Abstract
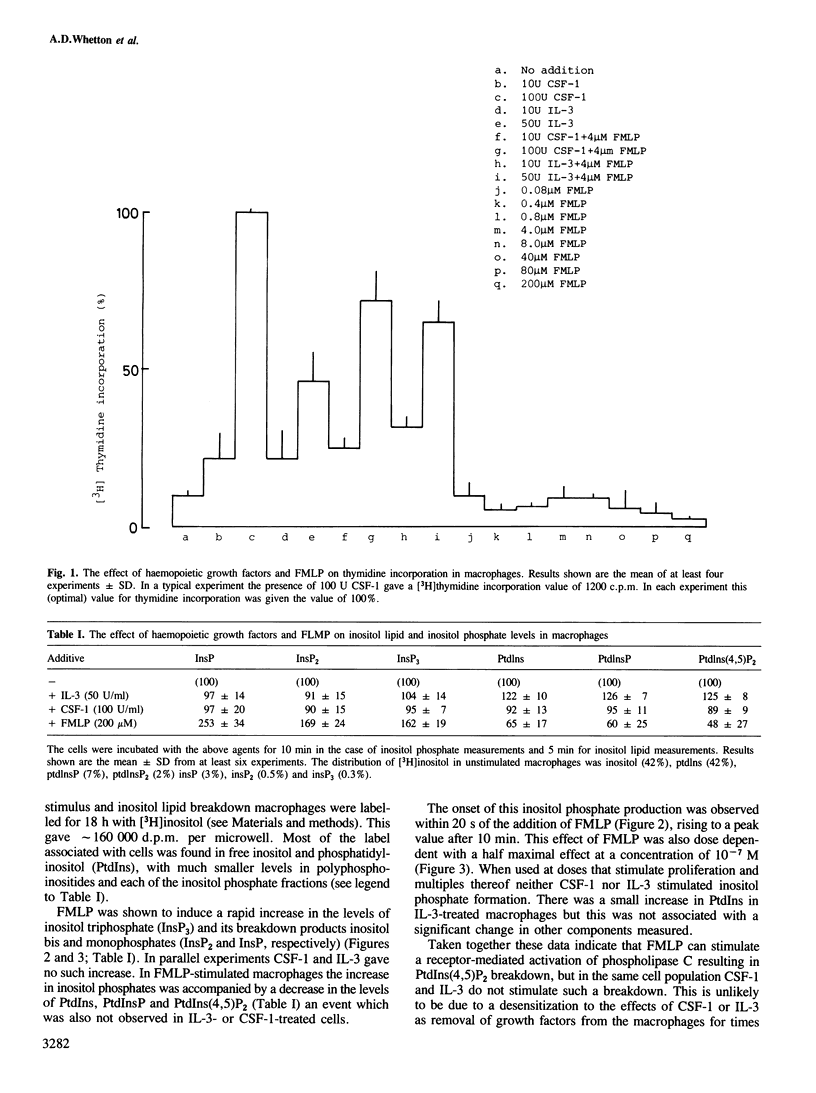
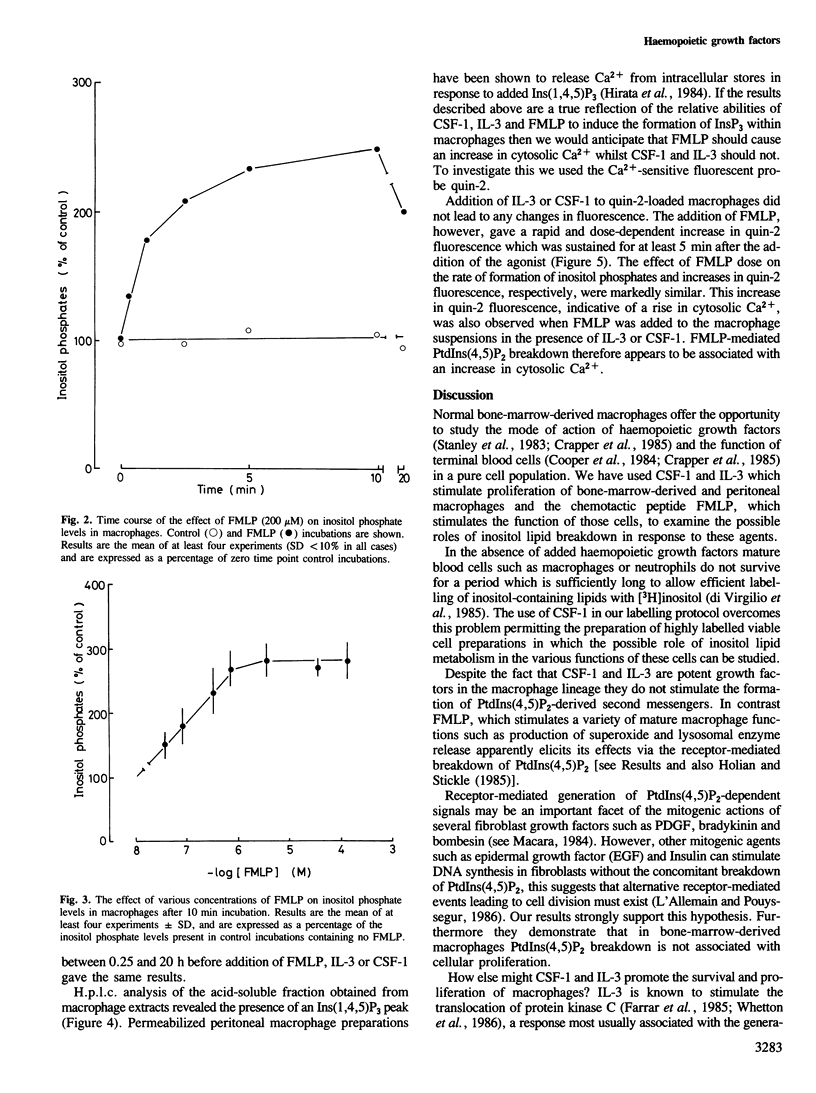
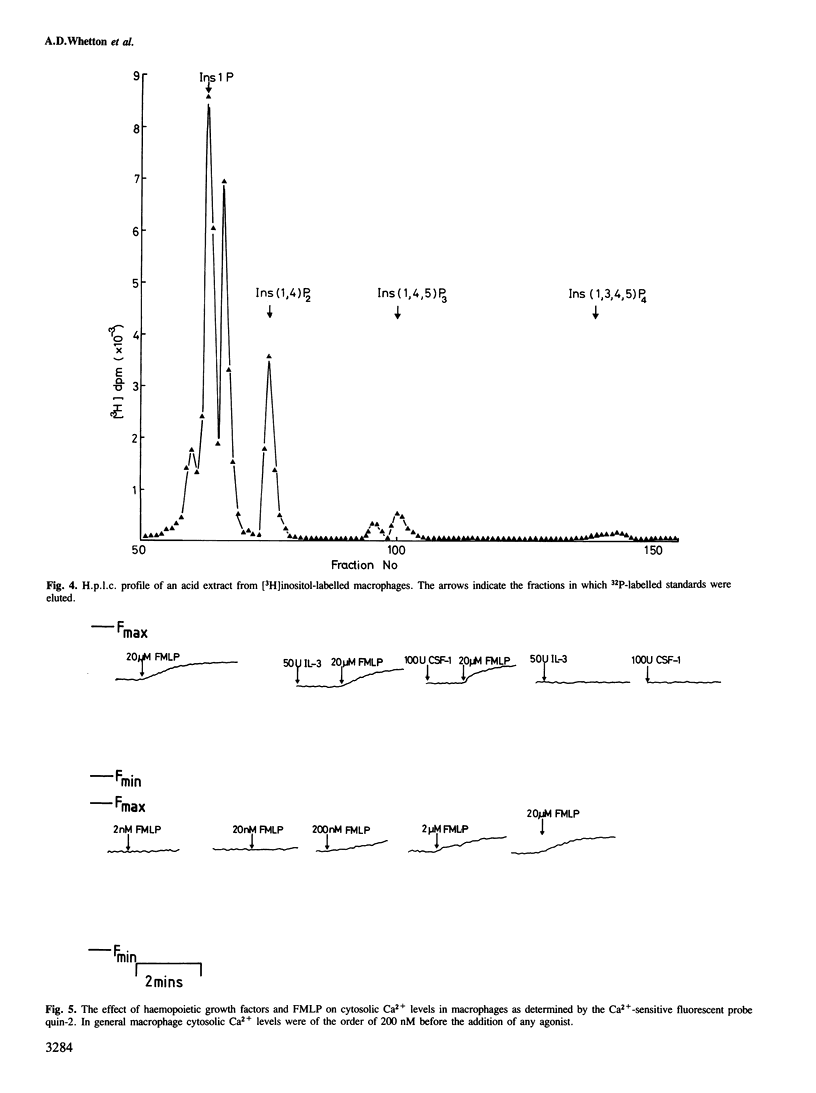
The haemopoietic growth factors interleukin 3 (IL-3) and colony stimulating factor-1 (CSF-1) stimulate the survival and proliferation of murine normal bone-marrow-derived macrophages. To establish whether these growth factors elicit their effects via the hydrolysis of phosphatidylinositol(4,5)bisphosphate [PtdIns(4,5)P2] to form the second messengers inositol (1,4,5)trisphosphate [Ins(1,4,5)P3] and diacylglycerol, macrophages were labelled with tracer quantities of [3H]inositol. Treatment of these cells with either IL-3 or CSF-1 did not alter the levels of PtdIns(4,5)P2 or Ins(1,4,5)P3. However, addition of the chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine (FMLP) which does not stimulate proliferation in macrophages caused a marked and rapid increase in the levels of Ins(1,4,5)P3, inositol bisphosphate and inositol monophosphate, and a decrease in the amount of PtdIns(4,5)P2. FMLP also evoked a rapid increase in intracellular cytosolic Ca2+ levels, as measured with quin 2 the Ca2+-sensitive fluorescent probe, whereas IL-3 and CSF-1 had no such effect. These results suggest that FMLP stimulates the hydrolysis of PtdIns(4,5)P2 to form the second messenger Ins(1,4,5)P3 which acts to increase the levels of cytosolic Ca2+, and that IL-3- and CSF-1-stimulated proliferation in macrophages is not associated with the formation of PtdIns(4,5)P2-derived second messengers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazill G. W., Haynes M., Garland J., Dexter T. M. Characterization and partial purification of a haemopoietic cell growth factor in WEHI-3 cell conditioned medium. Biochem J. 1983 Mar 15;210(3):747–759. doi: 10.1042/bj2100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Brown D. M., Stewart J. C. The structure of triphosphoinositide from beef brain. Biochim Biophys Acta. 1966 Dec 7;125(3):413–421. doi: 10.1016/0005-2760(66)90029-4. [DOI] [PubMed] [Google Scholar]
- Cooper P. H., Mayer P., Baggiolini M. Stimulation of phagocytosis in bone marrow-derived mouse macrophages by bacterial lipopolysaccharide: correlation with biochemical and functional parameters. J Immunol. 1984 Aug;133(2):913–922. [PubMed] [Google Scholar]
- Crapper R. M., Vairo G., Hamilton J. A., Clark-Lewis I., Schrader J. W. Stimulation of bone marrow-derived and peritoneal macrophages by a T lymphocyte-derived hemopoietic growth factor, persisting cell-stimulating factor. Blood. 1985 Oct;66(4):859–865. [PubMed] [Google Scholar]
- Das S. K., Stanley E. R. Structure-function studies of a colony stimulating factor (CSF-1). J Biol Chem. 1982 Nov 25;257(22):13679–13684. [PubMed] [Google Scholar]
- Di Virgilio F., Vicentini L. M., Treves S., Riz G., Pozzan T. Inositol phosphate formation in fMet-Leu-Phe-stimulated human neutrophils does not require an increase in the cytosolic free Ca2+ concentration. Biochem J. 1985 Jul 15;229(2):361–367. doi: 10.1042/bj2290361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Wusteman M. M. Breakdown of polyphosphoinositides and not phosphatidylinositol accounts for muscarinic agonist-stimulated inositol phospholipid metabolism in rat parotid glands. Biochem J. 1983 Dec 15;216(3):633–640. doi: 10.1042/bj2160633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese R. V., Davis J. S., Barnes D. E., Standaert M. L., Babischkin J. S., Hock R., Rosic N. K., Pollet R. J. The de novo phospholipid effect of insulin is associated with increases in diacylglycerol, but not inositol phosphates or cytosolic Ca2+. Biochem J. 1985 Oct 15;231(2):269–278. doi: 10.1042/bj2310269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. L., Thomas T. P., Anderson W. B. Altered cytosol/membrane enzyme redistribution on interleukin-3 activation of protein kinase C. Nature. 1985 May 16;315(6016):235–237. doi: 10.1038/315235a0. [DOI] [PubMed] [Google Scholar]
- Guy G. R., Gordon J., Michell R. H., Brown G. Synergism between diacylglycerols and calcium ionophore in the induction of human B cell proliferation mimics the inositol lipid polyphosphate breakdown signals induced by crosslinking surface immunoglobulin. Biochem Biophys Res Commun. 1985 Aug 30;131(1):484–491. doi: 10.1016/0006-291x(85)91828-5. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Dientsman S. R. Induction of macrophage DNA synthesis by phorbol esters. J Cell Physiol. 1981 Mar;106(3):445–450. doi: 10.1002/jcp.1041060314. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Stanley E. R., Burgess A. W., Shadduck R. K. Stimulation of macrophage plasminogen activator activity by colony-stimulating factors. J Cell Physiol. 1980 Jun;103(3):435–445. doi: 10.1002/jcp.1041030309. [DOI] [PubMed] [Google Scholar]
- Hapel A. J., Fung M. C., Johnson R. M., Young I. G., Johnson G., Metcalf D. Biologic properties of molecularly cloned and expressed murine interleukin-3. Blood. 1985 Jun;65(6):1453–1459. [PubMed] [Google Scholar]
- Hasegawa-Sasaki H., Sasaki T. Phytohemagglutinin induces rapid degradation of phosphatidylinositol 4,5-bisphosphate and transient accumulation of phosphatidic acid and diacylglycerol in a human T lymphoblastoid cell line, CCRF-CEM. Biochim Biophys Acta. 1983 Dec 20;754(3):305–314. [PubMed] [Google Scholar]
- Hirata M., Suematsu E., Hashimoto T., Hamachi T., Koga T. Release of Ca2+ from a non-mitochondrial store site in peritoneal macrophages treated with saponin by inositol 1,4,5-trisphosphate. Biochem J. 1984 Oct 1;223(1):229–236. doi: 10.1042/bj2230229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holian A., Stickle D. F. Calcium regulation of phosphatidyl inositol turnover in macrophage activation by formyl peptides. J Cell Physiol. 1985 Apr;123(1):39–45. doi: 10.1002/jcp.1041230107. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S., Rettenmier C. W., Sherr C. J., Rock C. O. A guanine nucleotide-dependent phosphatidylinositol 4,5-diphosphate phospholipase C in cells transformed by the v-fms and v-fes oncogenes. J Biol Chem. 1986 Apr 15;261(11):4978–4985. [PubMed] [Google Scholar]
- Kindler V., Thorens B., de Kossodo S., Allet B., Eliason J. F., Thatcher D., Farber N., Vassalli P. Stimulation of hematopoiesis in vivo by recombinant bacterial murine interleukin 3. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1001–1005. doi: 10.1073/pnas.83.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Allemain G., Pouysségur EGF and insulin action in fibroblasts. Evidence that phosphoinositide hydrolysis is not an essential mitogenic signalling pathway. FEBS Lett. 1986 Mar 3;197(1-2):344–348. doi: 10.1016/0014-5793(86)80354-4. [DOI] [PubMed] [Google Scholar]
- Lord B. I., Molineux G., Testa N. G., Kelly M., Spooncer E., Dexter T. M. The kinetic response of haemopoietic precursor cells, in vivo, to highly purified, recombinant interleukin-3. Lymphokine Res. 1986 Spring;5(2):97–104. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Sharps E. S., McCarl R. L. A high-performance liquid chromatographic method to measure 32P incorporation into phosphorylated metabolites in cultured cells. Anal Biochem. 1982 Aug;124(2):421–424. doi: 10.1016/0003-2697(82)90059-8. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Cifone M., Heard P. M., Defendi V. Factors regulating macrophage production and growth: identity of colony-stimulating factor and macrophage growth factor. J Exp Med. 1976 Mar 1;143(3):631–647. doi: 10.1084/jem.143.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J., Tushinski R. J., Bartelmez S. H. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Till J. E., McCulloch E. A. Hemopoietic stem cell differentiation. Biochim Biophys Acta. 1980 Nov 26;605(4):431–459. doi: 10.1016/0304-419x(80)90009-8. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi M., Yassin R., Naccache P. H., Sha'afi R. I. Chemotactic factor causes rapid decreases in phosphatidylinositol,4,5-bisphosphate and phosphatidylinositol 4-monophosphate in rabbit neutrophils. Biochem Biophys Res Commun. 1983 May 16;112(3):957–964. doi: 10.1016/0006-291x(83)91711-4. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Heyworth C. M., Dexter T. M. Phorbol esters activate protein kinase C and glucose transport and can replace the requirement for growth factor in interleukin-3-dependent multipotent stem cells. J Cell Sci. 1986 Aug;84:93–104. doi: 10.1242/jcs.84.1.93. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


