Abstract
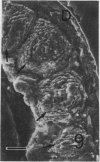
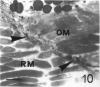
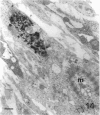
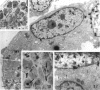
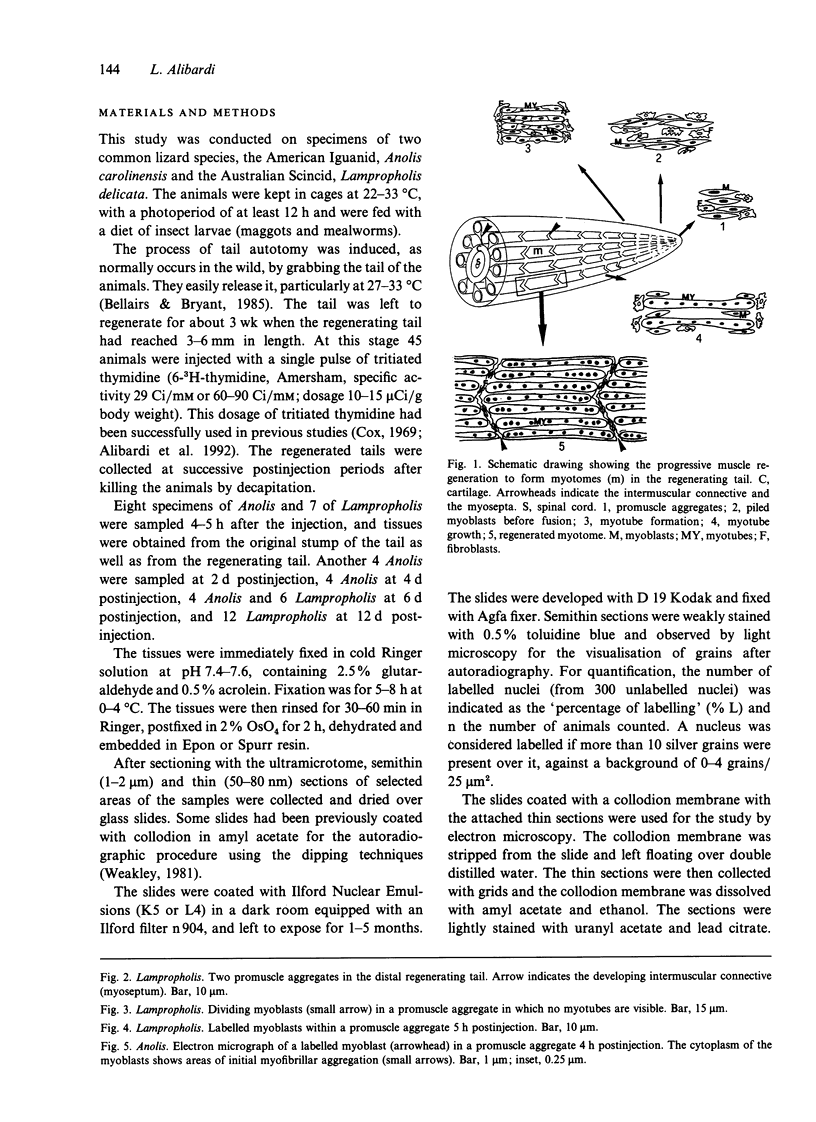
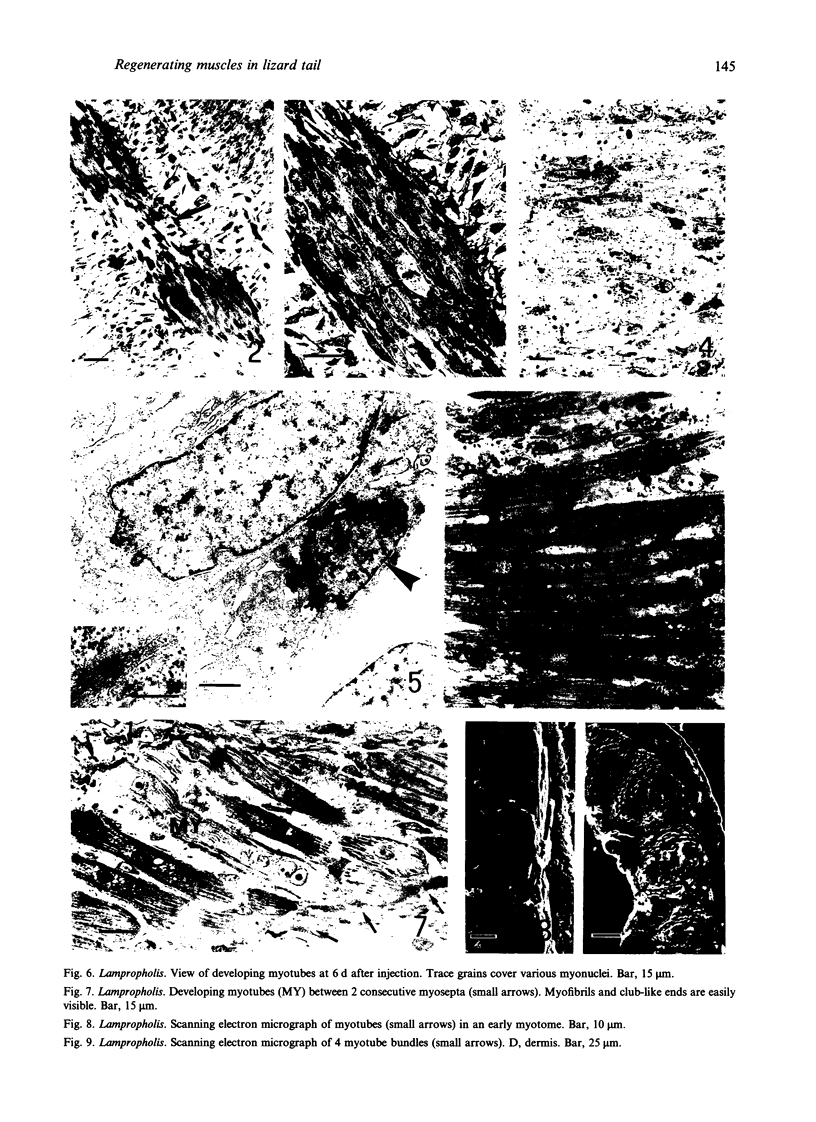
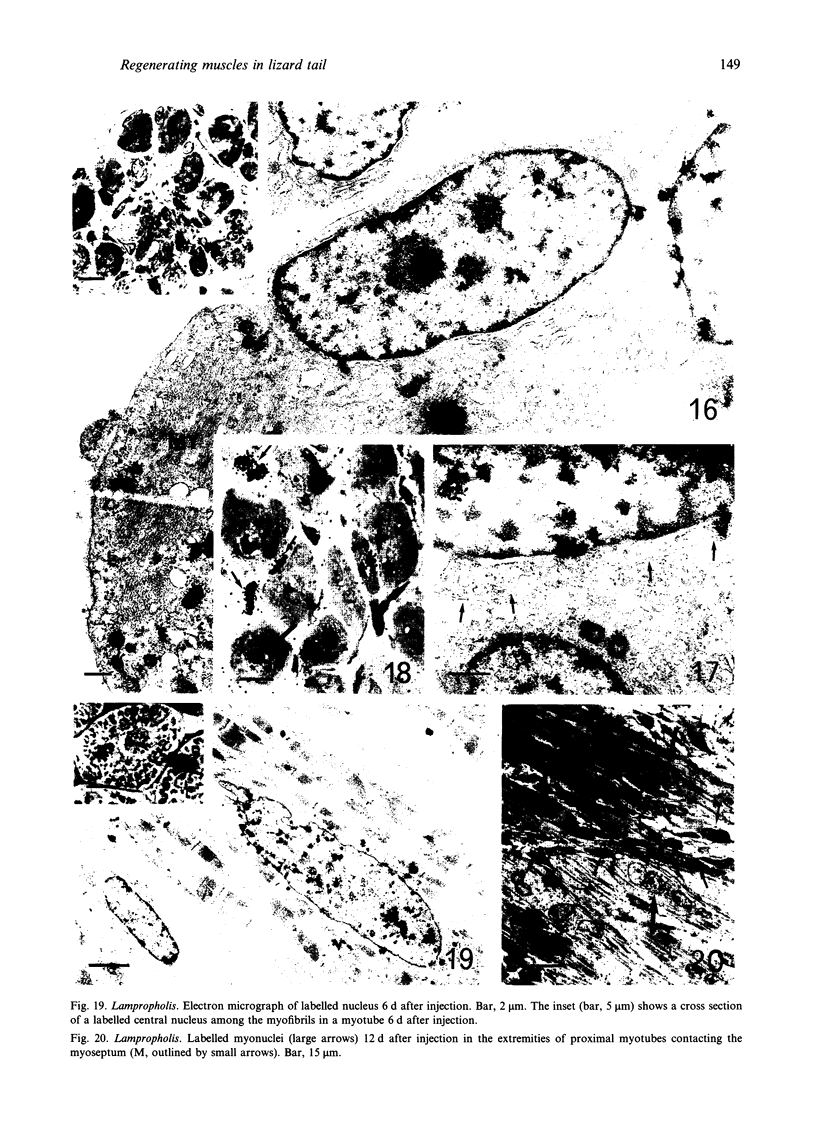
The differentiation of muscles in the lizards Anolis and Lampropholis with tails that had regenerated for 21-50 d was investigated by light and electron microscope autoradiography using tritiated thymidine. At the apex of the regenerating tail, groups of 4-8 myoblasts of the promuscle aggregates fused to produce bundles of myotubes whose multiple labelled and unlabelled nuclei appeared to be distributed at random. The formation of the first myotubes and their growth is responsible for the formation of the myotome primordia and their separation from the intermuscular connective myosepta. More nuclei were added with the lengthening of the myotubes--up to 14-18 nuclei in the oldest proximal myotubes. At 4-5 h after injection labelled nuclei were found outside the myotubes while at 2-6 d after injection many labelled nuclei were observed in the myotubes, particularly near the two ends of the myotubular sarcoplasm contacting the myoseptum. This change from the initial distribution suggests that the growth of the myotubes takes place mostly at their terminals. There is an apparent correlation between the number of nuclei and the final length of the myotubes and myotome. The insertion of fibres with a similar number of nuclei and lengths into the pinnated connective myoseptum of the original musculature, the autotomy plane, probably determines the wave-like shape of the muscles within the regenerated myotomes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alibardi L., Gibbons J., Simpson S., Jr Fine structure of cells in the young regenerating spinal cord of the lizard Anolis carolinensis after H3-thymidine administration. Biol Struct Morphog. 1992;4(2):45–52. [PubMed] [Google Scholar]
- Boudjelida H., Muntz L. Multinucleation during myogenesis of the myotome of Xenopus laevis: a qualitative study. Development. 1987 Nov;101(3):583–590. doi: 10.1242/dev.101.3.583. [DOI] [PubMed] [Google Scholar]
- Chlebowski J. S., Przbylski R. J., Cox P. G. Ultrastructural studies of lizard (Anolis carolinensis) myogenesis in vitro. Dev Biol. 1973 Jul;33(1):80–99. doi: 10.1016/0012-1606(73)90166-8. [DOI] [PubMed] [Google Scholar]
- González Santander R., Toledo Lobo M. V., Martínez Alonso F. J., Martínez Cuadrado G. Fusion mechanism of the myoblasts in the myotome of the chick embryo. Histol Histopathol. 1993 Jul;8(3):471–490. [PubMed] [Google Scholar]
- KITIYAKARA A., ANGEVINE D. M. A STUDY OF THE PATTERN OF POSTEMBRYONIC GROWTH OF M. GRACILIS IN MICE. Dev Biol. 1963 Dec;8:322–340. doi: 10.1016/0012-1606(63)90033-2. [DOI] [PubMed] [Google Scholar]
- Kahn E. B., Simpson S. B., Jr Satellite cells in mature, uninjured skeletal muscle of the lizard tail. Dev Biol. 1974 Mar;37(1):219–223. doi: 10.1016/0012-1606(74)90181-x. [DOI] [PubMed] [Google Scholar]
- Marusich M. F., Simpson S. B., Jr Changes in cell surface antigens during in vitro lizard myogenesis. Dev Biol. 1983 Jun;97(2):313–328. doi: 10.1016/0012-1606(83)90089-1. [DOI] [PubMed] [Google Scholar]
- Moss F. P., Leblond C. P. Nature of dividing nuclei in skeletal muscle of growing rats. J Cell Biol. 1970 Feb;44(2):459–462. doi: 10.1083/jcb.44.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss F. P., Leblond C. P. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971 Aug;170(4):421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Muntz L. Myogenesis in the trunk and leg during development of the tadpole of Xenopus laevis (Daudin 1802). J Embryol Exp Morphol. 1975 Jun;33(3):757–774. [PubMed] [Google Scholar]
- Waterman R. E. Development of the lateral musculature in the teleost, Brachydanio rerio: a fine structural study. Am J Anat. 1969 Aug;125(4):457–493. doi: 10.1002/aja.1001250406. [DOI] [PubMed] [Google Scholar]