Abstract
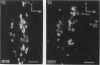
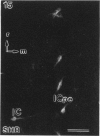
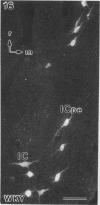
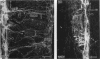
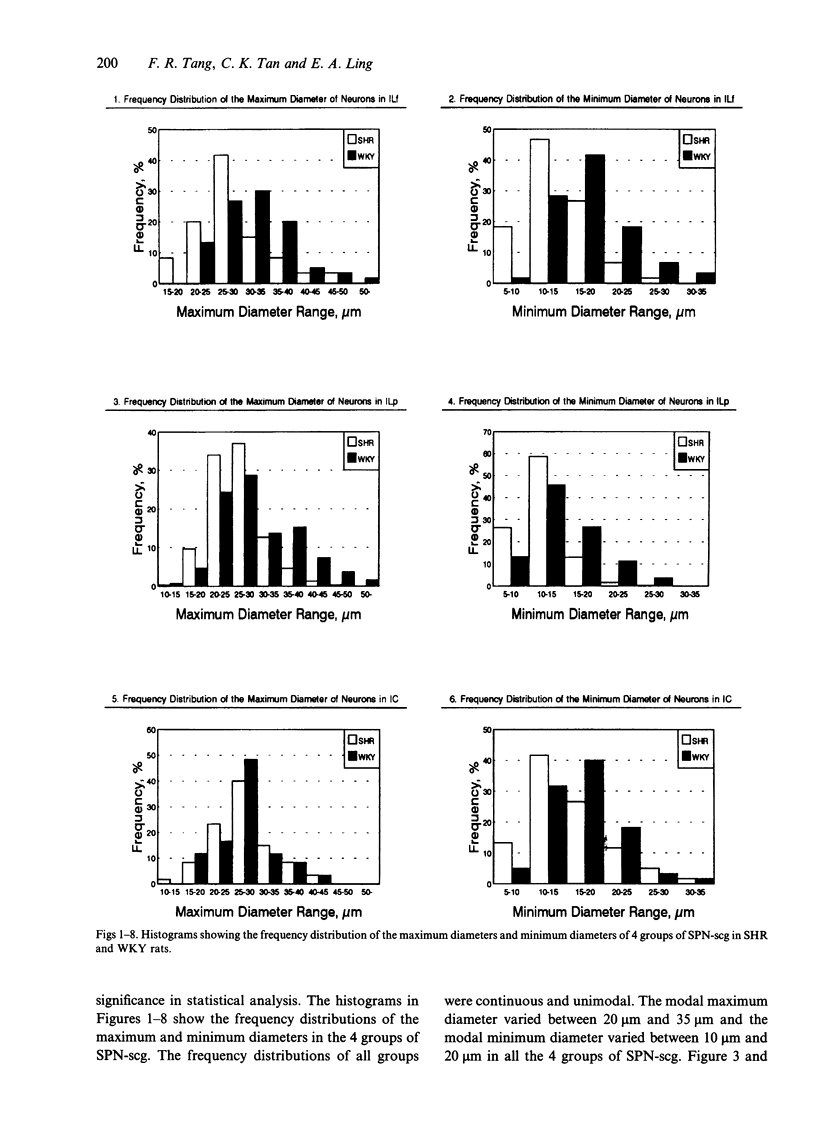
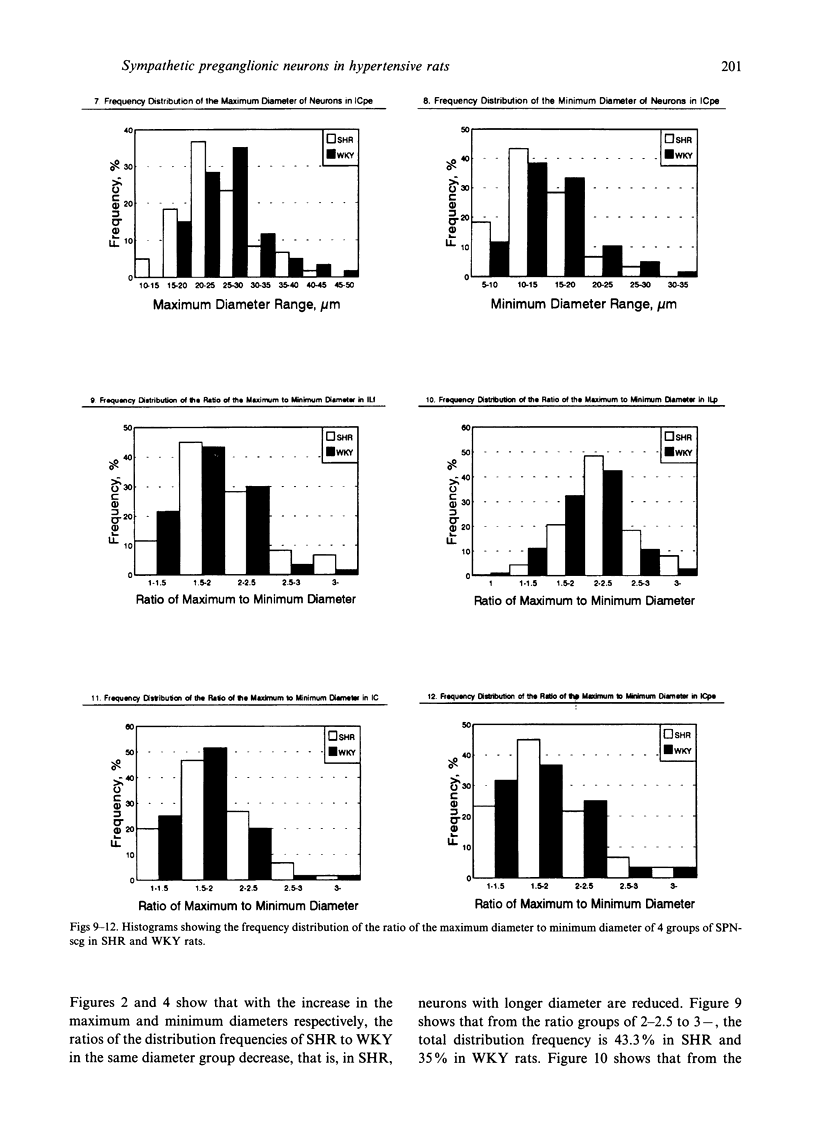
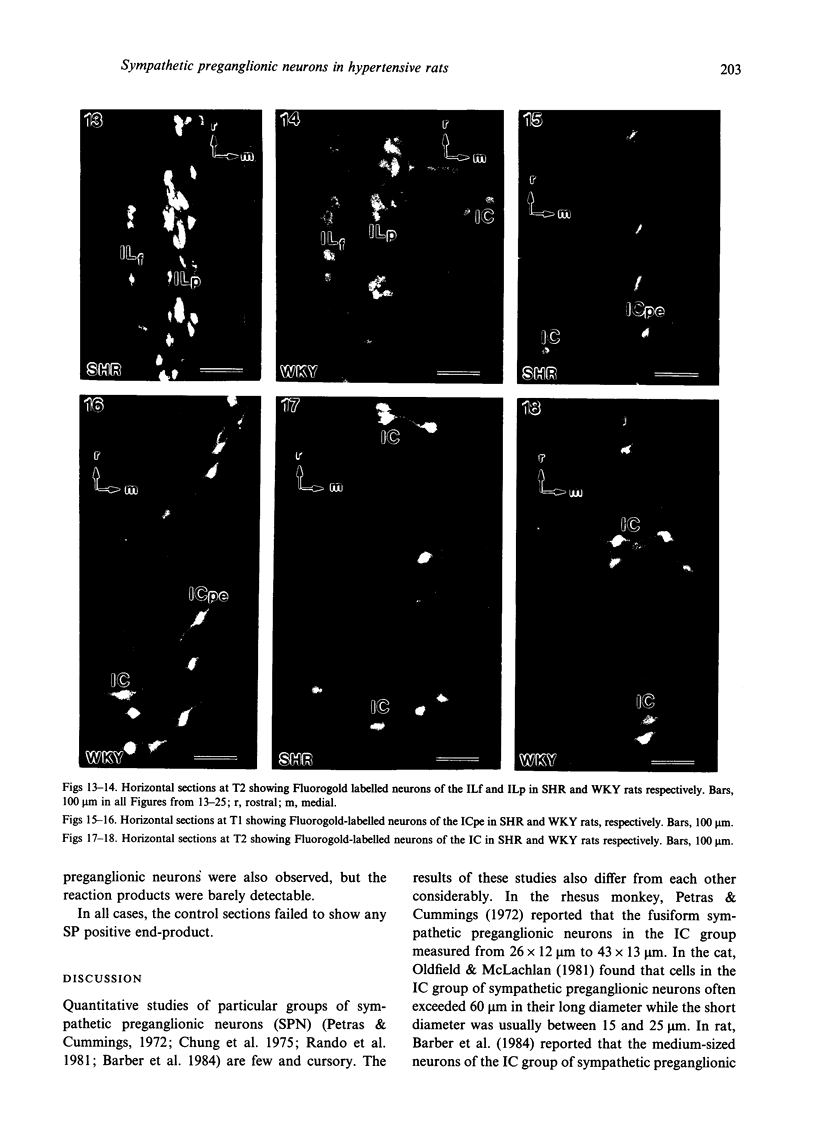
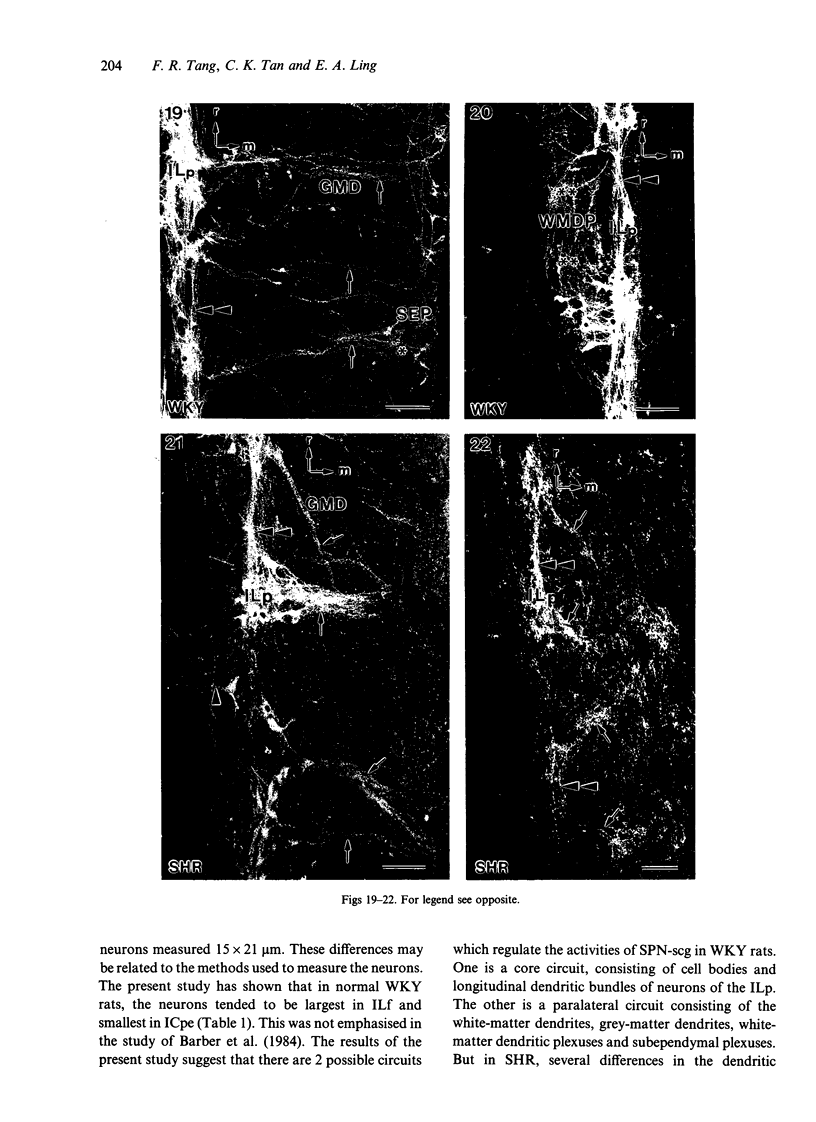
A comparative morphological study of the sympathetic preganglionic neurons that innervate the superior cervical ganglion (SPN-scg) was made in spontaneously hypertensive rats (SHR) and age-matched normotensive Wistar-Kyoto (WKY) rats. The cytoarchitectonics and dendroarchitectonics of the SPN-scg were studied following retrograde transport of choleragen subunit B horseradish peroxidase conjugate (CB-HRP) and Fluorogold. Significant differences were observed in the maximum and minimum diameters of neurons of the nucleus intermediolateralis pars principalis (ILp) and in the minimum diameter of neurons in the nucleus intermediolateralis pars funicularis (ILf) between SHR and WKY rats (P < 0.01). These diameters were decreased in neurons of SHR. The distribution patterns of dendrites of SPN-scg also showed differences between SHR and WKY rats. The dendritic distribution patterns showed the following changes in SH rats: (1) the mediolaterally oriented dendrites were reduced in number, (2) the ladder-like configuration of the medially oriented grey-matter dendrites was less prominent, (3) the medially oriented dendrites formed a triangular or dome-like configuration, and (4) the white matter dendritic plexuses and subependymal plexuses were reduced. Similar differences between SHR and WKY rats were also observed in our immunohistochemical study of substance P-like fibres. In addition, the SP study has also shown a close association of SP fibres with the central canal both in SHR and WKY rats; some of the SP fibres penetrated the ependymal lining to run longitudinally up or down the central canal. This finding suggests the presence of substance P-positive neurons contacting the cerebrospinal fluid.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud F. M. The sympathetic system in hypertension. State-of-the-art review. Hypertension. 1982 May-Jun;4(3 Pt 2):208–225. [PubMed] [Google Scholar]
- Abel P. W., Hermsmeyer K. Sympathetic cross-innervation of SHR and genetic controls suggests a trophic influence on vascular muscle membranes. Circ Res. 1981 Dec;49(6):1311–1318. doi: 10.1161/01.res.49.6.1311. [DOI] [PubMed] [Google Scholar]
- Amendt K., Czachurski J., Dembowsky K., Seller H. Bulbospinal projections to the intermediolateral cell column: a neuroanatomical study. J Auton Nerv Syst. 1979 Oct;1(1):103–107. doi: 10.1016/0165-1838(79)90009-2. [DOI] [PubMed] [Google Scholar]
- Appel N. M., Wessendorf M. W., Elde R. P. Thyrotropin-releasing hormone in spinal cord: coexistence with serotonin and with substance P in fibers and terminals apposing identified preganglionic sympathetic neurons. Brain Res. 1987 Jul 7;415(1):137–143. doi: 10.1016/0006-8993(87)90276-9. [DOI] [PubMed] [Google Scholar]
- Barber R. P., Phelps P. E., Houser C. R., Crawford G. D., Salvaterra P. M., Vaughn J. E. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984 Nov 1;229(3):329–346. doi: 10.1002/cne.902290305. [DOI] [PubMed] [Google Scholar]
- Barber R. P., Vaughn J. E., Slemmon J. R., Salvaterra P. M., Roberts E., Leeman S. E. The origin, distribution and synaptic relationships of substance P axons in rat spinal cord. J Comp Neurol. 1979 Mar 15;184(2):331–351. doi: 10.1002/cne.901840208. [DOI] [PubMed] [Google Scholar]
- Campbell G. R., Chamley-Campbell J., Short N., Robinson R. B., Hermsmeyer K. Effect of cross-transplantation on normotensive and spontaneously hypertensive rat arterial muscle membrane. Hypertension. 1981 Sep-Oct;3(5):534–543. doi: 10.1161/01.hyp.3.5.534. [DOI] [PubMed] [Google Scholar]
- Chung J. M., Chung K., Wurster R. D. Sympathetic preganglionic neurons of the cat spinal cord: horseradish peroxidase study. Brain Res. 1975 Jun 20;91(1):126–131. doi: 10.1016/0006-8993(75)90471-0. [DOI] [PubMed] [Google Scholar]
- Fein J. M. Hypertension and the central nervous system. Clin Neurosurg. 1982;29:666–721. doi: 10.1093/neurosurgery/29.cn_suppl_1.666. [DOI] [PubMed] [Google Scholar]
- Folkow B. The haemodynamic consequences of adaptive structural changes of the resistance vessels in hypertension. Clin Sci. 1971 Jul;41(1):1–12. doi: 10.1042/cs0410001. [DOI] [PubMed] [Google Scholar]
- Galeno T. M., Van Hoesen G. W., Maixner W., Johnson A. K., Brody M. J. Contribution of the amygdala to the development of spontaneous hypertension. Brain Res. 1982 Aug 19;246(1):1–6. doi: 10.1016/0006-8993(82)90136-6. [DOI] [PubMed] [Google Scholar]
- Gilbey M. P., McKenna K. E., Schramm L. P. Effects of substance P on sympathetic preganglionic neurones. Neurosci Lett. 1983 Oct 31;41(1-2):157–159. doi: 10.1016/0304-3940(83)90239-2. [DOI] [PubMed] [Google Scholar]
- Gómez Sánchez E. P. What is the role of the central nervous system in mineralocorticoid hypertension? Am J Hypertens. 1991 Apr;4(4 Pt 1):374–381. doi: 10.1093/ajh/4.4.374. [DOI] [PubMed] [Google Scholar]
- Hart M. N., Heistad D. D., Brody M. J. Effect of chronic hypertension and sympathetic denervation on wall/lumen ratio of cerebral vessels. Hypertension. 1980 Jul-Aug;2(4):419–423. doi: 10.1161/01.hyp.2.4.419. [DOI] [PubMed] [Google Scholar]
- Helke C. J., Neil J. J., Massari V. J., Loewy A. D. Substance P neurons project from the ventral medulla to the intermediolateral cell column and ventral horn in the rat. Brain Res. 1982 Jul 8;243(1):147–152. doi: 10.1016/0006-8993(82)91128-3. [DOI] [PubMed] [Google Scholar]
- Hermsmeyer K. Cellular basis for increased sensitivity of vascular smooth muscle in spontaneously hypertensive rats. Circ Res. 1976 Jun;38(6 Suppl 2):53–57. doi: 10.1161/01.res.38.6.53. [DOI] [PubMed] [Google Scholar]
- Holets V., Elde R. The differential distribution and relationship of serotoninergic and peptidergic fibers to sympathoadrenal neurons in the intermediolateral cell column of the rat: a combined retrograde axonal transport and immunofluorescence study. Neuroscience. 1982 May;7(5):1155–1174. doi: 10.1016/0306-4522(82)91123-x. [DOI] [PubMed] [Google Scholar]
- Johansson O., Hökfelt T., Pernow B., Jeffcoate S. L., White N., Steinbusch H. W., Verhofstad A. A., Emson P. C., Spindel E. Immunohistochemical support for three putative transmitters in one neuron: coexistence of 5-hydroxytryptamine, substance P- and thyrotropin releasing hormone-like immunoreactivity in medullary neurons projecting to the spinal cord. Neuroscience. 1981;6(10):1857–1881. doi: 10.1016/0306-4522(81)90028-2. [DOI] [PubMed] [Google Scholar]
- Jänig W., McLachlan E. M. Identification of distinct topographical distributions of lumbar sympathetic and sensory neurons projecting to end organs with different functions in the cat. J Comp Neurol. 1986 Apr 1;246(1):104–112. doi: 10.1002/cne.902460107. [DOI] [PubMed] [Google Scholar]
- Katholi R. E., Winternitz S. R., Oparil S. Role of the renal nerves in the pathogenesis of one-kidney renal hypertension in the rat. Hypertension. 1981 Jul-Aug;3(4):404–409. doi: 10.1161/01.hyp.3.4.404. [DOI] [PubMed] [Google Scholar]
- Komatsu K., Tanaka I., Funai T., Ichiyama A., Yoshimi T. Increased level of atrial natriuretic peptide messenger RNA in the hypothalamus and brainstem of spontaneously hypertensive rats. J Hypertens. 1992 Jan;10(1):17–23. doi: 10.1097/00004872-199201000-00004. [DOI] [PubMed] [Google Scholar]
- Krukoff T. L., Ciriello J., Calaresu F. R. Segmental distribution of peptide- and 5HT-like immunoreactivity in nerve terminals and fibers of the thoracolumbar sympathetic nuclei of the cat. J Comp Neurol. 1985 Oct 1;240(1):103–116. doi: 10.1002/cne.902400108. [DOI] [PubMed] [Google Scholar]
- Krukoff T. L., Ciriello J., Calaresu F. R. Segmental distribution of peptide-like immunoreactivity in cell bodies of the thoracolumbar sympathetic nuclei of the cat. J Comp Neurol. 1985 Oct 1;240(1):90–102. doi: 10.1002/cne.902400107. [DOI] [PubMed] [Google Scholar]
- Krukoff T. L. Decreased hexokinase activity in paraventricular nucleus of adult SHR and renal hypertensive rats. Am J Physiol. 1988 Mar;254(3 Pt 2):R508–R512. doi: 10.1152/ajpregu.1988.254.3.R508. [DOI] [PubMed] [Google Scholar]
- Kuo D. C., Oravitz J. J., DeGroat W. C. Tracing of afferent and efferent pathways in the left inferior cardiac nerve of the cat using retrograde and transganglionic transport of horseradish peroxidase. Brain Res. 1984 Oct 29;321(1):111–118. doi: 10.1016/0006-8993(84)90686-3. [DOI] [PubMed] [Google Scholar]
- LaMotte C. C. Vasoactive intestinal polypeptide cerebrospinal fluid-contacting neurons of the monkey and cat spinal central canal. J Comp Neurol. 1987 Apr 22;258(4):527–541. doi: 10.1002/cne.902580405. [DOI] [PubMed] [Google Scholar]
- Loewy A. D., McKellar S., Saper C. B. Direct projections from the A5 catecholamine cell group to the intermediolateral cell column. Brain Res. 1979 Oct 5;174(2):309–314. doi: 10.1016/0006-8993(79)90852-7. [DOI] [PubMed] [Google Scholar]
- Loizou L. A. Projections of the nucleus locus coeruleus in the albino rat. Brain Res. 1969 Oct;15(2):563–566. doi: 10.1016/0006-8993(69)90185-1. [DOI] [PubMed] [Google Scholar]
- Mark A. L., Kerber R. E. Augmentation of cardiopulmonary baroreflex control of forearm vascular resistance in borderline hypertension. Hypertension. 1982 Jan-Feb;4(1):39–46. doi: 10.1161/01.hyp.4.1.39. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M., Rosene D. L. Sensitivity in horseradish peroxidase neurohistochemistry: a comparative and quantitative study of nine methods. J Histochem Cytochem. 1979 Mar;27(3):763–773. doi: 10.1177/27.3.113450. [DOI] [PubMed] [Google Scholar]
- Okamoto K. Spontaneous hypertension in rats. Int Rev Exp Pathol. 1969;7:227–270. [PubMed] [Google Scholar]
- Oldfield B. J., McLachlan E. M. An analysis of the sympathetic preganglionic neurons projecting from the upper thoracic spinal roots of the cat. J Comp Neurol. 1981 Feb 20;196(2):329–345. doi: 10.1002/cne.901960211. [DOI] [PubMed] [Google Scholar]
- Oldfield B. J., Sheppard A., Nilaver G. A study of the substance P innervation of the intermediate zone of the thoracolumbar spinal cord. J Comp Neurol. 1985 Jun 1;236(1):127–140. doi: 10.1002/cne.902360111. [DOI] [PubMed] [Google Scholar]
- Petras J. M., Cummings J. F. Autonomic neurons in the spinal cord of the Rhesus monkey: a correlation of the findings of cytoarchitectonics and sympathectomy with fiber degeneration following dorsal rhizotomy. J Comp Neurol. 1972 Oct;146(2):189–218. doi: 10.1002/cne.901460205. [DOI] [PubMed] [Google Scholar]
- Rando T. A., Bowers C. W., Zigmond R. E. Localization of neurons in the rat spinal cord which project to the superior cervical ganglion. J Comp Neurol. 1981 Feb 10;196(1):73–83. doi: 10.1002/cne.901960107. [DOI] [PubMed] [Google Scholar]
- Reis D. J., Doba N. The central nervous system and neurogenic hypertension. Prog Cardiovasc Dis. 1974 Jul-Aug;17(1):51–71. doi: 10.1016/0033-0620(74)90038-3. [DOI] [PubMed] [Google Scholar]
- Saper C. B., Loewy A. D. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980 Sep 22;197(2):291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Saper C. B., Loewy A. D., Swanson L. W., Cowan W. M. Direct hypothalamo-autonomic connections. Brain Res. 1976 Nov 26;117(2):305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- Schärer K., Benninger C., Heimann A., Rascher W. Involvement of the central nervous system in renal hypertension. Eur J Pediatr. 1993 Jan;152(1):59–63. doi: 10.1007/BF02072518. [DOI] [PubMed] [Google Scholar]
- Sharif N. A., Burt D. R. Micromolar substance P reduces spinal receptor binding for thyrotropin-releasing hormone--possible relevance to neuropeptide coexistence? Neurosci Lett. 1983 Dec 30;43(2-3):245–251. doi: 10.1016/0304-3940(83)90196-9. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., McKellar S. The distribution of oxytocin- and neurophysin-stained fibers in the spinal cord of the rat and monkey. J Comp Neurol. 1979 Nov 1;188(1):87–106. doi: 10.1002/cne.901880108. [DOI] [PubMed] [Google Scholar]
- Vigh B., Vigh-Teichmann I. Cytochemistry of CSF-contacting neurons and pinealocytes. Prog Brain Res. 1992;91:299–306. doi: 10.1016/s0079-6123(08)62346-8. [DOI] [PubMed] [Google Scholar]
- Vigh B., Vigh-Teichmann I., Manzano e Silva M. J., van den Pol A. N. Cerebrospinal fluid-contacting neurons of the central canal and terminal ventricle in various vertebrates. Cell Tissue Res. 1983;231(3):615–621. doi: 10.1007/BF00218119. [DOI] [PubMed] [Google Scholar]
- Yamori Y., Okamoto K. Hypothalamic tonic regulation of blood pressure in spontaneously hypertensive rats. Jpn Circ J. 1969 May;33(5):509–519. doi: 10.1253/jcj.33.509. [DOI] [PubMed] [Google Scholar]
- Zhang Y. L., Tan C. K., Wong W. C. The ciliary ganglion of the cat: a light and electron microscopic study. Anat Embryol (Berl) 1993 Jun;187(6):591–599. doi: 10.1007/BF00214438. [DOI] [PubMed] [Google Scholar]