | |
| Homma et al 2002 (PICSS) 29 | warfarin | 203 | 57.9 ± 133 | 120 | 28.1 ± 5.0 | N/A | N/A | 108 | N/A | 43 | N/A | N/A | N/A | 55 | N/A |
| aspirin | 398 | 59.6 ± 11.6 | 212 | 28.7 ± 6 .4 | N/A | N/A | 249 | N/A | 127 | N/A | N/A | N/A | 117 | N/A | |
| Kamel et al 2024 (ARCADIA) 15 | apixaban | 507 | 67.8 ± 10.8 | 235 (46.3) | N/A | 1 ± 0.3 | N/A | 396 (78.1) | 36 (7.1) | 156 (30.8) | N/A | 58 (11.4) | 97 (19.1) | 230 (45.4) | N/A |
| aspirin | 508 | 68.2 ± 11.0 | 229 (45.1) | N/A | 1 ± 0.3 | N/A | 388 (76.4) | 35 (6.9) | 159 (31.3) | N/A | 46 (9.1) | 100 (19.7) | 200 (39.4) | N/A | |
| Mas et al 2017 (CLOSE) 16 | warfarin | 187 | 43.8 ± 9.5 | 104 (55.6) | 20 ± 10.7 | N/A | N/A | 15 (8.0) | N/A | 2 (1.1) | N/A | N/A | N/A | 54 (28.9) | 4 (2.1) |
| aspirin | 409 | 44.18 ± 10.5 | 244 (59.65) | 51 ± 27 | N/A | N/A | 43 (24.04) | N/A | 16 (3.9) | N/A | N/A | N/A | 119 (29.09) | 7 (1.71) | |
| Shariat et al 2012 28 | warfarin | 21 | 60.6 ± 4.3 | 10 (47.6) | N/A | N/A | N/A | 9 (42.9) | N/A | 2 (9.5) | N/A | 5 (23.8) | N/A | 7 (33.3) | N/A |
| aspirin | 23 | 63.0 ± 4.7 | 18 (78.3) | N/A | N/A | N/A | 9 (39.1) | N/A | 3 (13) | N/A | 4 (17.4) | N/A | 10 (43.5) | N/A | |
Abbreviations: BMI, body mass index; NIHSS, National Health Institute Stroke Severity Score; AF, atrial fibrillation; HTN, hypertension; HF, heart failure; DM, diabetes mellitus; CAD, coronary heart disease; IHD, ischemic heart disease; TIA, transient ischemic attack; DVT, deep vein thrombosis; PE, pulmonary embolism.
Risk of Bias
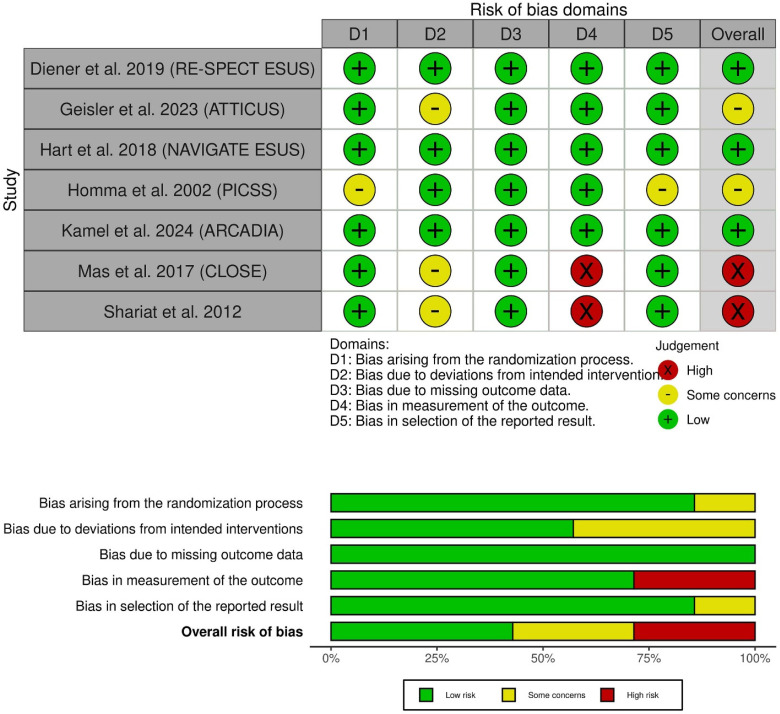
Three of the included RCTs, including the RE-SPECT ESUS trial, NAVIGATE ESUS, and ARCADIA trial,15,27,30 demonstrated an overall low risk of bias. However, ATTICUS showed overall some concerns due to the open-label treatments. 21 Also, PICSS 29 showed some concerns due to a lack of information about allocation concealment. Finally, CLOSE 16 and Shariat et al 28 exhibited a high risk of bias mainly due to the open-label assessment of outcomes.16,28 The risk of bias in the included studies is detailed in (Figure 2).
Figure 2.
Quality assessment of risk of bias in the included trials. The upper panel presents a schematic representation of risks (low = green, unclear = yellow, and high = red) for specific types of biases of each of the studies in the review. The lower panel presents risks (low = green, unclear = yellow, and high = red) for the subtypes of biases of the combination of studies included in this review.
DOACs Versus Warfarin Versus Aspirin
Efficacy Outcomes
All-Cause Mortality
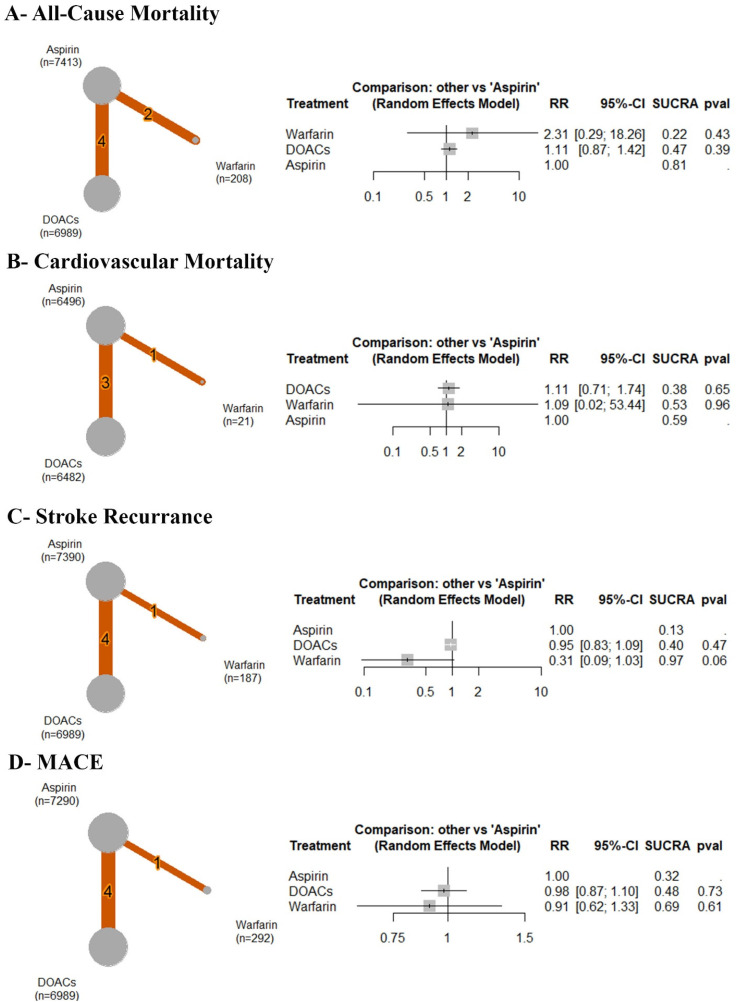
Based on the analysis of six RCTs, compared to aspirin, there was no significant difference observed between aspirin and warfarin (RR: 2.31 with 95% CI (0.29, 18.26), P = .43) or DOACs (RR: 1.11 with 95% CI (0.87, 1.42), P = .39) (Figure 3A). After ranking the treatment arms, warfarin has the highest risk, followed by DOACs and aspirin (Table 3). Low heterogeneity was observed (I2 = 0%).
Figure 3.
Forest and network plots of the efficacy outcomes, comparing warfarin versus DOACs versus aspirin. DOACs: direct oral anticoagulants, MACE: major adverse cardiac events, RR: risk ratio, CI: confidence interval.
Table 3.
Ranking Table row for DOACs Versus Warfarin Versus Aspirin Network meta-Analysis.
| All-Cause Mortality. | ||
| Warfarin | ||
| 2.08 (0.26; 16.65) | DOACs | |
| 2.31 (0.29; 18.26) | 1.11 (0.87; 1.42) | Aspirin |
| Cardiovascular Mortality. | ||
| DOACs | ||
| 1.02 (0.02; 50.97) | Warfarin | |
| 1.11 (0.71; 1.74) | 1.09 (0.02; 53.44) | Aspirin |
| Stroke Recurrence. | ||
| Aspirin | ||
| 1.05 (0.92; 1.20) | DOACs | |
| 3.20 (0.97; 10.60) | 3.05 (0.91; 10.17) | Warfarin |
| MACE. | ||
| Aspirin | ||
| 1.02 (0.91; 1.15) | DOACs | |
| 1.10 (0.75; 1.61) | 1.08 (0.72; 1.61) | Warfarin |
| Ischemic Stroke. | ||
| Aspirin | ||
| 1.09 (0.95; 1.26) | DOACs | |
| 7.78 (0.45; 134.15) | 7.14 (0.41; 123.46) | Warfarin |
| Myocardial Infarction. | ||
| Warfarin | ||
| 2.18 (0.04; 111.51) | Aspirin | |
| 2.36 (0.04; 124.31) | 1.08 (0.66; 1.77) | DOACs |
| Combined Systemic Thromboembolism. | ||
| Aspirin | ||
| 1.00 (0.83; 1.19) | DOACs | |
| 1.89 (0.89; 4.00) | 1.89 (0.87; 4.11) | Warfarin |
| Any Serious Adverse Events. | ||
| DOACs | ||
| 1.04 (0.77; 1.41) | Warfarin | |
| 1.03 (0.92; 1.16) | 0.99 (0.75; 1.31) | Aspirin |
| Major Bleeding. | ||
| Warfarin | ||
| 1.45 (0.40; 5.35) | DOACs | |
| 2.29 (0.70; 7.51) | 1.58 (0.92; 2.70) | Aspirin |
| Gastrointestinal Bleeding. | ||
| DOACs | ||
| 0.93 (0.18; 4.79) | Warfarin | |
| 1.26 (0.73; 2.19) | 1.36 (0.29; 6.40) | Aspirin |
| Intracranial Hemorrhage. | ||
| DOACs | ||
| 1.03 (0.01; 93.16) | Warfarin | |
| 1.12 (0.31; 4.04) | 1.09 (0.01; 82.41) | Aspirin |
Abbreviations: DOACs, direct oral anticoagulants; MACE, major adverse cardiac events.
Cardiovascular Mortality
Based on the analysis of four RCTs, compared to aspirin, no significant difference was observed between aspirin and DOACs (RR: 1.11 with 95% CI (0.71, 1.74), P = .65) or warfarin (RR: 1.09 with 95% CI (0.02, 53.44), P = .96) (Figure 3B). After ranking the treatment arms, DOACs had the highest risk, followed by warfarin and aspirin (Table 3). Low heterogeneity was observed (I2 = 14.9%).
Stroke Recurrence
Based on the analysis of five RCTs, compared to aspirin, no significant difference was observed between aspirin and DOACs (RR: 0.95 with 95% CI (0.83, 1.09), P = .47) or warfarin (RR: 0.31 with 95% CI (0.09, 1.03), P = .06) (Figure 3C). After ranking the treatment arms, aspirin had the highest risk, followed by DOACs and warfarin (Table 3). Low heterogeneity was observed (I2 = 0%).
Major Adverse Cardiac Events
Based on the analysis of five RCTs, compared to aspirin, no significant difference was observed between aspirin and DOACs (RR: 0.98 with 95% CI (0.87, 1.10), P = .73) or warfarin (RR: 0.91 with 95% CI (0.62, 1.33), P = .61) (Figure 3D). After ranking the treatment arms, aspirin had the highest risk, followed by DOACs and warfarin (Table 3). Low heterogeneity was observed (I2 = 0%).
Ischemic Stroke
Based on the analysis of four RCTs, compared to aspirin, no significant difference was observed between aspirin and DOACs (RR: 0.92 with 95% CI (0.79, 1.06), P = .23) or warfarin (RR: 0.13 with 95% CI (0.01, 2.21), P = .16) (Figure S1-A). After ranking the treatment arms, aspirin had the highest risk, followed by DOACs and warfarin (Table 3). Low heterogeneity was observed (I2 = 0%).
Myocardial Infarction
Based on the analysis of four RCTs, compared to aspirin, no significant difference was observed between aspirin and warfarin (RR: 2.18 with 95% CI (0.04, 111.51), P = 0.70) or DOACs (RR: 0.93 with 95% CI (0.56, 1.52), P = .76) (Figure S1-B). After ranking the treatment arms, warfarin had the highest risk, followed by aspirin and DOACs (Table 3). Low heterogeneity was observed (I2 = 16.1%).
Combined Systemic Thromboembolism
Based on the analysis of five RCTs, compared to aspirin, no significant difference was observed between aspirin and DOACs (RR: 1.00 with 95% CI (0.84, 1.20), P = .96) or warfarin (RR: 0.53 with 95% CI (0.25, 1.13), P = .10) (Figure S1C). After ranking the treatment arms, aspirin had the highest risk, followed by DOACs and warfarin (Table 3). Low heterogeneity was observed (I2 = 0%).
Safety Outcomes
Major Bleeding
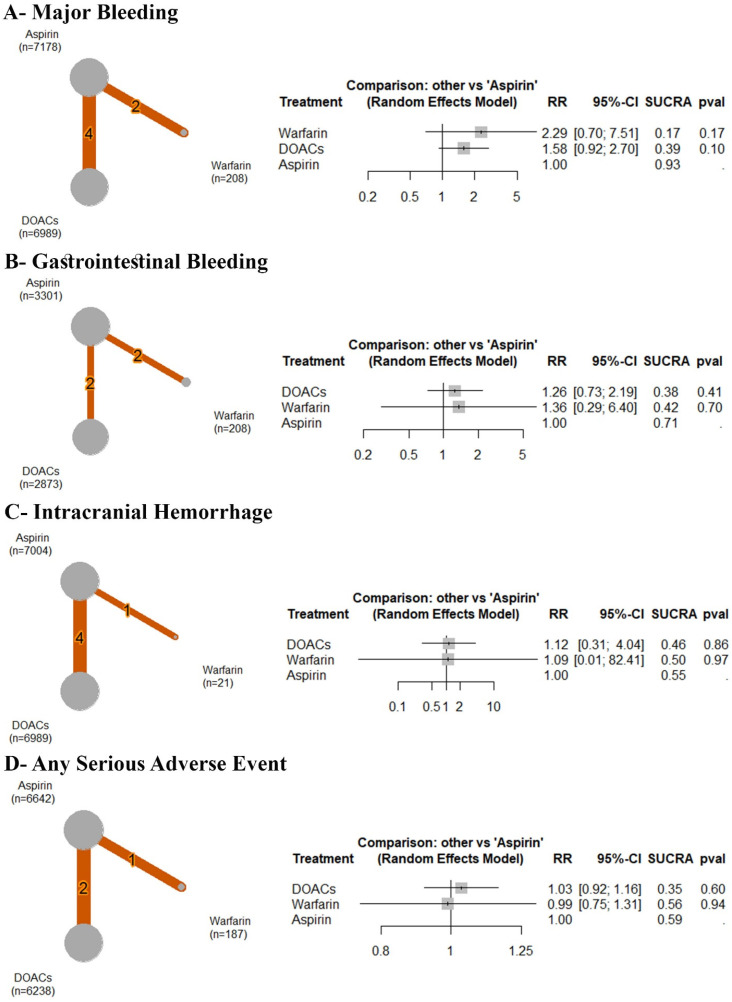
Based on the analysis of six RCTs, compared to aspirin, no significant difference was observed between aspirin and warfarin (RR: 2.29 with 95% CI (0.70, 7.51), P = .17) or DOACs (RR: 1.58 with 95% CI (0.92, 2.70), P = .10) (Figure 4A). After ranking the treatment arms, warfarin had the highest risk, followed by DOACs and aspirin (Table 3). High heterogeneity was observed (I2 = 50.2%).
Figure 4.
Forest and network plots of the safety outcomes, comparing warfarin versus DOACs versus aspirin. DOACs: direct oral anticoagulants, RR: risk ratio, CI: confidence interval.
Gastrointestinal Bleeding
Based on the analysis of four RCTs, compared to aspirin, no significant difference was observed between aspirin and DOACs (RR: 1.26 with 95% CI (0.73, 2.19), P = .41) or warfarin (RR: 1.36 with 95% CI (0.29, 6.40), P = .70) (Figure 4B). After ranking the treatment arms, DOACs had the highest risk, followed by warfarin and aspirin (Table 3). Low heterogeneity was observed (I2 = 0%).
Intracranial Hemorrhage
Based on the analysis of five RCTs, compared to aspirin, no significant difference was observed between aspirin and DOACs (RR: 1.12 with 95% CI (0.31, 4.04), P = .86) or warfarin (RR: 1.09 with 95% CI (0.01, 82.41), P = .97) (Figure 4C). After ranking the treatment arms, DOACs had the highest risk, followed by warfarin and aspirin (Table 3). High heterogeneity was observed (I2 = 70.7%).
Any Serious Adverse Events
Based on the analysis of three RCTs, compared to aspirin, no significant difference was observed between aspirin and DOACs (RR: 1.03 with 95% CI (0.92, 1.16), P = 0.60) or warfarin (RR: 0.99 with 95% CI (0.75, 1.31), P = .94) (Figure 4D). After ranking the treatment arms, DOACs had the highest risk, followed by warfarin and aspirin (Table 3). High heterogeneity was observed (I2 = 62.9%).
Individual DOACs Versus Warfarin Versus Aspirin
Efficacy Outcomes
All-Cause Mortality
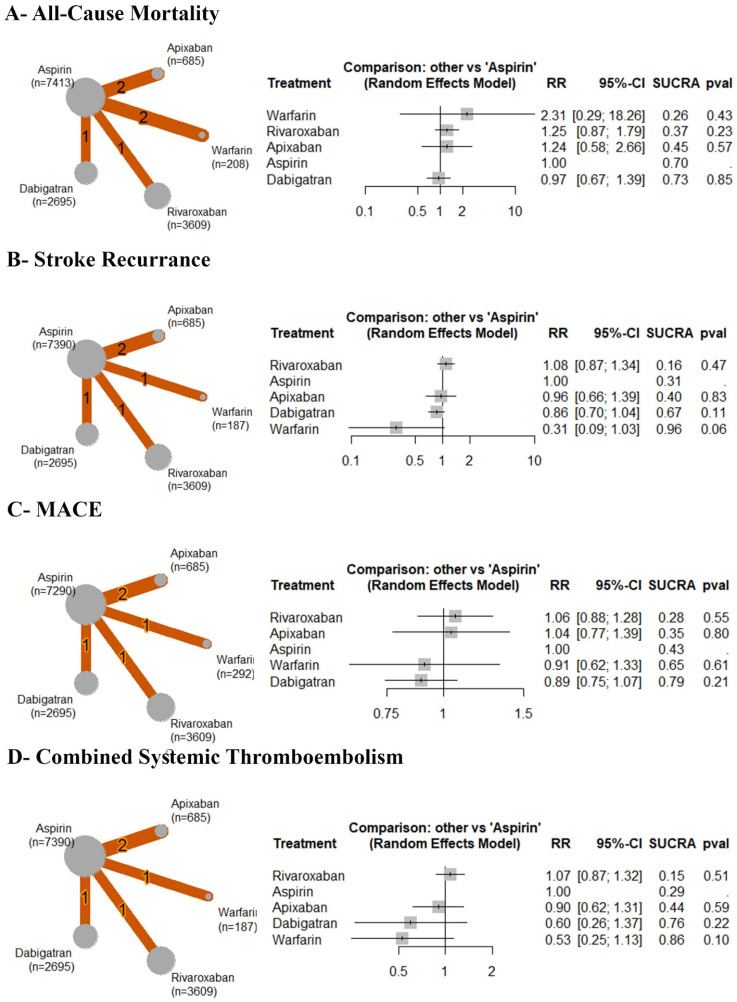
Based on the analysis of six RCTs, compared to aspirin, there was no significant difference observed between aspirin and warfarin (RR: 2.31 with 95% CI (0.29, 18.26), P = .43) or rivaroxaban (RR: 1.25 with 95% CI (0.87, 1.79), P = .23) or apixaban (RR: 1.24 with 95% CI (0.58, 2.66), P = .57), or dabigatran (RR: 0.97 with 95% CI (0.67, 1.39), P = .85) (Figure 5A). After ranking the treatment arms, warfarin has the highest risk, followed by rivaroxaban, apixaban, aspirin, and dabigatran (Table 4). Low heterogeneity was observed (I2 = 0%).
Figure 5.
Forest and network plots of the efficacy outcomes, comparing various OACs versus aspirin. OACs: oral anticoagulants, RR: risk ratio, CI: confidence interval.
Table 4.
Ranking Table row for Individual OACs Versus Warfarin Versus Aspirin Network meta-Analysis.
| All-Cause Mortality. | ||||
| Warfarin | ||||
| 1.85 (0.23; 15.09) | Rivaroxaban | |||
| 1.86 (0.21; 16.80) | 1.00 (0.43; 2.33) | Apixaban | ||
| 2.31 (0.29; 18.26) | 1.25 (0.87; 1.79) | 1.24 (0.58; 2.66) | Aspirin | |
| 2.40 (0.29; 19.52) | 1.29 (0.77; 2.16) | 1.29 (0.55; 2.99) | 1.04 (0.72; 1.49) | Dabigatran |
| Stroke Recurrence. | ||||
| Rivaroxaban | ||||
| 1.08 (0.87; 1.34) | Aspirin | |||
| 1.13 (0.74; 1.72) | 1.04 (0.72; 1.51) | Apixaban | ||
| 1.26 (0.95; 1.68) | 1.17 (0.96; 1.42) | 1.12 (0.74; 1.70) | Dabigatran | |
| 3.46 (1.03; 11.67) | 3.20 (0.97; 10.60) | 3.07 (0.88; 10.75) | 2.74 (0.81; 9.20) | Warfarin |
| MACE. | ||||
| Rivaroxaban | ||||
| 1.02 (0.72; 1.45) | Apixaban | |||
| 1.06 (0.88; 1.28) | 1.04 (0.77; 1.39) | Aspirin | ||
| 1.17 (0.76; 1.79) | 1.14 (0.71; 1.85) | 1.10 (0.75; 1.61) | Warfarin | |
| 1.19 (0.91; 1.54) | 1.16 (0.82; 1.64) | 1.12 (0.94; 1.34) | 1.02 (0.67; 1.55) | Dabigatran |
| Combined Systemic Thromboembolism. | ||||
| Rivaroxaban | ||||
| 1.07 (0.87; 1.32) | Aspirin | |||
| 1.19 (0.77; 1.83) | 1.11 (0.76; 1.61) | Apixaban | ||
| 1.79 (0.76; 4.19) | 1.67 (0.73; 3.80) | 1.50 (0.61; 3.72) | Dabigatran | |
| 2.02 (0.93; 4.42) | 1.89 (0.89; 4.00) | 1.70 (0.73; 3.95) | 1.13 (0.37; 3.46) | Warfarin |
| Major Bleeding. | ||||
| Rivaroxaban | ||||
| 1.17 (0.38; 3.62) | Warfarin | |||
| 2.24 (1.26; 3.99) | 1.91 (0.65; 5.60) | Dabigatran | ||
| 2.70 (0.79; 9.16) | 2.30 (0.50; 10.56) | 1.21 (0.37; 3.90) | Apixaban | |
| 2.69 (1.67; 4.33) | 2.30 (0.83; 6.40) | 1.20 (0.87; 1.67) | 1.00 (0.32; 3.08) | Aspirin |
| Gastrointestinal Bleeding. | ||||
| Apixaban | ||||
| 2.16 (0.06; 75.01) | Warfarin | |||
| 2.39 (0.09; 61.18) | 1.11 (0.21; 5.75) | Dabigatran | ||
| 2.93 (0.12; 71.50) | 1.36 (0.29; 6.40) | 1.23 (0.70; 2.15) | Aspirin | |
| Intracranial Hemorrhage. | ||||
| Rivaroxaban | ||||
| 3.65 (0.04; 325.00) | Warfarin | |||
| 3.99 (0.39; 40.40) | 1.09 (0.01; 89.64) | Dabigatran | ||
| 3.99 (0.70; 22.82) | 1.09 (0.02; 68.34) | 1.00 (0.22; 4.58) | Aspirin | |
| 22.10 (1.01; 481.37) | 6.05 (0.05; 775.39) | 5.53 (0.29; 106.95) | 5.53 (0.44; 70.20) | Apixaban |
Stroke Recurrence
Based on the analysis of five RCTs, compared to aspirin, there was no significant difference observed between aspirin and rivaroxaban (RR: 1.08 with 95% CI (0.87, 1.34), P = .47) or apixaban (RR: 0.96 with 95% CI (0.66, 1.39), P = .83) or dabigatran (RR: 0.86 with 95% CI (0.70, 1.04), P = .11), or warfarin (RR: 0.31 with 95% CI (0.09, 1.03), P = .06) (Figure 5B). After ranking the treatment arms, rivaroxaban has the highest risk, followed by aspirin, apixaban, dabigatran, and warfarin (Table 4. Low heterogeneity was observed (I2 = 0%). Compared to warfarin, rivaroxaban was significantly associated with an increased incidence of stroke recurrence (RR: 3.46 with 95% CI (1.03, 11.67) (Table 4).
Major Adverse Cardiac Events
Based on the analysis of five RCTs, compared to aspirin, there was no significant difference observed between aspirin and rivaroxaban (RR: 1.06 with 95% CI (0.88, 1.28), P = .55) or apixaban (RR: 1.04 with 95% CI (0.77, 1.39), P = .80) or warfarin (RR: 0.91 with 95% CI (0.62, 1.33), P = .61), or dabigatran (RR: 0.89 with 95% CI (0.75, 1.07), P = .21) (Figure 5C). After ranking the treatment arms, rivaroxaban has the highest risk, followed by apixaban, aspirin, warfarin, and dabigatran (Table 4). Low heterogeneity was observed (I2 = 0%).
Combined Systemic Thromboembolism
Based on the analysis of five RCTs, compared to aspirin, there was no significant difference observed between aspirin and rivaroxaban (RR: 1.07 with 95% CI (0.87, 1.32), P = .16) or apixaban (RR: 0.90 with 95% CI (0.62, 1.31), P = .45) or dabigatran (RR: 0.60 with 95% CI (0.26, 1.37), P = .75), or warfarin (RR: 0.53 with 95% CI (0.25, 1.13), P = .84) (Figure 5D). After ranking the treatment arms, rivaroxaban has the highest risk, followed by aspirin, apixaban, dabigatran, and warfarin (Table 4). Low heterogeneity was observed (I2 = 0%).
Safety Outcomes
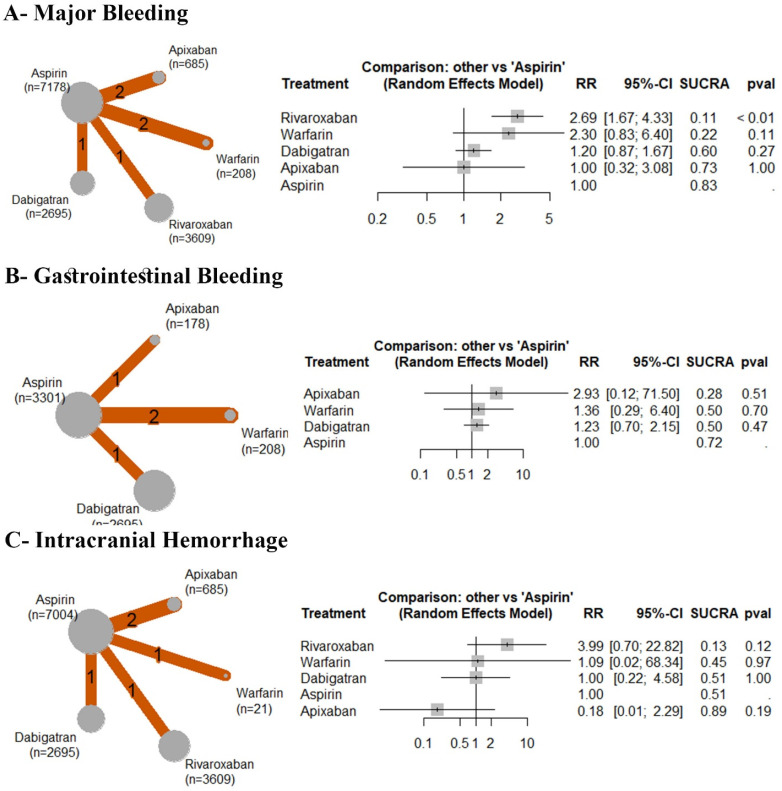
Major Bleeding
Based on the analysis of six RCTs, compared to aspirin, rivaroxaban was significantly associated with an increased incidence of major bleeding (RR: 2.69 with 95% CI (1.67, 4.33), P < 0.01); however, there was no significant difference observed between aspirin and warfarin (RR: 2.30 with 95% CI (0.83, 6.40), P = .11) or dabigatran (RR: 1.20 with 95% CI (0.87, 1.67), P = .27), or apixaban (RR: 1.00 with 95% CI (0.32, 3.08), P = 1.00) (Figure 6A). After ranking the treatment arms, rivaroxaban has the highest risk, followed by warfarin, dabigatran, apixaban, and aspirin (Table 4). Low heterogeneity was observed (I2 = 0%). Compared to dabigatran, rivaroxaban was significantly associated with an increased incidence of major bleeding (RR: 2.24 with 95% CI (1.26, 3.99) (Table 4).
Figure 6.
Forest and network plots of the safety outcomes, comparing various OACs versus aspirin. OACs: oral anticoagulants, MACE: major adverse cardiac events, RR: risk ratio, CI: confidence interval.
Gastrointestinal Bleeding
Based on the analysis of four RCTs, compared to aspirin, there was no significant difference observed between aspirin and apixaban (RR: 2.93 with 95% CI (0.12, 71.50), P = .51) or dabigatran (RR: 1.23 with 95% CI (0.70, 2.15), P = .47), or warfarin (RR: 1.36 with 95% CI (0.29, 6.40), P = .70) (Figure 6B). After ranking the treatment arms, apixaban has the highest risk, followed by warfarin, dabigatran, and aspirin (Table 4). Low heterogeneity was observed (I2 = 0%).
Intracranial Hemorrhage
Based on the analysis of five RCTs, compared to aspirin, there was no significant difference observed between aspirin and rivaroxaban (RR: 3.99 with 95% CI (0.70, 22.82), P = .12) or warfarin (RR: 1.09 with 95% CI (0.02, 68.34), P = .97) or dabigatran (RR: 1.00 with 95% CI (0.22, 4.58), P = 1.00), or apixaban (RR: 0.18 with 95% CI (0.01, 2.29), P = .19) (Figure 6C). After ranking the treatment arms, rivaroxaban has the highest risk, followed by warfarin, dabigatran, aspirin, and apixaban (Table 3). Low heterogeneity was observed (I2 = 15%). Compared to apixaban, rivaroxaban was significantly associated with an increased incidence of intracranial hemorrhage (RR: 22.10 with 95% CI (1.01, 481.37) (Table 4).
Discussion
Two previous meta-analyses revealed the benefits of using OACs over aspirin in reducing the rate of stroke recurrence without any significant difference in major bleeding.19,20 On the other hand, another meta-analysis conveyed no net benefit of OACs over antiplatelet therapy in reducing stroke recurrence with a non-significant increase in bleeding risk. 18 The present network meta-analysis comparing warfarin, direct-acting oral anticoagulants (DOACs), and aspirin revealed no significant benefit of anticoagulants over aspirin in mortality rates, stroke recurrence, bleeding, or cardiovascular events. The lack of benefit of anticoagulants over aspirin, especially in preventing recurrent ischemic events or strokes, can have numerous explanations. All the included RCTs showed no superiority of anticoagulation over antiplatelet therapy. The trials included different anticoagulants, including warfarin, apixaban, dabigatran, and rivaroxaban, compared to aspirin.15,16,21,27–30
Strokes constitute a significant cause of morbidity and mortality worldwide, with a high risk of recurrence unless secondary prevention is implied. Proper secondary prevention requires identifying the underlying stroke mechanism and risk factors. 31 Cryptogenic strokes are believed to be caused by cardio-embolism in the context of atrial cardiopathy or PFO.7,8,17 Since antithrombotic agents comprise a cornerstone in the secondary prevention of stroke cases, many trials investigated the efficacy of OACs in cryptogenic strokes. For instance, in cases with PFO, closure of the PFO plus antiplatelet therapy was superior to antiplatelet therapy alone but not OACs alone.16,18,32 Thus, OACs can be hypothesized to be used in cases where only medical treatment is considered. 19 Although there are published meta-analyses on the efficacy and safety of OACs compared to antiplatelets,18–20 to the authors’ knowledge, this is the first network meta-analysis to evaluate the use of different OACs and antiplatelets in cryptogenic strokes with the inclusion of recently published large trials.15,21
The primary hypothesis for using anticoagulants is that they would be superior to antiplatelets in cryptogenic stroke due to the presumed embolic origin of stroke, especially in the presence of factors promoting embolism, such as occult AF and PFO. 30 Yet, occult AF was detected in only 3% to 25% of trials where extensive electrocardiographic monitoring was initiated.15,21,30 Notably, the diagnosis of occult AF requires such patients to crossover to anticoagulant therapy if they were randomized to antiplatelet, which can affect the precision and interpretation of the results. 15 The presence of PFO among patients in some trials did not favor using anticoagulants either.16,28–30 Indeed, the presence of PFO and co-occurrence of stroke can be debated as to be a cryptogenic stroke. PFO can be found incidentally without clinical implications. 28 Homma et al, comparing warfarin to aspirin in stroke cases, showed higher rates of cryptogenic strokes with the presence and larger size of PFO. However, neither the presence nor the size affected the patients’ response to the medical therapy. 29 The indifference between medical treatments in cryptogenic stroke and PFO may suggest that PFO causes the first stroke but does not have any causal role in stroke recurrence. 33 This is consistent with American Heart Association/American Stroke Association guidelines, which state that antiplatelet therapy is reasonable in patients with an ischemic stroke or transient ischemic attack with a PFO. 31
Although a comprehensive diagnostic evaluation for a source of stroke was required for eligibility for the trials, the heterogeneous underlying sources of the embolic strokes (arterial, cardiogenic, or paradoxical) with variation in the composition of emboli may have resulted in enrolling patients that would not have benefited from anticoagulants. 30 Furthermore, most recurrent strokes occur in the same cerebral arterial territory as the index cryptogenic stroke, a finding that suggests an upstream atherosclerotic source rather than a central cardioembolic source. 34 The lack of benefit of anticoagulation over aspirin in trial participants despite selection for underlying cardiac abnormalities suggests that a substantial proportion of cryptogenic strokes may arise from non-stenosing atherosclerosis. 15
All anticoagulants have an inherent risk of bleeding in different anatomical sites with variable severity. 35 Despite the non-significant differences, we found that aspirin had lower risks of major bleeding, intracranial hemorrhage (ICH), or gastrointestinal bleeding. Furthermore, warfarin seemed to have higher risks for major bleeding, whereas, for ICH and gastrointestinal bleeding, DOACs ranked first in the risk assessment. Although DOACs are considered more tolerable with a better safety profile than warfarin, controversial results have been published regarding specific bleeding patterns, eg, major bleeding, ICH, and gastrointestinal bleeding, according to the populations’ characteristics and comorbidities.36–38 The clinical decision for either DOACs or warfarin should consider the notable differences between each of DOACs.
Regarding major bleeding and ICH, rivaroxaban had the highest risk, followed by warfarin, while apixaban had lower risks, even lower than aspirin. The trial by Hart et al, comparing rivaroxaban 15 mg to aspirin 100 mg daily, was early terminated due to the increased bleeding with the rivaroxaban group along with the lack of beneficial effect regarding stroke risk. 30 On the other hand, another recent trial showed that apixaban had a lower incidence of symptomatic ICH, although such a finding is limited to the small number of events. 15 Similarly, a meta-analysis of RCTs and real-world data suggested that apixaban can be safer than rivaroxaban.19,39
Strengths & Limitations
Although this is the first network meta-analysis to evaluate the use of different anticoagulants compared to antiplatelets for patients after cryptogenic stroke with possible sources of PFO in which we implemented frequentist methodology and adhered to the PRISMA recommendations for reviews evaluating randomized trials, it has some limitations. Some studies may differ in defining some outcome measures or diagnostic criteria. Small sample sizes of some studies may reduce the generalizability of the results. 28 Furthermore, the confidence intervals were wide, implying insufficient precision. Thus, our results are suggestive rather than definitive or conclusive. However, we included only RCTs that inherently provide the highest quality of evidence and precise and relevant interpretation, and the implications of such results should be in light of the real-world data. Also, the mean/median follow-up of the included trials was not more than two years.15,16,21,27–30 Finally, the aspirin dosage differed across the included trials, but most trials used low-dose aspirin (75-100 mg); thus, this is unlikely to affect the results.
Implications for Future Research
More extensive studies with large sample sizes and long follow-up durations are needed to conclude recommendations for using anticoagulants primarily over antiplatelets in cryptogenic strokes. Continuous monitoring of patients enrolling in all studies of cryptogenic stroke should be considered to reveal any possible occult conditions. More investigation should be performed into the underlying pathophysiology of cryptogenic strokes. Due to the difference in the characteristics of each anticoagulant, future studies may focus on investigating and comparing each extensively, which can lead to more precise and clinically relevant interpretations. So far, there is no sufficient evidence to recommend the use of anticoagulants in cryptogenic strokes with or without PFO.
Conclusion
There was no difference between various OACs and aspirin regarding efficacy and safety outcomes. Considering the higher risks and costs of anticoagulation, requiring regular laboratory checks and involving an increased probability of intracranial hemorrhage, we propose that anticoagulation has no benefits over antiplatelet therapy in patients with cryptogenic stroke. Further research is still warranted to define a personalized strategy for selecting antithrombotic strategies after cryptogenic stroke on a case-by-case basis.
Supplemental Material
Supplemental material, sj-docx-1-cat-10.1177_10760296241309639 for Optimal Antithrombotic Regimen After Cryptogenic Stroke: A Systematic Review and Network Meta-Analysis by Mohamed Abuelazm, Ahmed Mazen Amin, Hossam Tharwat Ali, Mohammed Ayyad, Abubakar Nazir, Mohammad Tanashat, Shrouk Ramadan, Basel Abdelazeem and James Robert Brašić in Clinical and Applied Thrombosis/Hemostasis
List of Abbreviations
- CVAs
Cerebrovascular accidents
- CENTRAL
Cochrane Central Register of Controlled Trials
- CI
Confidence interval
- DOACs
Direct oral anticoagulant
- ESUS
Embolic stroke of undetermined source
- ICH
Intracranial hemorrhage
- MACE
Major adverse cardiac events
- OACs
Oral anticoagulants
- PFO
Patent foramen ovale
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- RCTs
Randomized clinical trials
- RR
Risk ratio
- SUCRA
Surface Under the Cumulative Ranking Curve
Footnotes
Author Contributions: M.Ab. conceived the idea. M.Ab. designed the research workflow. B.A. and M.Ab. searched the databases. A.N., M.Ay., S.R., and M.T. screened the retrieved records, extracted relevant data, assessed the quality of evidence, and M.Ab. resolved the conflicts. AMA. performed the analysis. H.T., M.Ab., and AMA. wrote the final manuscript. J.R.B., M.Ab., and B.A. supervised the project. All authors have read and agreed to the final version of the manuscript.
Availability of Data and Materials: The data is available upon reasonable request from the corresponding author.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Abubakar Nazir https://orcid.org/0000-0002-6650-6982
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Laurent D, Small CN, Goutnik M, Hoh B. Ischemic stroke. In: Acute care neurosurg by case manag pearls pitfalls [internet]. 2022. [cited 2024 Apr 14]:159-172. https://www.ncbi.nlm.nih.gov/books/NBK499997/ [Google Scholar]
- 2.Shatri G, Senst B. Acute Stroke. In: StatPearls [Internet]. 2023. [cited 2024 Apr 14]. https://www.ncbi.nlm.nih.gov/books/NBK535369/ [Google Scholar]
- 3.Tiu C, Băjenaru O, Radu R, Terecoasa O. Etiologic classification of ischemic stroke: Where do we stand ? Clin Neurol Neurosurg [Internet]. 2017;159:93-106. https://pdf.sciencedirectassets.com/271158/1-s2.0-S0303846717X00073/1-s2.0-S030384671730149X/main.pdf?X-Amz-Security-Token=AgoJb3JpZ2luX2VjEMX%2F%2F%2F%2F%2F%2F%2F%2F%2F%2FwEaCXVzLWVhc3QtMSJIMEYCIQCG%2BS0gu0%2FhRkqNK%2FDLUtyQukd2n4umjdbxlihGlxYZQAIhAKlbH2 [DOI] [PubMed] [Google Scholar]
- 4.Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: The NINCDS stroke data bank. Ann Neurol. 1989;25:382-390. [DOI] [PubMed] [Google Scholar]
- 5.Donnan GA. Cryptogenic stroke. Int J Stroke. 2017;12:919. [DOI] [PubMed] [Google Scholar]
- 6.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35-41. [DOI] [PubMed] [Google Scholar]
- 7.Goette A, Kalman JM, Aguinaga L, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace. 2016;18:1455-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsh BJ, Copeland-Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism. J Am Coll Cardiol. 2015;65:2239-2251. [DOI] [PubMed] [Google Scholar]
- 9.Dhakal P, Verma V, Bhatt VR. Cryptogenic stroke. N Engl J Med [Internet]. 2016. [cited 2024 Apr 14];375:e26. http://www.ncbi.nlm.nih.gov/pubmed/27626544 [DOI] [PubMed] [Google Scholar]
- 10.Mazzucco S, Li L, Binney L, Rothwell PM. Prevalence of patent foramen ovale in cryptogenic transient ischaemic attack and non-disabling stroke at older ages: A population-based study, systematic review, and meta-analysis. Lancet Neurol. 2018;17:609-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GBD 2016 Menigitis Collaborators. Global, regional, and national burden of stroke, 1990–2016 : A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2016;17:1061-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosconi MG, Paciaroni M. Treatments in ischemic stroke: Current and future. Eur Neurol. 2022;85:349-366. [DOI] [PubMed] [Google Scholar]
- 13.Finsterer J. Management of cryptogenic stroke. Acta Neurol Belg. 2010;110:135-147. [PubMed] [Google Scholar]
- 14.Sacco RL, Prabhakaran S, Thompson JLP, et al. Comparison of warfarin versus aspirin for the prevention of recurrent stroke or death: Subgroup analyses from the warfarin-aspirin recurrent stroke study. Cerebrovasc Dis [Internet]. 2006;22:4-12. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L43875154%0Ahttp://dx.doi.org/10.1159/000092331 [DOI] [PubMed] [Google Scholar]
- 15.Kamel H, Longstreth T, Tirschwell DL, et al. Apixaban to prevent recurrence after cryptogenic stroke in patients with atrial cardiopathy the ARCADIA randomized clinical trial. Jama. 2024;331:573-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mas J-L, Derumeaux G, Guillon B, et al. Patent foramen Ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377:1011-1021. [DOI] [PubMed] [Google Scholar]
- 17.Saver JL, Mattle HP, Thaler D. Patent foramen ovale closure versus medical therapy for cryptogenic ischemic stroke a topical review. Stroke. 2018;49:1541-1548. [DOI] [PubMed] [Google Scholar]
- 18.Romoli M, Giannandrea D, Eusebi P, Cupini LM, Ricci S, Calabresi P. Aspirin or anticoagulation after cryptogenic stroke with patent foramen ovale: Systematic review and meta-analysis of randomized controlled trials. Neurol Sci. 2020;41:2819-2824. [DOI] [PubMed] [Google Scholar]
- 19.Huang WY, Ovbiagele B, Lee M. Oral anticoagulants vs antiplatelets in cryptogenic stroke with potential cardiac emboli: Meta-analysis. Eur J Intern Med. 2022;95:44-49. [DOI] [PubMed] [Google Scholar]
- 20.Sagris D, Georgiopoulos G, Perlepe K, et al. Antithrombotic treatment in cryptogenic stroke patients with patent foramen Ovale: systematic review and meta-analysis. Stroke. 2019;50:3135-3140. [DOI] [PubMed] [Google Scholar]
- 21.Geisler T, Keller T, Martus P, et al. Apixaban versus aspirin for embolic stroke of undetermined source. NEJM Evid. 2023;3. [DOI] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ [Internet]. 2021. [cited 2021 Aug 13];372. https://www.bmj.com/content/372/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann Intern Med. 2015;162:777-784. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Cochrane Handb Syst Rev Interv. 2019:1-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ [Internet]. 2019. [cited 2021 Jul 22];366. https://www.bmj.com/content/366/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 26.Mbuagbaw L, Rochwerg B, Jaeschke R, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diener H-C, Sacco RL, Easton JD, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. 2019;380:1906-1917. [DOI] [PubMed] [Google Scholar]
- 28.Shariat A, Yaghoubi E, Farazdaghi M, Aghasadeghi K, Haghighi AB. Comparison of medical treatments in cryptogenic stroke patients with patent foramen ovale: A randomized clinical trial. J Res Med Sci. 2013;18:94-98. [PMC free article] [PubMed] [Google Scholar]
- 29.Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: Patent foramen ovale in Cryptogenic Stroke Study. Circulation. 2002;105:2625-2631. [DOI] [PubMed] [Google Scholar]
- 30.Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378:2191-2201. [DOI] [PubMed] [Google Scholar]
- 31.Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:E364-E467. [DOI] [PubMed] [Google Scholar]
- 32.Søndergaard L, Kasner SE, Rhodes JF, et al. Patent foramen Ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2020;382:978-978. [DOI] [PubMed] [Google Scholar]
- 33.Mono M-L, Geister L, Galimanis A, et al. Patent foramen ovale may be causal for the first stroke but unrelated to subsequent ischemic events. Stroke [Internet]. 2011;42:2891-2895. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed13&NEWS=N&AN=51562810 [DOI] [PubMed] [Google Scholar]
- 34.Veltkamp R, Pearce LA, Korompoki E, et al. Characteristics of recurrent ischemic stroke after embolic stroke of undetermined source: Secondary analysis of a randomized clinical trial. JAMA Neurol. 2020;77:1233-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ballestri S, Romagnoli E, Arioli D, et al. Risk and management of bleeding complications with direct oral anticoagulants in patients with atrial fibrillation and venous thromboembolism: A narrative review. Adv Ther. 2023;40:41-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abuelazm M, Abdelazeem B, Katamesh BE, et al. The Efficacy and Safety of Direct Oral Anticoagulants versus Standard of Care in Patients without an Indication of Anticoagulants after Transcatheter Aortic Valve Replacement: A Meta-Analysis of Randomized Controlled Trials. J Clin Med. 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abuelazm M, Mahmoud A, Ali S, et al. The efficacy and safety of direct factor Xa inhibitors versus vitamin K antagonists for atrial fibrillation in patients on hemodialysis: A meta-analysis of randomized controlled trials. Baylor Univ Med Cent Proc. 2023;36:736-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng S, Zheng Y, Jiang J, Ma J, Zhu W, Cai X. Effectiveness and safety of DOACs vs. Warfarin in patients with atrial fibrillation and frailty: A systematic review and meta-analysis. Front Cardiovasc Med. 2022;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin SY, Kuo CH, Yeh SJ, et al. Real-World rivaroxaban and apixaban levels in Asian patients with atrial fibrillation. Clin Pharmacol Ther. 2020;107:278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cat-10.1177_10760296241309639 for Optimal Antithrombotic Regimen After Cryptogenic Stroke: A Systematic Review and Network Meta-Analysis by Mohamed Abuelazm, Ahmed Mazen Amin, Hossam Tharwat Ali, Mohammed Ayyad, Abubakar Nazir, Mohammad Tanashat, Shrouk Ramadan, Basel Abdelazeem and James Robert Brašić in Clinical and Applied Thrombosis/Hemostasis