Abstract
There is clear evidence that tissues related to the intervertebral disc, such as articular cartilage, contain several phenotypically different chondrocytic cell populations. Histological data for the disc suggest the same may be true for the annulus fibrosus and nucleus pulposus, but this has not been shown directly. For the first time, cells from adult human nondegenerative nucleus pulposus and annulus fibrosus were recovered after enzymatic digestion and maintained in an alginate bead culture system for up to 6 wk. The cells remained viable and produced matrix, but did not divide. Cultured cells were stained simultaneously for the presence of chondroitin sulphate and keratan sulphate, or types I and II collagen. The majority of the cells from both the annulus fibrosus and the nucleus pulposus produced both keratan sulphate and chondroitin sulphate (> 60%), a few only detectable levels of one or the other, but a significant population produced neither. This is an indication of a population of cells with a nonchondrocytic phenotype. In nondegenerative discs, the majority of the annulus fibrosus cells produced both types I and II collagen but the majority of nucleus pulposus cells produced only type II collagen. These observations are consistent with the presence of at least 2 phenotypically stable populations of cells in the adult human intervertebral disc and with the view that the phenotype of the major population of the annulus is different from that of the nucleus.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benya P. D., Shaffer J. D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982 Aug;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bradford D. S., Cooper K. M., Oegema T. R., Jr Chymopapain, chemonucleolysis, and nucleus pulposus regeneration. J Bone Joint Surg Am. 1983 Dec;65(9):1220–1231. [PubMed] [Google Scholar]
- Caterson B., Christner J. E., Baker J. R., Couchman J. R. Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed Proc. 1985 Feb;44(2):386–393. [PubMed] [Google Scholar]
- Caterson B., Mahmoodian F., Sorrell J. M., Hardingham T. E., Bayliss M. T., Carney S. L., Ratcliffe A., Muir H. Modulation of native chondroitin sulphate structure in tissue development and in disease. J Cell Sci. 1990 Nov;97(Pt 3):411–417. doi: 10.1242/jcs.97.3.411. [DOI] [PubMed] [Google Scholar]
- Chelberg M. K., McCarthy J. B., Skubitz A. P., Furcht L. T., Tsilibary E. C. Characterization of a synthetic peptide from type IV collagen that promotes melanoma cell adhesion, spreading, and motility. J Cell Biol. 1990 Jul;111(1):261–270. doi: 10.1083/jcb.111.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelberg M. K., Tsilibary E. C., Hauser A. R., McCarthy J. B. Type IV collagen-mediated melanoma cell adhesion and migration: involvement of multiple, distinct domains of the collagen molecule. Cancer Res. 1989 Sep 1;49(17):4796–4802. [PubMed] [Google Scholar]
- Eyre D. R., Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta. 1977 May 27;492(1):29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Fernandez M. P., Selmin O., Martin G. R., Yamada Y., Pfäffle M., Deutzmann R., Mollenhauer J., von der Mark K. The structure of anchorin CII, a collagen binding protein isolated from chondrocyte membrane. J Biol Chem. 1990 May 15;265(14):8344–8344. [PubMed] [Google Scholar]
- Gannon J. M., Walker G., Fischer M., Carpenter R., Thompson R. C., Jr, Oegema T. R., Jr Localization of type X collagen in canine growth plate and adult canine articular cartilage. J Orthop Res. 1991 Jul;9(4):485–494. doi: 10.1002/jor.1100090404. [DOI] [PubMed] [Google Scholar]
- Guo J. F., Jourdian G. W., MacCallum D. K. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res. 1989;19(2-4):277–297. doi: 10.3109/03008208909043901. [DOI] [PubMed] [Google Scholar]
- Humzah M. D., Soames R. W. Human intervertebral disc: structure and function. Anat Rec. 1988 Apr;220(4):337–356. doi: 10.1002/ar.1092200402. [DOI] [PubMed] [Google Scholar]
- Häuselmann H. J., Aydelotte M. B., Schumacher B. L., Kuettner K. E., Gitelis S. H., Thonar E. J. Synthesis and turnover of proteoglycans by human and bovine adult articular chondrocytes cultured in alginate beads. Matrix. 1992 Apr;12(2):116–129. doi: 10.1016/s0934-8832(11)80053-3. [DOI] [PubMed] [Google Scholar]
- Jones K. H., Senft J. A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985 Jan;33(1):77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- Ludwigs U., Elgavish A., Esko J. D., Meezan E., Rodén L. Reaction of unsaturated uronic acid residues with mercuric salts. Cleavage of the hyaluronic acid disaccharide 2-acetamido-2-deoxy-3-O-(beta-D-gluco-4-enepyranosyluronic acid)-D-glucose. Biochem J. 1987 Aug 1;245(3):795–804. doi: 10.1042/bj2450795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons G., Eisenstein S. M., Sweet M. B. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981 Apr 3;673(4):443–453. doi: 10.1016/0304-4165(81)90476-1. [DOI] [PubMed] [Google Scholar]
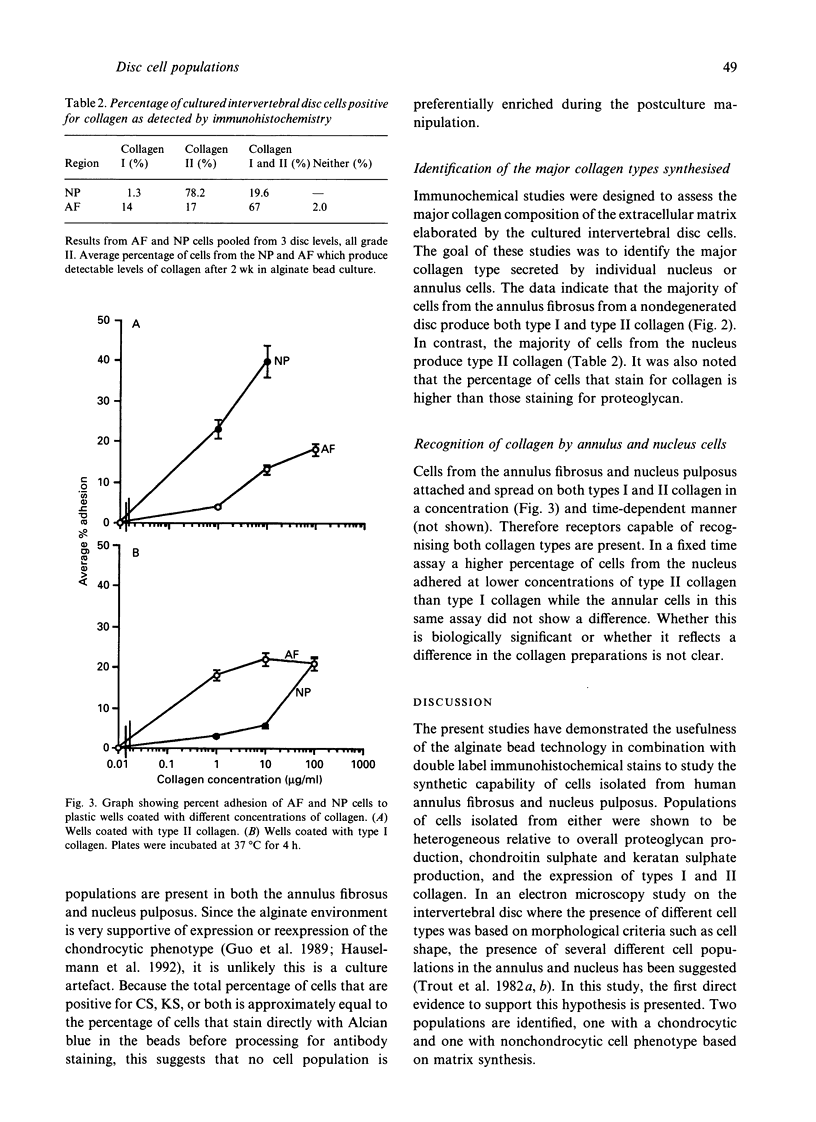
- Maldonado B. A., Oegema T. R., Jr Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 1992 Sep;10(5):677–690. doi: 10.1002/jor.1100100510. [DOI] [PubMed] [Google Scholar]
- McCarthy J. B., Chelberg M. K., Mickelson D. J., Furcht L. T. Localization and chemical synthesis of fibronectin peptides with melanoma adhesion and heparin binding activities. Biochemistry. 1988 Feb 23;27(4):1380–1388. doi: 10.1021/bi00404a044. [DOI] [PubMed] [Google Scholar]
- Mehmet H., Scudder P., Tang P. W., Hounsell E. F., Caterson B., Feizi T. The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve sulphated hepta- or larger oligosaccharides of the poly(N-acetyllactosamine) series. Eur J Biochem. 1986 Jun 2;157(2):385–391. doi: 10.1111/j.1432-1033.1986.tb09680.x. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Rhodes R. K. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;82(Pt A):33–64. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- Mörgelin M., Paulsson M., Malmström A., Heinegård D. Shared and distinct structural features of interstitial proteoglycans from different bovine tissues revealed by electron microscopy. J Biol Chem. 1989 Jul 15;264(20):12080–12090. [PubMed] [Google Scholar]
- Neame P. J., Choi H. U., Rosenberg L. C. The primary structure of the core protein of the small, leucine-rich proteoglycan (PG I) from bovine articular cartilage. J Biol Chem. 1989 May 25;264(15):8653–8661. [PubMed] [Google Scholar]
- Oegema T. R., Jr, Bradford D. S., Cooper K. M. Aggregated proteoglycan synthesis in organ cultures of human nucleus pulposus. J Biol Chem. 1979 Nov 10;254(21):10579–10581. [PubMed] [Google Scholar]
- Plaas A. H., Ison A. L., Ackland J. Synthesis of small proteoglycans substituted with keratan sulfate by rabbit articular chondrocytes. J Biol Chem. 1989 Aug 25;264(24):14447–14454. [PubMed] [Google Scholar]
- Scott J. E., Bosworth T. R., Cribb A. M., Taylor J. R. The chemical morphology of age-related changes in human intervertebral disc glycosaminoglycans from cervical, thoracic and lumbar nucleus pulposus and annulus fibrosus. J Anat. 1994 Feb;184(Pt 1):73–82. [PMC free article] [PubMed] [Google Scholar]
- Thompson J. P., Pearce R. H., Schechter M. T., Adams M. E., Tsang I. K., Bishop P. B. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine (Phila Pa 1976) 1990 May;15(5):411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- Trout J. J., Buckwalter J. A., Moore K. C., Landas S. K. Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell. 1982;14(2):359–369. doi: 10.1016/0040-8166(82)90033-7. [DOI] [PubMed] [Google Scholar]
- Trout J. J., Buckwalter J. A., Moore K. C. Ultrastructure of the human intervertebral disc: II. Cells of the nucleus pulposus. Anat Rec. 1982 Dec;204(4):307–314. doi: 10.1002/ar.1092040403. [DOI] [PubMed] [Google Scholar]
- Vaughan L., Mendler M., Huber S., Bruckner P., Winterhalter K. H., Irwin M. I., Mayne R. D-periodic distribution of collagen type IX along cartilage fibrils. J Cell Biol. 1988 Mar;106(3):991–997. doi: 10.1083/jcb.106.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon-Roberts B., Pirie C. J. Degenerative changes in the intervertebral discs of the lumbar spine and their sequelae. Rheumatol Rehabil. 1977 Feb;16(1):13–21. doi: 10.1093/rheumatology/16.1.13. [DOI] [PubMed] [Google Scholar]