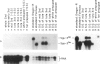Abstract
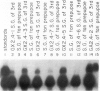
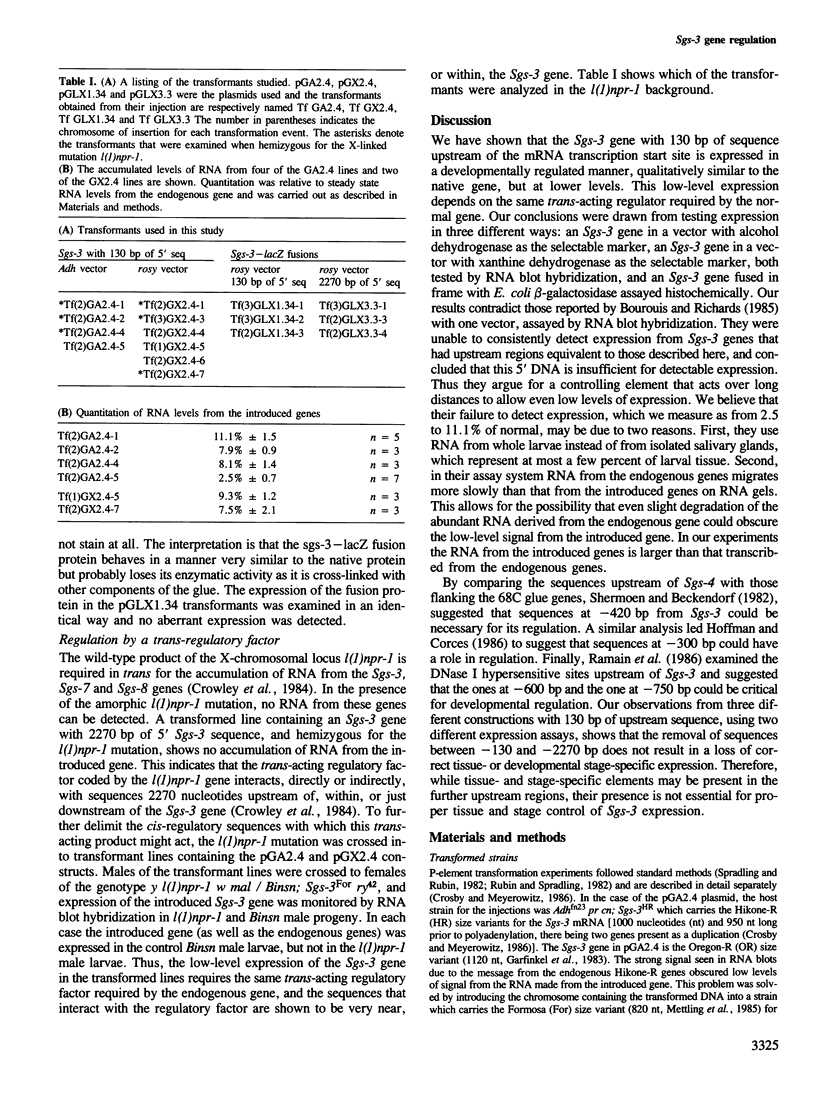
The Drosophila melanogaster 68C chromosomal locus is the site of a prominent polytene chromosome puff that harbors the genes Sgs-3, Sgs-7 and Sgs-8. These genes code for proteins that are part of the salivary glue that Drosophila larvae secrete as a means of fixing themselves to an external substrate for the duration of the pre-pupal and pupal period. The 68C glue genes are regulated by the steroid hormone ecdysterone, with the hormone required for both initiation and cessation of gene expression during the third larval instar. Previous work has defined sequences sufficient for expression of abundant levels of Sgs-3 mRNA at the correct time and in the correct tissue. We show here that sequences sufficient for normal tissue- and stage-specific accumulation of Sgs-3 RNA, but adequate only for low levels of expression, lie within 130 bp of the 5' end of the gene, or within the gene.
Full text
PDF
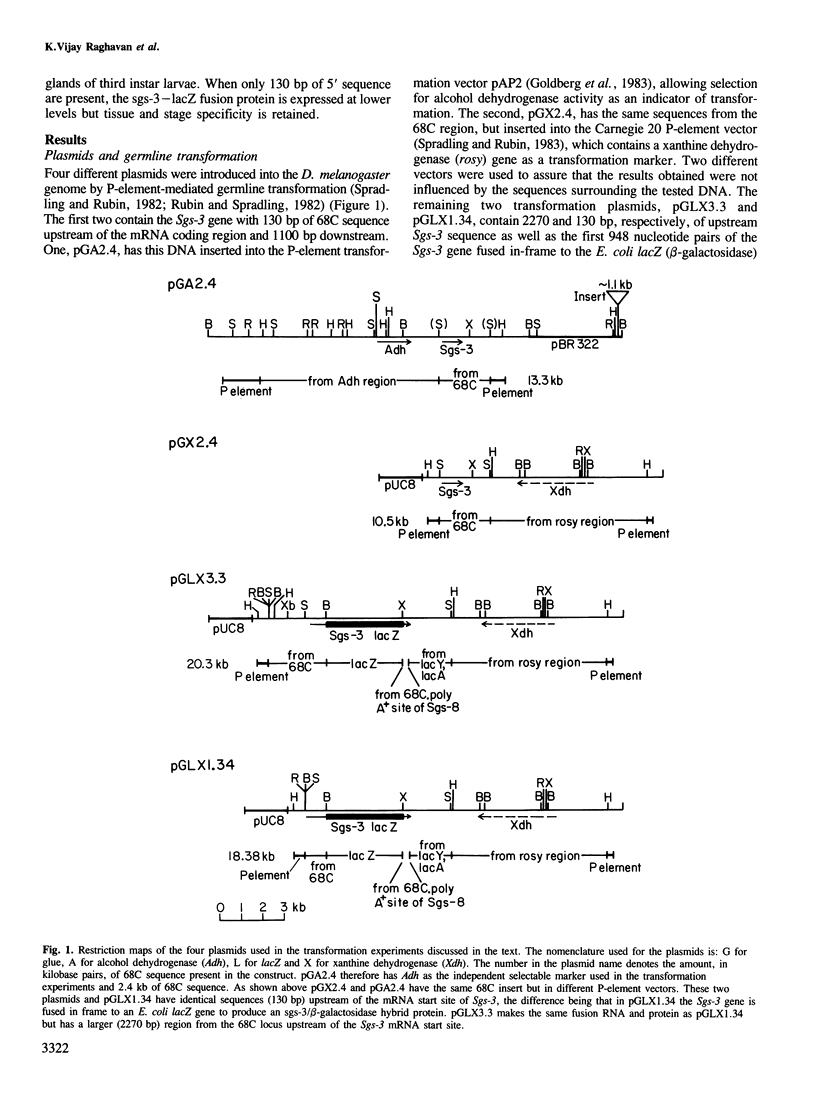



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. E., Roberts D. B., Richards G. P., Ashburner M. Drosophila: the genetics of two major larval proteins. Cell. 1978 Feb;13(2):215–225. doi: 10.1016/0092-8674(78)90190-3. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Richards G. Sequential gene activation by ecdysone in polytene chromosomes of Drosophila melanogaster. III. Consequences of ecdysone withdrawal. Dev Biol. 1976 Dec;54(2):241–255. doi: 10.1016/0012-1606(76)90302-x. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Sequential gene activation by ecdysone in polytene chromosomes of Drosophila melanogaster. I. Dependence upon ecdysone concentration. Dev Biol. 1973 Nov;35(1):47–61. doi: 10.1016/0012-1606(73)90006-7. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Sequential gene activation by ecdysone in polytene chromosomes of Drosophila melanogaster. II. The effects of inhibitors of protein synthesis. Dev Biol. 1974 Jul;39(1):141–157. doi: 10.1016/s0012-1606(74)80016-3. [DOI] [PubMed] [Google Scholar]
- Beckendorf S. K., Kafatos F. C. Differentiation in the salivary glands of Drosophila melanogaster: characterization of the glue proteins and their developmental appearance. Cell. 1976 Nov;9(3):365–373. doi: 10.1016/0092-8674(76)90081-7. [DOI] [PubMed] [Google Scholar]
- Bourouis M., Richards G. Remote regulatory sequences of the Drosophila glue gene sgs3 as revealed by P-element transformation. Cell. 1985 Feb;40(2):349–357. doi: 10.1016/0092-8674(85)90149-7. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley T. E., Bond M. W., Meyerowitz E. M. The structural genes for three Drosophila glue proteins reside at a single polytene chromosome puff locus. Mol Cell Biol. 1983 Apr;3(4):623–634. doi: 10.1128/mcb.3.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley T. E., Mathers P. H., Meyerowitz E. M. A trans-acting regulatory product necessary for expression of the Drosophila melanogaster 68C glue gene cluster. Cell. 1984 Nov;39(1):149–156. doi: 10.1016/0092-8674(84)90200-9. [DOI] [PubMed] [Google Scholar]
- Crowley T. E., Meyerowitz E. M. Steroid regulation of RNAs transcribed from the Drosophila 68c polytene chromosome puff. Dev Biol. 1984 Mar;102(1):110–121. doi: 10.1016/0012-1606(84)90179-9. [DOI] [PubMed] [Google Scholar]
- Dworniczak B., Seidel R., Pongs O. Puffing activities and binding of ecdysteroid to polytene chromosomes of Drosophila melanogaster. EMBO J. 1983;2(8):1323–1330. doi: 10.1002/j.1460-2075.1983.tb01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel M. D., Pruitt R. E., Meyerowitz E. M. DNA sequences, gene regulation and modular protein evolution in the Drosophila 68C glue gene cluster. J Mol Biol. 1983 Aug 25;168(4):765–789. doi: 10.1016/s0022-2836(83)80074-6. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A., Posakony J. W., Maniatis T. Correct developmental expression of a cloned alcohol dehydrogenase gene transduced into the Drosophila germ line. Cell. 1983 Aug;34(1):59–73. doi: 10.1016/0092-8674(83)90136-8. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H., Pongs O. Localization of ecdysterone on polytene chromosomes of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2108–2112. doi: 10.1073/pnas.77.4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild G. M., Shore E. M. Larval salivary gland secretion proteins in Drosophila. Identification and characterization of the Sgs-5 structural gene. J Mol Biol. 1984 Nov 5;179(3):289–314. doi: 10.1016/0022-2836(84)90067-6. [DOI] [PubMed] [Google Scholar]
- Hoffman E., Corces V. Sequences involved in temperature and ecdysterone-induced transcription are located in separate regions of a Drosophila melanogaster heat shock gene. Mol Cell Biol. 1986 Feb;6(2):663–673. doi: 10.1128/mcb.6.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge G. Chromosome puff activity and protein synthesis in larval salivary glands of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4550–4554. doi: 10.1073/pnas.72.11.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge G. Direct correlation between a chromosome puff and the synthesis of a larval saliva protein in Drosophila melanogaster. Chromosoma. 1977 Jul 5;62(2):155–174. doi: 10.1007/BF00292637. [DOI] [PubMed] [Google Scholar]
- Korge G. Larval saliva in Drosophila melanogaster: production, composition, and relationship to chromosome puffs. Dev Biol. 1977 Jul 15;58(2):339–355. doi: 10.1016/0012-1606(77)90096-3. [DOI] [PubMed] [Google Scholar]
- Meyerowitz E. M., Crosby M. A., Garfinkel M. D., Martin C. H., Mathers P. H., VijayRaghavan K. The 68C glue puff of Drosophila. Cold Spring Harb Symp Quant Biol. 1985;50:347–353. doi: 10.1101/sqb.1985.050.01.044. [DOI] [PubMed] [Google Scholar]
- Meyerowitz E. M., Hogness D. S. Molecular organization of a Drosophila puff site that responds to ecdysone. Cell. 1982 Jan;28(1):165–176. doi: 10.1016/0092-8674(82)90386-5. [DOI] [PubMed] [Google Scholar]
- Muskavitch M. A., Hogness D. S. Molecular analysis of a gene in a developmentally regulated puff of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7362–7366. doi: 10.1073/pnas.77.12.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramain P., Bourouis M., Dretzen G., Richards G., Sobkowiak A., Bellard M. Changes in the chromatin structure of Drosophila glue genes accompany developmental cessation of transcription in wild type and transformed strains. Cell. 1986 May 23;45(4):545–553. doi: 10.1016/0092-8674(86)90286-2. [DOI] [PubMed] [Google Scholar]
- Richards G., Cassab A., Bourouis M., Jarry B., Dissous C. The normal developmental regulation of a cloned sgs3 'glue' gene chromosomally integrated in Drosophila melanogaster by P element transformation. EMBO J. 1983;2(12):2137–2142. doi: 10.1002/j.1460-2075.1983.tb01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Shermoen A. W., Beckendorf S. K. A complex of interacting DNAase I-hypersensitive sites near the Drosophila glue protein gene, Sgs4. Cell. 1982 Jun;29(2):601–607. doi: 10.1016/0092-8674(82)90176-3. [DOI] [PubMed] [Google Scholar]
- Singh M. B., Knox R. B. Quantitative cytochemistry of beta-galactosidase in normal and enzyme deficient (gal) pollen of Brassica campestris: application of the indigogenic method. Histochem J. 1984 Dec;16(12):1273–1296. doi: 10.1007/BF01003726. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. The effect of chromosomal position on the expression of the Drosophila xanthine dehydrogenase gene. Cell. 1983 Aug;34(1):47–57. doi: 10.1016/0092-8674(83)90135-6. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Velissariou V., Ashburner M. Cytogenetic and genetic mapping of a salivary gland secretion protein in Drosophila melanogaster. Chromosoma. 1981;84(2):173–185. doi: 10.1007/BF00399129. [DOI] [PubMed] [Google Scholar]
- Velissariou V., Ashburner M. The secretory proteins of the larval salivary gland of Drosophila melanogaster: Cytogenetic correlation of a protein and a puff. Chromosoma. 1980;77(1):13–27. doi: 10.1007/BF00292038. [DOI] [PubMed] [Google Scholar]