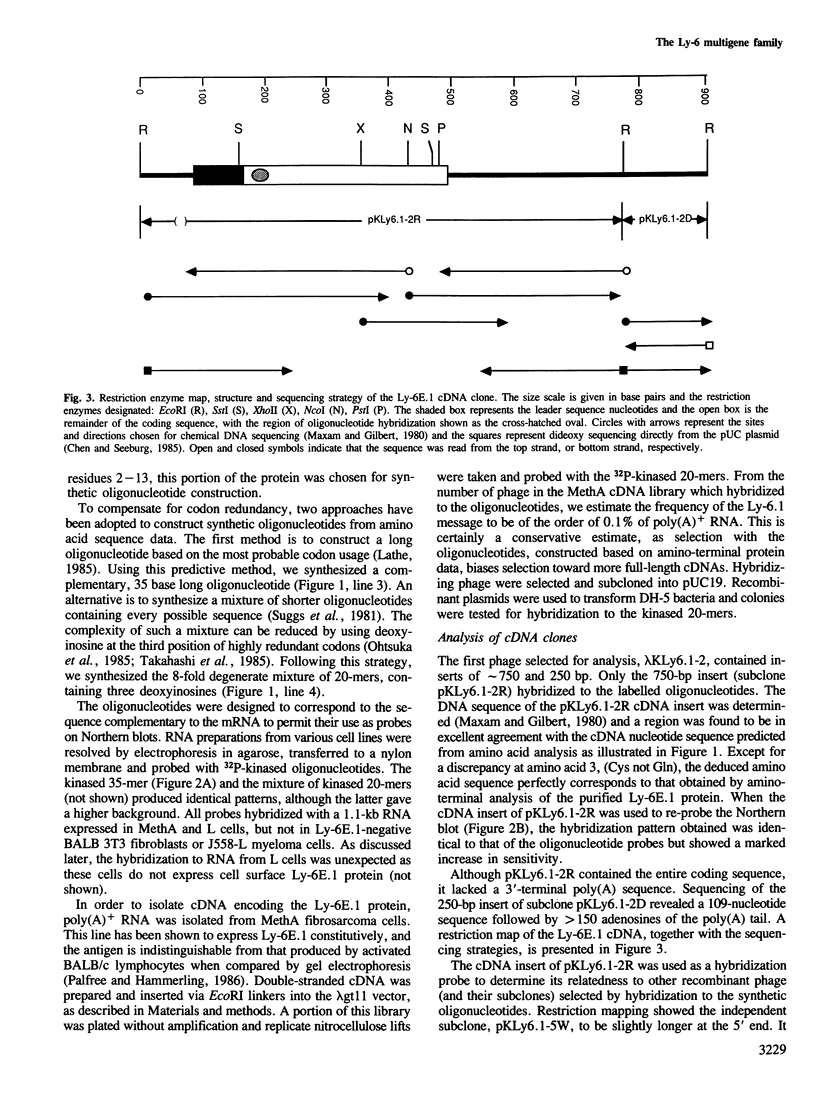
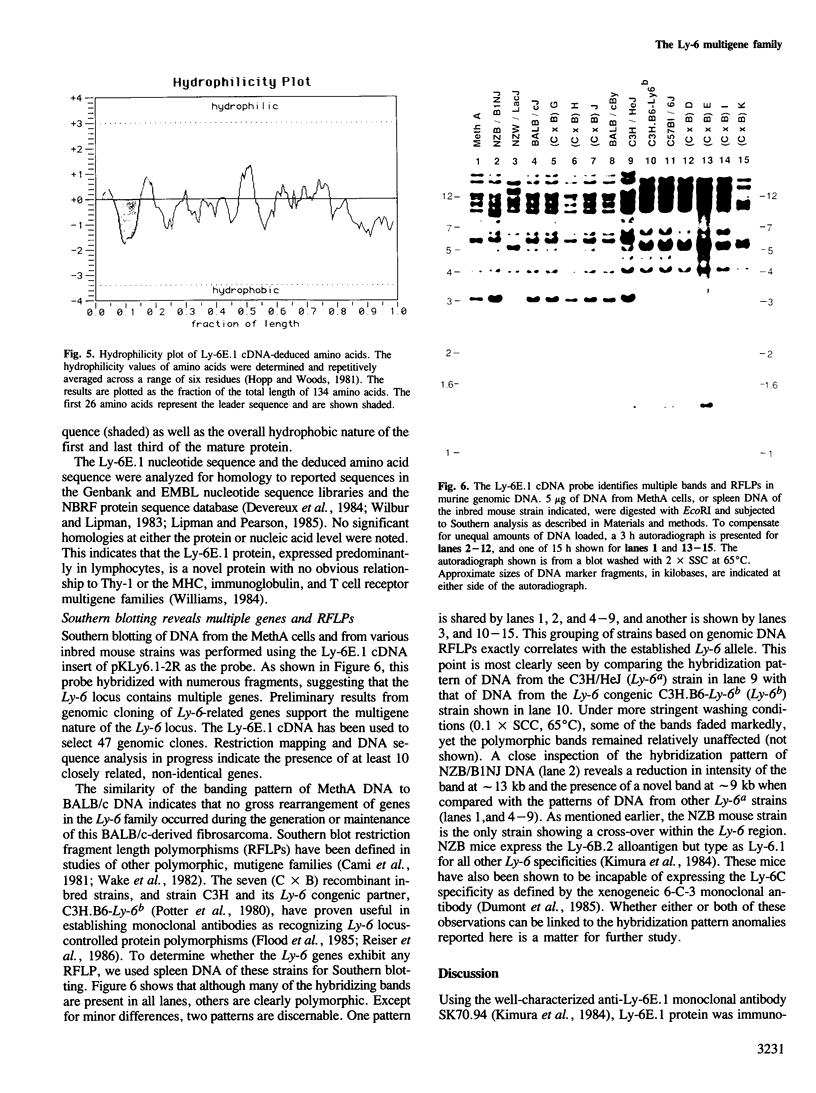
Abstract
The Ly-6 alloantigens have been shown to play a critical role in T lymphocyte activation. To isolate a Ly-6 cDNA, synthetic oligonucleotides, based on the partial amino acid sequence of purified Ly-6E.1 protein, were used to probe a cDNA library. The synthetic oligonucleotides or the isolated cDNA detected a 1.1-kb RNA species. Sequence analysis of the cDNA clone revealed that the Ly-6E.1 protein consists of a 26-amino acid leader followed by a 108-residue, cysteine-rich, core protein with no N-linked glycosylation sites. Southern blot analysis of genomic DNAs revealed multiple bands indicating a family of related genes. Using recombinant inbred and Ly-6 congenic strains of mice, restriction fragment length polymorphisms were demonstrable, and correlated with the Ly-6 allotype of the DNA donors. This probe will enable further molecular genetic analysis of the role of Ly-6-linked proteins in the process of T lymphocyte activation. Isolation of Ly-6 genomic clones may promote a further understanding of the complex tissue-specific expression patterns characteristic of Ly-6-linked genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. P., Lanier L. L. Identification of antigen receptor-associated structures on murine T cells. Nature. 1985 Mar 7;314(6006):107–109. doi: 10.1038/314107a0. [DOI] [PubMed] [Google Scholar]
- Auchincloss H., Jr, Ozato K., Sachs D. H. Two distinct murine differentiation antigens determined by genes linked to the Ly-6 locus. J Immunol. 1981 Nov;127(5):1839–1843. [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Cami B., Brégégère F., Abastado J. P., Kourilsky P. Multiple sequences related to classical histocompatibility antigens in the mouse genome. Nature. 1981 Jun 25;291(5817):673–675. doi: 10.1038/291673a0. [DOI] [PubMed] [Google Scholar]
- Campbell D. G., Gagnon J., Reid K. B., Williams A. F. Rat brain Thy-1 glycoprotein. The amino acid sequence, disulphide bonds and an unusual hydrophobic region. Biochem J. 1981 Apr 1;195(1):15–30. doi: 10.1042/bj1950015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont F. J., Coker L. Z., Habbersett R. C., Treffinger J. A. Xenogeneic monoclonal antibody to an Ly-6-linked murine cell surface antigen: differential reactivity with T cell subpopulations and bone marrow cells. J Immunol. 1985 Apr;134(4):2357–2365. [PubMed] [Google Scholar]
- Flood P. M., Murphy D. B., Horowitz M., LeClair K. P., Smith F. R., Stockert E., Palladino M. A., Jr, DeLeo A. B. A monoclonal antibody that recognizes an Ly-6-linked antigen inhibits the generation of functionally active T cell subsets. J Immunol. 1985 Jul;135(1):63–72. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gunter K. C., Malek T. R., Shevach E. M. T cell-activating properties of an anti-Thy-1 monoclonal antibody. Possible analogy to OKT3/Leu-4. J Exp Med. 1984 Mar 1;159(3):716–730. doi: 10.1084/jem.159.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs M. L., Hogarth P. M., Scott B. M., Harris R. A., McKenzie I. F. Monoclonal antibody to murine neutrophils: identification of the Gm-2.2 specificity. J Immunol. 1984 Nov;133(5):2619–2623. [PubMed] [Google Scholar]
- Hogarth P. M., Houlden B. A., Latham S. E., Sutton V. R., McKenzie I. F. Definition of new alloantigens encoded by genes in the Ly-6 complex. Immunogenetics. 1984;20(1):57–69. doi: 10.1007/BF00373447. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton M. A., Hetherington C. M. Genetic linkage of Ly-6 and Thy-1 loci in the mouse. Immunogenetics. 1980;11(5):521–525. doi: 10.1007/BF01567820. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Hyman R. Genetic basis for Ly-6- defect: complementation between Ly-6- and Thy-1- mutant cell lines. Immunogenetics. 1983;17(3):261–270. doi: 10.1007/BF00364410. [DOI] [PubMed] [Google Scholar]
- Houlden B. A., Hogarth P. M., McKenzie I. F. Interrelationships of the "Ly-6 complex" antigens. Immunogenetics. 1986;23(4):226–232. doi: 10.1007/BF00373017. [DOI] [PubMed] [Google Scholar]
- Kaye J., Janeway C. A., Jr The Fab fragment of a directly activating monoclonal antibody that precipitates a disulfide-linked heterodimer from a helper T cell clone blocks activation by either allogeneic Ia or antigen and self-Ia. J Exp Med. 1984 May 1;159(5):1397–1412. doi: 10.1084/jem.159.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Tada N., Liu-Lam Y., Hämmerling U. Studies of the mouse Ly-6 alloantigen system. II. Complexities of the Ly-6 region. Immunogenetics. 1984;20(1):47–56. doi: 10.1007/BF00373446. [DOI] [PubMed] [Google Scholar]
- Kroczek R. A., Gunter K. C., Germain R. N., Shevach E. M. Thy-1 functions as a signal transduction molecule in T lymphocytes and transfected B lymphocytes. Nature. 1986 Jul 10;322(6075):181–184. doi: 10.1038/322181a0. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meruelo D., Offer M., Rossomando A. Evidence for a major cluster of lymphocyte differentiation antigens on murine chromosome 2. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7460–7464. doi: 10.1073/pnas.79.23.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Cantrell D. A., Hodgdon J. C., Schlossman S. F., Smith K. A., Reinherz E. L. Triggering of the T3-Ti antigen-receptor complex results in clonal T-cell proliferation through an interleukin 2-dependent autocrine pathway. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1509–1513. doi: 10.1073/pnas.81.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettgen H. C., Pettey C. L., Maloy W. L., Terhorst C. A T3-like protein complex associated with the antigen receptor on murine T cells. Nature. 1986 Mar 20;320(6059):272–275. doi: 10.1038/320272a0. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Matsuki S., Ikehara M., Takahashi Y., Matsubara K. An alternative approach to deoxyoligonucleotides as hybridization probes by insertion of deoxyinosine at ambiguous codon positions. J Biol Chem. 1985 Mar 10;260(5):2605–2608. [PubMed] [Google Scholar]
- Palfree R. G., Dumont F. J., Hammerling U. Ly-6A.2 and Ly-6E.1 molecules are antithetical and identical to MALA-1. Immunogenetics. 1986;23(3):197–207. doi: 10.1007/BF00373821. [DOI] [PubMed] [Google Scholar]
- Palfree R. G., Hämmerling U. Biochemical characterization of the murine activated lymphocyte alloantigen Ly-6E.1 controlled by the Ly-6 locus. J Immunol. 1986 Jan;136(2):594–600. [PubMed] [Google Scholar]
- Potter T. A., McKenzie I. F., Morgan G. M., Cherry M. Murine lymphocyte alloantigens. I. The Ly-6 locus. J Immunol. 1980 Aug;125(2):541–545. [PubMed] [Google Scholar]
- Reinach F. C., Fischman D. A. Recombinant DNA approach for defining the primary structure of monoclonal antibody epitopes. The analysis of a conformation-specific antibody to myosin light chain 2. J Mol Biol. 1985 Feb 5;181(3):411–422. doi: 10.1016/0022-2836(85)90229-3. [DOI] [PubMed] [Google Scholar]
- Reiser H., Yeh E. T., Gramm C. F., Benacerraf B., Rock K. L. Gene encoding T-cell-activating protein TAP maps to the Ly-6 locus. Proc Natl Acad Sci U S A. 1986 May;83(9):2954–2958. doi: 10.1073/pnas.83.9.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Yeh E. T., Gramm C. F., Haber S. I., Reiser H., Benacerraf B. TAP, a novel T cell-activating protein involved in the stimulation of MHC-restricted T lymphocytes. J Exp Med. 1986 Feb 1;163(2):315–333. doi: 10.1084/jem.163.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson L. E., Harford J. B., Klausner R. D. Identification of the components of the murine T cell antigen receptor complex. Cell. 1985 Nov;43(1):223–231. doi: 10.1016/0092-8674(85)90027-3. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Harford J., Schwartz R. H., Klausner R. D. A 20-kDa protein associated with the murine T-cell antigen receptor is phosphorylated in response to activation by antigen or concanavalin A. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1969–1973. doi: 10.1073/pnas.82.7.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Speculations on RNA splicing. Cell. 1981 Mar;23(3):643–646. doi: 10.1016/0092-8674(81)90425-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffen D., Bird S., Rowe W. P., Weinberg R. A. Identification of DNA fragments carrying ecotropic proviruses of AKR mice. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4554–4558. doi: 10.1073/pnas.76.9.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Kato K., Hayashizaki Y., Wakabayashi T., Ohtsuka E., Matsuki S., Ikehara M., Matsubara K. Molecular cloning of the human cholecystokinin gene by use of a synthetic probe containing deoxyinosine. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1931–1935. doi: 10.1073/pnas.82.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei F. Biochemical characterization of H9/25, an allospecificity encoded by the Ly-6 region. Immunogenetics. 1982;16(3):201–208. doi: 10.1007/BF00343309. [DOI] [PubMed] [Google Scholar]
- Takei F., Galfrè G., Alderson T., Lennox E. S., Milstein C. H 9/25 monoclonal antibody recognizes a new allospecificity of mouse lymphocyte subpopulations: strain and tissue distribution. Eur J Immunol. 1980 Apr;10(4):241–246. doi: 10.1002/eji.1830100404. [DOI] [PubMed] [Google Scholar]
- Takei F. MALA-1: a surface antigen expressed on activated murine T and B lymphocytes. J Immunol. 1984 Jul;133(1):345–350. [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]
- Wake C. T., Long E. O., Mach B. Allelic polymorphism and complexity of the genes for HLA-DR beta-chains--direct analysis by DNA-DNA hybridization. Nature. 1982 Nov 25;300(5890):372–374. doi: 10.1038/300372a0. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F. The immunoglobulin superfamily takes shape. Nature. 1984 Mar 1;308(5954):12–13. doi: 10.1038/308012a0. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yutoku M., Grossberg A. L., Pressman D. A cell surface antigenic determinant present on mouse plasmacytes and only about half of mouse thymocytes. J Immunol. 1974 May;112(5):1774–1781. [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]