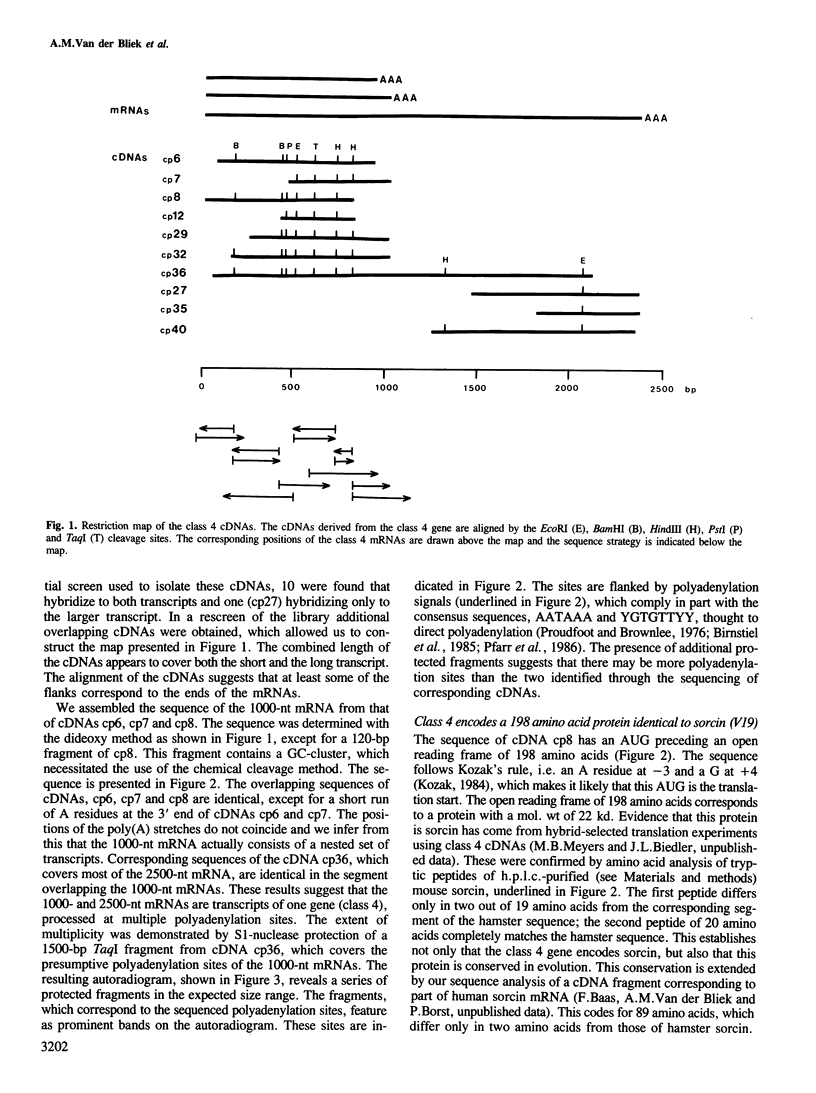
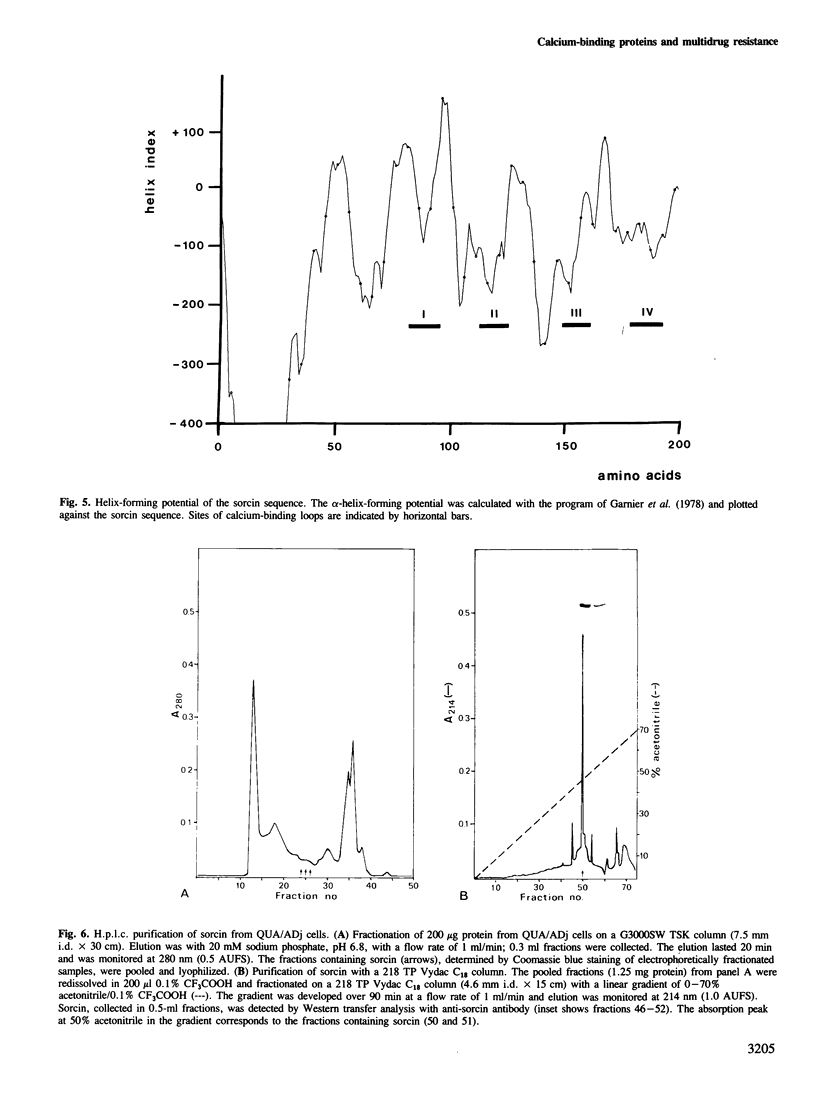
Abstract
We have previously shown that at least five linked genes are co-amplified and overexpressed in the multi-drug resistant (MDR) Chinese hamster ovary cell line CHRC5. We show here that one of these genes (class 4) codes for a small phosphorylated, cytosolic protein, sorcin/V19, known to be overproduced by many MDR cell lines. The class 4 gene codes for a nested set of mRNAs, varying in size between 1000 and 2500 nucleotides. Sequence analysis of complementary DNAs shows that these mRNAs encode a protein of 198 amino acids. The identity of this protein with sorcin was established by comparison with the amino acid sequence of two peptides from mouse sorcin. Hamster sorcin is a 22-kd protein with four 'E-F hand' structures typical of calcium-binding sites and it has substantial homology with the light chain of calpain. Two of the calcium-binding sites contain putative recognition sites for cAMP-dependent protein kinase. These may account for the known phosphorylation of sorcin. The unknown function of sorcin might therefore be controlled by both calcium and cAMP levels. The contribution of sorcin to multidrug resistance, if any, remains to be tested.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Klee C. B., Cohen P. The structure of the B subunit of calcineurin. Eur J Biochem. 1984 Mar 15;139(3):663–671. doi: 10.1111/j.1432-1033.1984.tb08055.x. [DOI] [PubMed] [Google Scholar]
- Beck W. T. Cellular pharmacology of Vinca alkaloid resistance and its circumvention. Adv Enzyme Regul. 1984;22:207–227. doi: 10.1016/0065-2571(84)90015-3. [DOI] [PubMed] [Google Scholar]
- Beck W. T., Mueller T. J., Tanzer L. R. Altered surface membrane glycoproteins in Vinca alkaloid-resistant human leukemic lymphoblasts. Cancer Res. 1979 Jun;39(6 Pt 1):2070–2076. [PubMed] [Google Scholar]
- Beck W. T. Vinca alkaloid-resistant phenotype in cultured human leukemic lymphoblasts. Cancer Treat Rep. 1983 Oct;67(10):875–882. [PubMed] [Google Scholar]
- Bell D. R., Gerlach J. H., Kartner N., Buick R. N., Ling V. Detection of P-glycoprotein in ovarian cancer: a molecular marker associated with multidrug resistance. J Clin Oncol. 1985 Mar;3(3):311–315. doi: 10.1200/JCO.1985.3.3.311. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Chang T. D., Meyers M. B., Peterson R. H., Spengler B. A. Drug resistance in Chinese hamster lung and mouse tumor cells. Cancer Treat Rep. 1983 Oct;67(10):859–867. [PubMed] [Google Scholar]
- Biedler J. L., Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Res. 1970 Apr;30(4):1174–1184. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973 Oct 25;323(3):466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- Debenham P. G., Kartner N., Siminovitch L., Riordan J. R., Ling V. DNA-mediated transfer of multiple drug resistance and plasma membrane glycoprotein expression. Mol Cell Biol. 1982 Aug;2(8):881–889. doi: 10.1128/mcb.2.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinta C., Persson B., Jörnvall H., von Heijne G. Sequence determinants of cytosolic N-terminal protein processing. Eur J Biochem. 1986 Jan 2;154(1):193–196. doi: 10.1111/j.1432-1033.1986.tb09378.x. [DOI] [PubMed] [Google Scholar]
- Fojo A. T., Whang-Peng J., Gottesman M. M., Pastan I. Amplification of DNA sequences in human multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7661–7665. doi: 10.1073/pnas.82.22.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariépy J., Hodges R. S. Primary sequence analysis and folding behavior of EF hands in relation to the mechanism of action of troponin C and calmodulin. FEBS Lett. 1983 Aug 22;160(1-2):1–6. doi: 10.1016/0014-5793(83)80924-7. [DOI] [PubMed] [Google Scholar]
- Garman D., Center M. S. Alterations in cell surface membranes in Chinese hamster lung cell resistant to adriamycin. Biochem Biophys Res Commun. 1982 Mar 15;105(1):157–163. doi: 10.1016/s0006-291x(82)80025-9. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Glass D. B., el-Maghrabi M. R., Pilkis S. J. Synthetic peptides corresponding to the site phosphorylated in 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase as substrates of cyclic nucleotide-dependent protein kinases. J Biol Chem. 1986 Feb 25;261(6):2987–2993. [PubMed] [Google Scholar]
- Gros P., Croop J., Roninson I., Varshavsky A., Housman D. E. Isolation and characterization of DNA sequences amplified in multidrug-resistant hamster cells. Proc Natl Acad Sci U S A. 1986 Jan;83(2):337–341. doi: 10.1073/pnas.83.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudkov A. V., Massino J. S., Chernova O. B., Kopnin B. P. Gene amplification in Djungarian hamster cell lines possessing decreased plasma membrane permeability for colchicine and some other drugs. Chromosoma. 1985;92(1):16–24. doi: 10.1007/BF00327241. [DOI] [PubMed] [Google Scholar]
- Gudkov A., Kopnin B. Gene amplification in multidrug-resistant cells: molecular and karyotypic events. Bioessays. 1985 Aug;3(2):68–71. doi: 10.1002/bies.950030207. [DOI] [PubMed] [Google Scholar]
- Harker W. G., Sikic B. I. Multidrug (pleiotropic) resistance in doxorubicin-selected variants of the human sarcoma cell line MES-SA. Cancer Res. 1985 Sep;45(9):4091–4096. [PubMed] [Google Scholar]
- Helson L. Calcium channel blocker enhancement of anticancer drug cytotoxicity--a review. Cancer Drug Deliv. 1984 Fall;1(4):353–361. doi: 10.1089/cdd.1984.1.353. [DOI] [PubMed] [Google Scholar]
- Inaba M., Fujikura R., Sakurai Y. Active efflux common to vincristine and daunorubicin in vincristine-resistant P388 leukemia. Biochem Pharmacol. 1981 Jul 1;30(13):1863–1865. doi: 10.1016/0006-2952(81)90027-7. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kartner N., Evernden-Porelle D., Bradley G., Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. 1985 Aug 29-Sep 4Nature. 316(6031):820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- Kartner N., Riordan J. R., Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983 Sep 23;221(4617):1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- Kartner N., Shales M., Riordan J. R., Ling V. Daunorubicin-resistant Chinese hamster ovary cells expressing multidrug resistance and a cell-surface P-glycoprotein. Cancer Res. 1983 Sep;43(9):4413–4419. [PubMed] [Google Scholar]
- Kessel D., Wilberding C. Anthracycline resistance in P388 murine leukemia and its circumvention by calcium antagonists. Cancer Res. 1985 Apr;45(4):1687–1691. [PubMed] [Google Scholar]
- Kessel D., Wilberding C. Interactions between calcium antagonists, calcium fluxes and anthracycline transport. Cancer Lett. 1984 Nov;25(1):97–101. doi: 10.1016/s0304-3835(84)80031-2. [DOI] [PubMed] [Google Scholar]
- Kessel D., Wilberding C. Mode of action of calcium antagonists which alter anthracycline resistance. Biochem Pharmacol. 1984 Apr 1;33(7):1157–1160. doi: 10.1016/0006-2952(84)90533-1. [DOI] [PubMed] [Google Scholar]
- Koch G., Smith M., Twentyman P., Wright K. Identification of a novel calcium-binding protein (CP22) in multidrug-resistant murine and hamster cells. FEBS Lett. 1986 Jan 20;195(1-2):275–279. doi: 10.1016/0014-5793(86)80176-4. [DOI] [PubMed] [Google Scholar]
- Kopnin B. P., Massino J. S., Gudkov A. V. Regular pattern of karyotypic alterations accompanying gene amplification in Djungarian hamster cells: study of colchicine, adriablastin, and methotrexate resistance. Chromosoma. 1985;92(1):25–36. doi: 10.1007/BF00327242. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ling V., Gerlach J., Kartner N. Multidrug resistance. Breast Cancer Res Treat. 1984;4(2):89–94. doi: 10.1007/BF01806390. [DOI] [PubMed] [Google Scholar]
- Ling V., Kartner N., Sudo T., Siminovitch L., Riordan J. R. Multidrug-resistance phenotype in Chinese hamster ovary cells. Cancer Treat Rep. 1983 Oct;67(10):869–874. [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Martinsson T., Dahllöf B., Wettergren Y., Leffler H., Levan G. Pleiotropic drug resistance and gene amplification in a SEWA mouse tumor cell line. Complex relations revealed by drug uptake data, and lipid and protein analysis. Exp Cell Res. 1985 Jun;158(2):382–394. doi: 10.1016/0014-4827(85)90463-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Meyers M. B., Biedler J. L. Increased synthesis of a low molecular weight protein in vincristine-resistant cells. Biochem Biophys Res Commun. 1981 Mar 16;99(1):228–235. doi: 10.1016/0006-291x(81)91736-8. [DOI] [PubMed] [Google Scholar]
- Meyers M. B., Spengler B. A., Chang T. D., Melera P. W., Biedler J. L. Gene amplification-associated cytogenetic aberrations and protein changes in vincristine-resistant Chinese hamster, mouse, and human cells. J Cell Biol. 1985 Feb;100(2):588–597. doi: 10.1083/jcb.100.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Emori Y., Imajoh S., Kawasaki H., Kisaragi M., Suzuki K. Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature. 1984 Dec 6;312(5994):566–570. doi: 10.1038/312566a0. [DOI] [PubMed] [Google Scholar]
- Ohno S., Emori Y., Suzuki K. Nucleotide sequence of a cDNA coding for the small subunit of human calcium-dependent protease. Nucleic Acids Res. 1986 Jul 11;14(13):5559–5559. [PMC free article] [PubMed] [Google Scholar]
- Peterson R. H., Biedler J. L. Plasma membrane proteins and glycoproteins from Chinese hamster cells sensitive and resistant to actinomycin D. J Supramol Struct. 1978;9(3):289–298. doi: 10.1002/jss.400090302. [DOI] [PubMed] [Google Scholar]
- Peterson R. H., Meyers M. B., Spengler B. A., Biedler J. L. Alteration of plasma membrane glycopeptides and gangliosides of Chinese hamster cells accompanying development of resistance to daunorubicin and vincristine. Cancer Res. 1983 Jan;43(1):222–228. [PubMed] [Google Scholar]
- Pfarr D. S., Rieser L. A., Woychik R. P., Rottman F. M., Rosenberg M., Reff M. E. Differential effects of polyadenylation regions on gene expression in mammalian cells. DNA. 1986 Apr;5(2):115–122. doi: 10.1089/dna.1986.5.115. [DOI] [PubMed] [Google Scholar]
- Polotskaia A. V., Gudkov A. V., Kopnin B. P. Giperproduktsiia spetsificheskogo belka v kletkakh, rezistentnykh k kholkhitsinu i adriablastinu. Biull Eksp Biol Med. 1983 Sep;96(9):95–96. [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Putkey J. A., Ts'ui K. F., Tanaka T., Lagacé L., Stein J. P., Lai E. C., Means A. R. Chicken calmodulin genes. A species comparison of cDNA sequences and isolation of a genomic clone. J Biol Chem. 1983 Oct 10;258(19):11864–11870. [PubMed] [Google Scholar]
- Ramu A., Glaubiger D., Fuks Z. Reversal of acquired resistance to doxorubicin in P388 murine leukemia cells by tamoxifen and other triparanol analogues. Cancer Res. 1984 Oct;44(10):4392–4395. [PubMed] [Google Scholar]
- Ramu A., Shan T. C., Glaubiger D. Enhancement of doxorubicin and vinblastine sensitivity in anthracycline-resistant P388 cells. Cancer Treat Rep. 1983 Oct;67(10):895–899. [PubMed] [Google Scholar]
- Ramu A., Spanier R., Rahamimoff H., Fuks Z. Restoration of doxorubicin responsiveness in doxorubicin-resistant P388 murine leukaemia cells. Br J Cancer. 1984 Oct;50(4):501–507. doi: 10.1038/bjc.1984.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Deuchars K., Kartner N., Alon N., Trent J., Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. 1985 Aug 29-Sep 4Nature. 316(6031):817–819. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Ling V. Genetic and biochemical characterization of multidrug resistance. Pharmacol Ther. 1985;28(1):51–75. doi: 10.1016/0163-7258(85)90082-8. [DOI] [PubMed] [Google Scholar]
- Robertson S. M., Ling V., Stanners C. P. Co-amplification of double minute chromosomes, multiple drug resistance, and cell surface P-glycoprotein in DNA-mediated transformants of mouse cells. Mol Cell Biol. 1984 Mar;4(3):500–506. doi: 10.1128/mcb.4.3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan A. M., Hamilton T. C., Young R. C., Klecker R. W., Jr, Ozols R. F. Reversal of adriamycin resistance by verapamil in human ovarian cancer. Science. 1984 Jun 1;224(4652):994–996. doi: 10.1126/science.6372095. [DOI] [PubMed] [Google Scholar]
- Roninson I. B., Abelson H. T., Housman D. E., Howell N., Varshavsky A. Amplification of specific DNA sequences correlates with multi-drug resistance in Chinese hamster cells. Nature. 1984 Jun 14;309(5969):626–628. doi: 10.1038/309626a0. [DOI] [PubMed] [Google Scholar]
- Roninson I. B., Chin J. E., Choi K. G., Gros P., Housman D. E., Fojo A., Shen D. W., Gottesman M. M., Pastan I. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4538–4542. doi: 10.1073/pnas.83.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. N., Horwitz S. B. A phosphoglycoprotein associated with taxol resistance in J774.2 cells. Cancer Res. 1985 Aug;45(8):3856–3863. [PubMed] [Google Scholar]
- Sakihama T., Kakidani H., Zenita K., Yumoto N., Kikuchi T., Sasaki T., Kannagi R., Nakanishi S., Ohmori M., Takio K. A putative Ca2+-binding protein: structure of the light subunit of porcine calpain elucidated by molecular cloning and protein sequence analysis. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6075–6079. doi: 10.1073/pnas.82.18.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotto K. W., Biedler J. L., Melera P. W. Amplification and expression of genes associated with multidrug resistance in mammalian cells. Science. 1986 May 9;232(4751):751–755. doi: 10.1126/science.2421411. [DOI] [PubMed] [Google Scholar]
- Shen D. W., Fojo A., Chin J. E., Roninson I. B., Richert N., Pastan I., Gottesman M. M. Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science. 1986 May 2;232(4750):643–645. doi: 10.1126/science.3457471. [DOI] [PubMed] [Google Scholar]
- Sirotnak F. M., Yang C. H., Mines L. S., Oribé E., Biedler J. L. Markedly altered membrane transport and intracellular binding of vincristine in multidrug-resistant Chinese hamster cells selected for resistance to vinca alkaloids. J Cell Physiol. 1986 Feb;126(2):266–274. doi: 10.1002/jcp.1041260217. [DOI] [PubMed] [Google Scholar]
- Skovsgaard T. Transport and binding of daunorubicin, adriamycin, and rubidazone in Ehrlich ascites tumour cells. Biochem Pharmacol. 1977 Feb 1;26(3):215–222. doi: 10.1016/0006-2952(77)90306-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sundaralingam M., Drendel W., Greaser M. Stabilization of the long central helix of troponin C by intrahelical salt bridges between charged amino acid side chains. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7944–7947. doi: 10.1073/pnas.82.23.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szebenyi D. M., Obendorf S. K., Moffat K. Structure of vitamin D-dependent calcium-binding protein from bovine intestine. Nature. 1981 Nov 26;294(5839):327–332. doi: 10.1038/294327a0. [DOI] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Cure of mice bearing P388 leukemia by vincristine in combination with a calcium channel blocker. Cancer Treat Rep. 1985 May;69(5):523–525. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Yamashiro M., Tsukagoshi S., Sakurai Y. Enhancement of vincristine- and adriamycin-induced cytotoxicity by verapamil in P388 leukemia and its sublines resistant to vincristine and adriamycin. Biochem Pharmacol. 1982 Oct 1;31(19):3138–3140. doi: 10.1016/0006-2952(82)90097-1. [DOI] [PubMed] [Google Scholar]
- Tsuruo T., Kawabata H., Nagumo N., Iida H., Kitatani Y., Tsukagoshi S., Sakurai Y. Potentiation of antitumor agents by calcium channel blockers with special reference to cross-resistance patterns. Cancer Chemother Pharmacol. 1985;15(1):16–19. doi: 10.1007/BF00257287. [DOI] [PubMed] [Google Scholar]
- Tsuruo T. Reversal of acquired resistance to vinca alkaloids and anthracycline antibiotics. Cancer Treat Rep. 1983 Oct;67(10):889–894. [PubMed] [Google Scholar]
- Van der Bliek A. M., Van der Velde-Koerts T., Ling V., Borst P. Overexpression and amplification of five genes in a multidrug-resistant Chinese hamster ovary cell line. Mol Cell Biol. 1986 May;6(5):1671–1678. doi: 10.1128/mcb.6.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]