Abstract
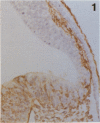
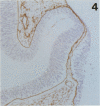
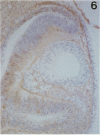
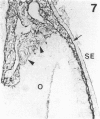
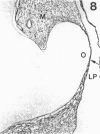
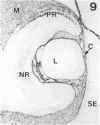
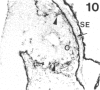
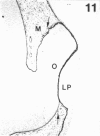
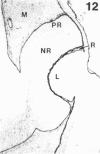
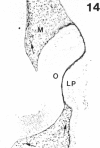
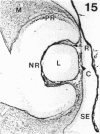
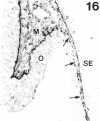
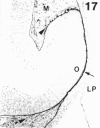
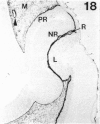
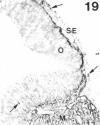
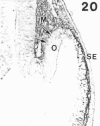
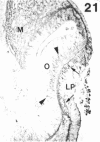
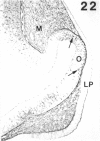
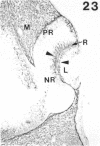
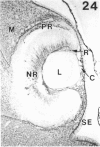
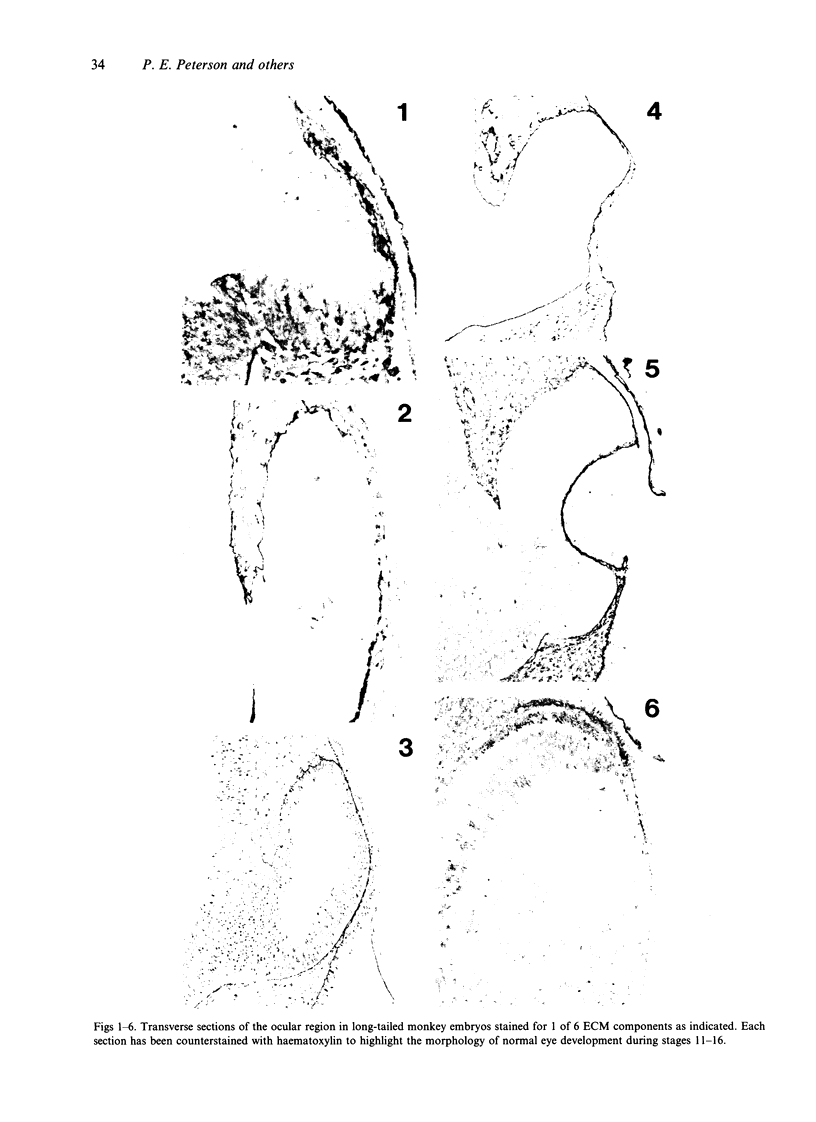
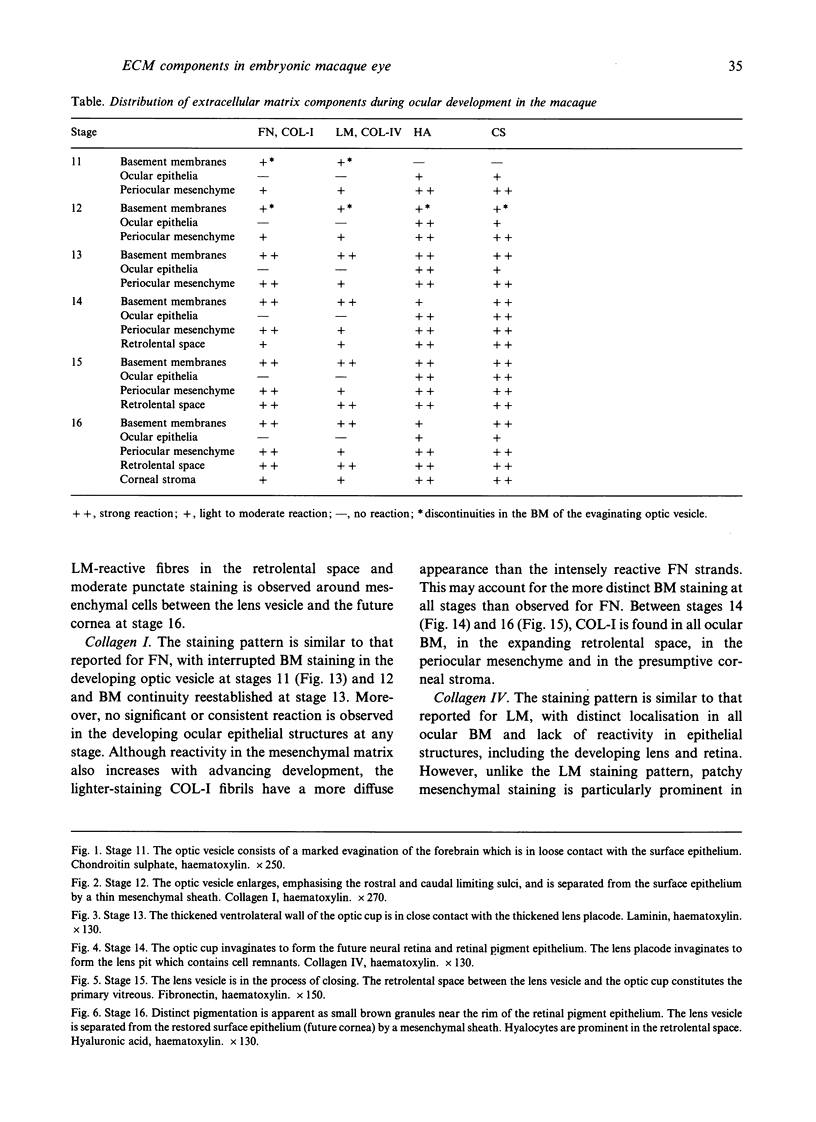
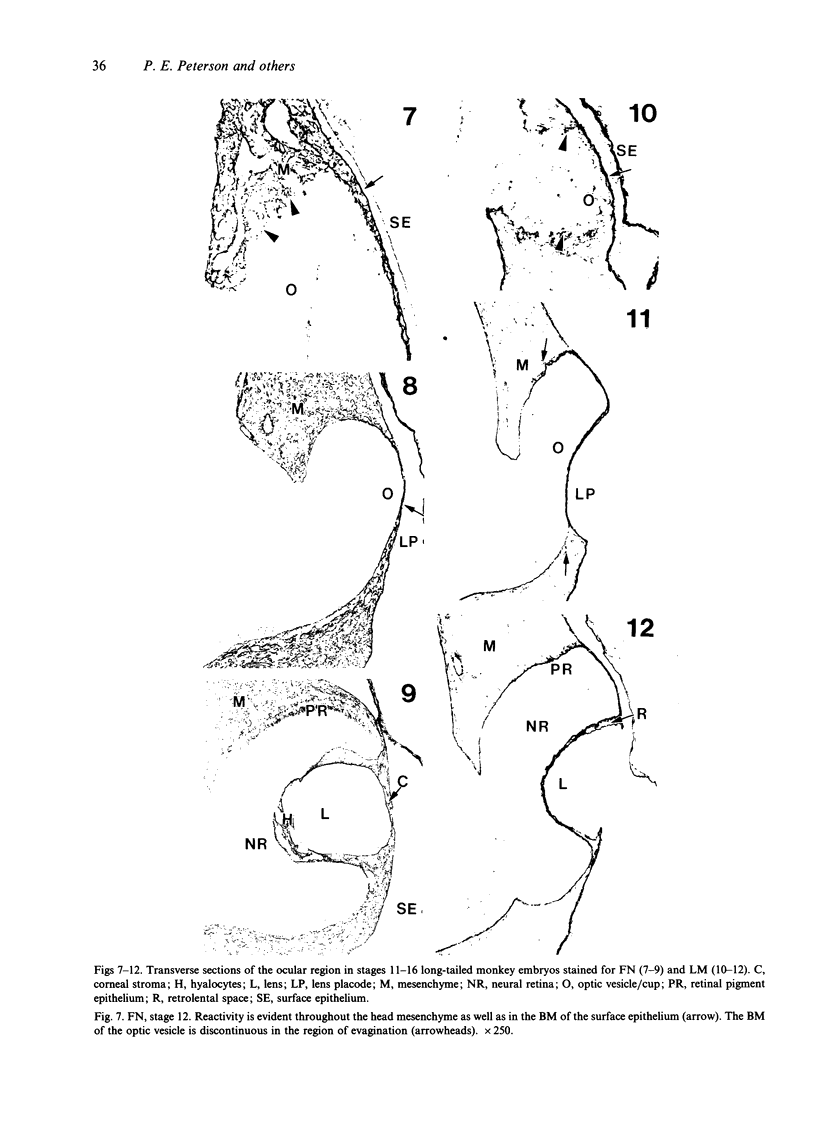
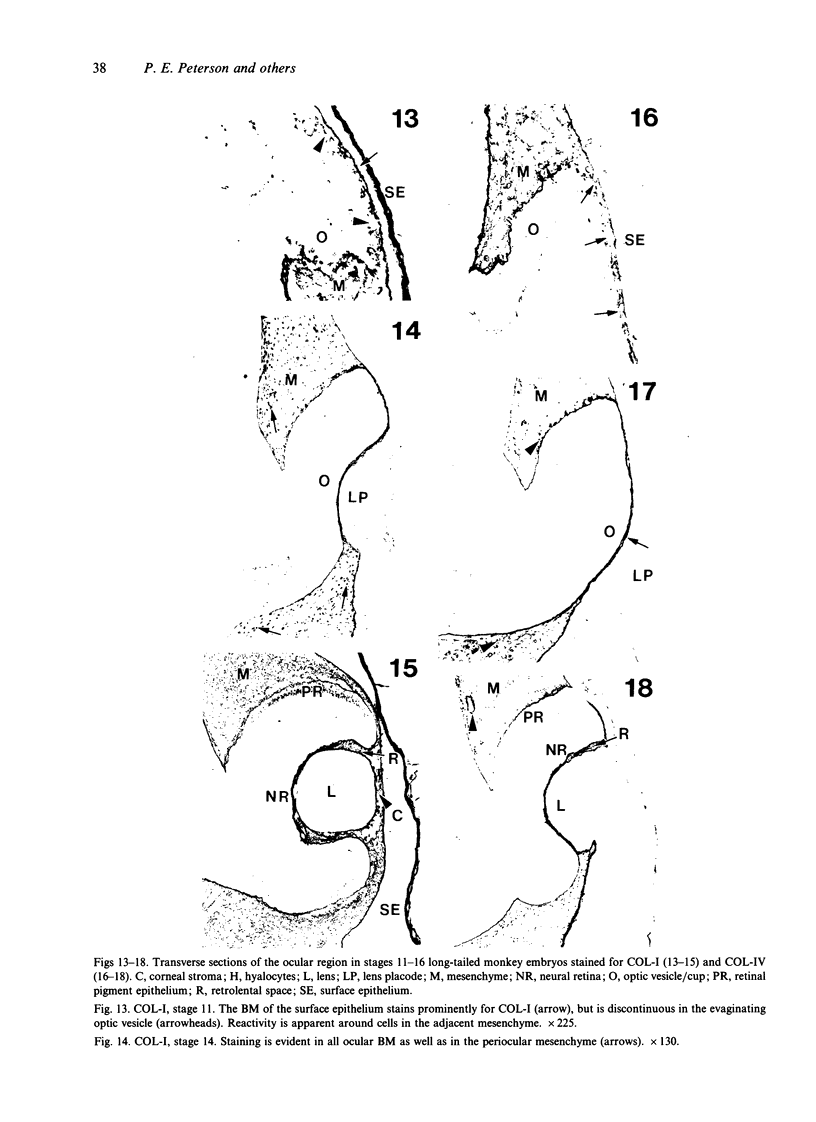
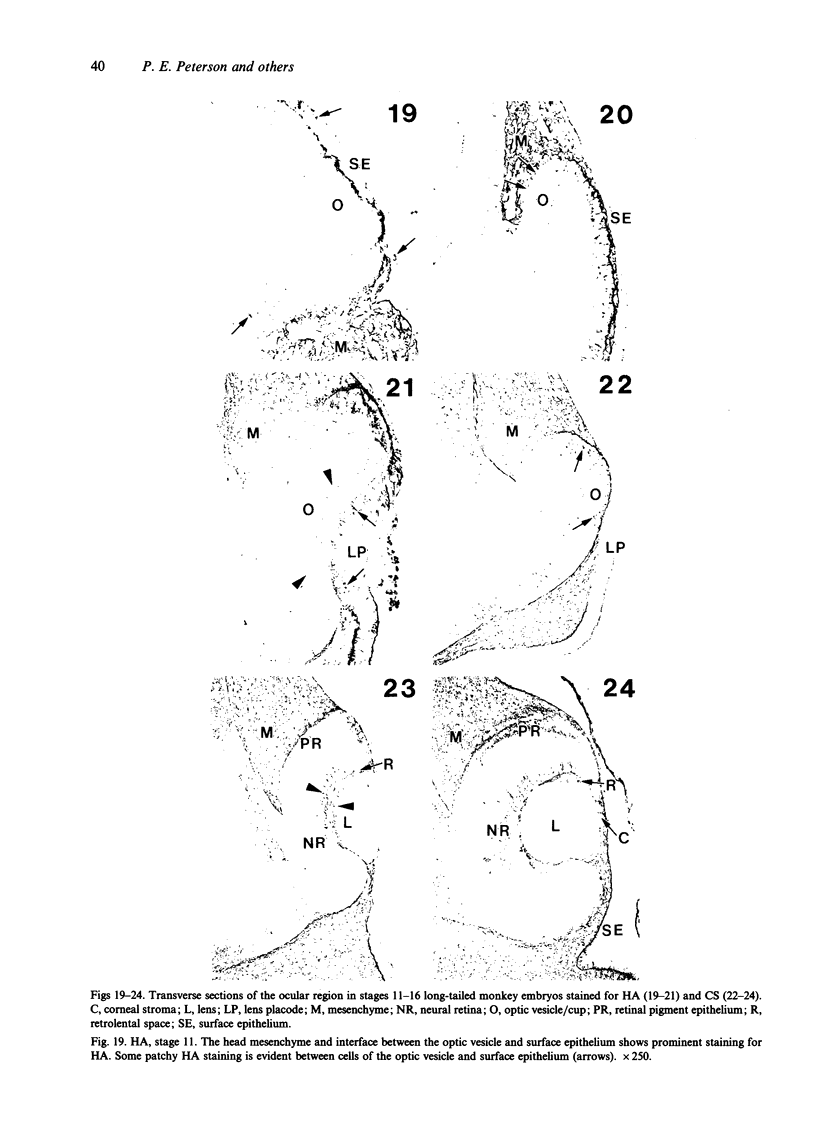
The composition of the extracellular matrix (ECM) was examined in the developing lens and optic cup (stages 11-16) of the long-tailed monkey (Macaca fascicularis) using peroxidase immunocytochemistry. The glycoproteins, fibronectin, laminin, and collagen types I and IV, were consistently associated with basement membranes (BM) of ocular epithelia at all stages examined. Discontinuity of the optic cup BM was observed during the early stages of evagination (stages 11 and 12); the even distribution of all 4 components was reestablished by stage 13 when the optic vesicle is closely apposed to the thickened lens placode. While fibronectin was most predominant in the mesenchymal matrix, all 4 glycoproteins were observed to variable degrees in the periocular mesenchyme. Particularly strong glycoprotein reactivity was observed in the interspace between the invaginating lens vesicle and optic cup whereas no significant reactivity occurred within the lens, developing retina or future corneal epithelium. Two glycosaminoglycans, hyaluronic acid and chondroitin sulphate, had virtually identical widespread staining patterns in all ocular BM and throughout the periocular mesenchyme and adjacent epithelial tissues, including the lens and retina. The observed temporal and regional staining patterns suggest that these ECM components are morphogenetic factors in the macaque eye, facilitating the complex series of integrated tissue interactions, movements and shape changes during the earliest stages of lens and optic vesicle morphogenesis. The macaque offers a valuable model to study these interactions due to the prolonged period of ocular development which is morphologically identical to humans.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avnur Z., Geiger B. Immunocytochemical localization of native chondroitin-sulfate in tissues and cultured cells using specific monoclonal antibody. Cell. 1984 Oct;38(3):811–822. doi: 10.1016/0092-8674(84)90276-9. [DOI] [PubMed] [Google Scholar]
- Bard J. B., Bansal M. K., Ross A. S. The extracellular matrix of the developing cornea: diversity, deposition and function. Development. 1988;103 (Suppl):195–205. doi: 10.1242/dev.103.Supplement.195. [DOI] [PubMed] [Google Scholar]
- Bilozur M. E., Hay E. D. Neural crest migration in 3D extracellular matrix utilizes laminin, fibronectin, or collagen. Dev Biol. 1988 Jan;125(1):19–33. doi: 10.1016/0012-1606(88)90055-3. [DOI] [PubMed] [Google Scholar]
- Bremer F. M. Histochemical study on glycosaminoglycans in the developing mouse vitreous. Histochemistry. 1987;87(6):579–583. doi: 10.1007/BF00492474. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Experimental analyses of the migration and cell lineage of avian neural crest cells. Cleft Palate J. 1990 Apr;27(2):110–120. doi: 10.1597/1545-1569(1990)027<0110:eaotma>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Cohn R. H., Banerjee S. D., Bernfield M. R. Basal lamina of embryonic salivary epithelia. Nature of glycosaminoglycan and organization of extracellular materials. J Cell Biol. 1977 May;73(2):464–478. doi: 10.1083/jcb.73.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp A. J., Bernfield M. Glycosaminoglycans vary in accumulation along the neuraxis during spinal neurulation in the mouse embryo. Dev Biol. 1988 Dec;130(2):573–582. doi: 10.1016/0012-1606(88)90352-1. [DOI] [PubMed] [Google Scholar]
- Davignon R. W., Parker R. M., Hendrickx A. G. Staging of the early embryonic brain in the baboon (Papio cynocephalus) and rhesus monkey (macaca mulatta). Anat Embryol (Berl) 1980;159(3):317–334. doi: 10.1007/BF00317654. [DOI] [PubMed] [Google Scholar]
- Derby M. A. Analysis of glycosaminoglycans within the extracellular environments encountered by migrating neural crest cells. Dev Biol. 1978 Oct;66(2):321–336. doi: 10.1016/0012-1606(78)90241-5. [DOI] [PubMed] [Google Scholar]
- Dong L. J., Chung A. E. The expression of the genes for entactin, laminin A, laminin B1 and laminin B2 in murine lens morphogenesis and eye development. Differentiation. 1991 Dec;48(3):157–172. doi: 10.1111/j.1432-0436.1991.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Engvall E., Davis G. E., Dickerson K., Ruoslahti E., Varon S., Manthorpe M. Mapping of domains in human laminin using monoclonal antibodies: localization of the neurite-promoting site. J Cell Biol. 1986 Dec;103(6 Pt 1):2457–2465. doi: 10.1083/jcb.103.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foellmer H. G., Madri J. A., Furthmayr H. Methods in laboratory investigation. Monoclonal antibodies to type IV collagen: probes for the study of structure and function of basement membranes. Lab Invest. 1983 May;48(5):639–649. [PubMed] [Google Scholar]
- Green S. J., Tarone G., Underhill C. B. Distribution of hyaluronate and hyaluronate receptors in the adult lung. J Cell Sci. 1988 May;90(Pt 1):145–156. doi: 10.1242/jcs.90.1.145. [DOI] [PubMed] [Google Scholar]
- Haloui Z., Jeanny J. C., Jonet L., Courtois Y., Laurent M. Immunochemical analysis of extracellular matrix during embryonic lens development of the Cat Fraser mouse. Exp Eye Res. 1988 Apr;46(4):463–474. doi: 10.1016/s0014-4835(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hendrickx A. G., Binkerd P. E. Nonhuman primates and teratological research. J Med Primatol. 1990;19(2):81–108. [PubMed] [Google Scholar]
- Hendrickx A. G., Cukierski M. A. Reproductive and developmental toxicology in nonhuman primates. Prog Clin Biol Res. 1987;235:73–88. [PubMed] [Google Scholar]
- Hendrix R. W., Zwaan J. The matrix of the optic vesicle-presumptive lens interface during induction of the lens in the chicken embryo. J Embryol Exp Morphol. 1975 Jul;33(4):1023–1049. [PubMed] [Google Scholar]
- Hilfer S. R., Randolph G. J. Immunolocalization of basal lamina components during development of chick otic and optic primordia. Anat Rec. 1993 Mar;235(3):443–452. doi: 10.1002/ar.1092350313. [DOI] [PubMed] [Google Scholar]
- McCarthy K. J., Accavitti M. A., Couchman J. R. Immunological characterization of a basement membrane-specific chondroitin sulfate proteoglycan. J Cell Biol. 1989 Dec;109(6 Pt 1):3187–3198. doi: 10.1083/jcb.109.6.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy R. A., Hay E. D. Collagen I, laminin, and tenascin: ultrastructure and correlation with avian neural crest formation. Int J Dev Biol. 1991 Dec;35(4):437–452. [PubMed] [Google Scholar]
- Morriss-Kay G., Tuckett F. Immunohistochemical localisation of chondroitin sulphate proteoglycans and the effects of chondroitinase ABC in 9- to 11-day rat embryos. Development. 1989 Aug;106(4):787–798. doi: 10.1242/dev.106.4.787. [DOI] [PubMed] [Google Scholar]
- Morriss G. M., Solursh M. Regional differences in mesenchymal cell morphology and glycosaminoglycans in early neural-fold stage rat embryos. J Embryol Exp Morphol. 1978 Aug;46:37–52. [PubMed] [Google Scholar]
- Parmigiani C., McAvoy J. Localisation of laminin and fibronectin during rat lens morphogenesis. Differentiation. 1984;28(1):53–61. doi: 10.1111/j.1432-0436.1984.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Peterson P. E., Pow C. S., Wilson D. B., Hendrickx A. G. Distribution of extracellular matrix components during early embryonic development in the macaque. Acta Anat (Basel) 1993;146(1):3–13. doi: 10.1159/000147414. [DOI] [PubMed] [Google Scholar]
- Poelmann R. E., Gittenberger-de Groot A. C., Mentink M. M., Delpech B., Girard N., Christ B. The extracellular matrix during neural crest formation and migration in rat embryos. Anat Embryol (Berl) 1990;182(1):29–39. doi: 10.1007/BF00187525. [DOI] [PubMed] [Google Scholar]
- Saha M. S., Spann C. L., Grainger R. M. Embryonic lens induction: more than meets the optic vesicle. Cell Differ Dev. 1989 Dec;28(3):153–171. doi: 10.1016/0922-3371(89)90001-4. [DOI] [PubMed] [Google Scholar]
- Sarthy V. Collagen IV mRNA expression during development of the mouse retina: an in situ hybridization study. Invest Ophthalmol Vis Sci. 1993 Jan;34(1):145–152. [PubMed] [Google Scholar]
- Smith B. S. Histochemical analysis of extracellular matrix material in embryonic trisomy 1 mouse eye. Dev Genet. 1989;10(4):287–291. doi: 10.1002/dvg.1020100402. [DOI] [PubMed] [Google Scholar]
- Solursh M., Morriss G. M. Glycosaminoglycan synthesis in rat embryos during the formation of the primary mesenchyme and neural folds. Dev Biol. 1977 May;57(1):75–86. doi: 10.1016/0012-1606(77)90355-4. [DOI] [PubMed] [Google Scholar]
- Svoboda K. K., O'Shea K. S. An analysis of cell shape and the neuroepithelial basal lamina during optic vesicle formation in the mouse embryo. Development. 1987 Jun;100(2):185–200. doi: 10.1242/dev.100.2.185. [DOI] [PubMed] [Google Scholar]
- Tripathi B. J., Tripathi R. C., Livingston A. M., Borisuth N. S. The role of growth factors in the embryogenesis and differentiation of the eye. Am J Anat. 1991 Dec;192(4):442–471. doi: 10.1002/aja.1001920411. [DOI] [PubMed] [Google Scholar]
- Tucker R. P. The distribution of J1/tenascin and its transcript during the development of the avian cornea. Differentiation. 1991 Nov;48(2):59–66. doi: 10.1111/j.1432-0436.1991.tb00243.x. [DOI] [PubMed] [Google Scholar]
- Tuckett F., Morriss-Kay G. M. The distribution of fibronectin, laminin and entactin in the neurulating rat embryo studied by indirect immunofluorescence. J Embryol Exp Morphol. 1986 Jun;94:95–112. [PubMed] [Google Scholar]
- Waterman R. E., Balian G. Indirect immunofluorescent staining of fibronectin associated with the floor of the foregut during formation and rupture of the oral membrane in the chick embryo. Anat Rec. 1980 Dec;198(4):619–635. doi: 10.1002/ar.1091980407. [DOI] [PubMed] [Google Scholar]
- Webster E. H., Jr, Silver A. F., Gonsalves N. I. Histochemical analysis of extracellular matrix material in embryonic mouse lens morphogenesis. Dev Biol. 1983 Nov;100(1):147–157. doi: 10.1016/0012-1606(83)90205-1. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Hendrickx A. G. Quantitative aspects of the cell cycle in the cranial neural tube of the rhesus monkey (Macaca mulatta) during early stages of gestation. J Craniofac Genet Dev Biol. 1986;6(4):363–368. [PubMed] [Google Scholar]
- Wilson D. B., Hendrickx A. G., Sawyer R. H. Distribution of [3H] thymidine in the lends of the rhesus monkey (Macaca mulatta) embryo. Exp Eye Res. 1976 Oct;23(4):417–423. doi: 10.1016/0014-4835(76)90170-6. [DOI] [PubMed] [Google Scholar]