Abstract
Background:
About 25% of palliative medication incidents involve continuous subcutaneous infusions. Complex structural and human factor issues make these risk-prone interventions. Detailed analysis of how this safety-critical care can be improved has not been undertaken. Understanding context, contributory factors and events leading to incidents is essential.
Aims:
(1) Understand continuous subcutaneous infusion safety incidents and their impact on patients and families; (2) Identify targets for system improvements by learning from recurrent events and contributory factors.
Design:
Following systematic identification and stratification by degree of harm, a mixed methods analysis of palliative medication incidents involving continuous subcutaneous infusions comprising quantitative descriptive analysis using the PatIent SAfety (PISA) classification system and qualitative narrative analysis of free-text reports.
Setting/participants:
Palliative medication incidents (n = 7506) reported to the National Reporting and Learning System, England and Wales (2016–2021).
Results:
About 1317/7506 incidents involved continuous subcutaneous infusions with 943 (72%) detailing harms. Primary incidents (most proximal to patient outcomes) leading to inappropriate medication use (including not using medication when it was needed) were underpinned by breakdowns in three major medication processes: monitoring and supply (405, 31%), administration (383, 29%) and prescribing (268, 20%). Recurring contributory factors included discontinuity of care within and between settings, inadequate time, inadequate staffing and unfamiliarity with protocols. Psychological harms for patients and families were identified.
Conclusions:
System infrastructure is needed to enable timely supply of medication and equipment, effective coordinated use of continuous subcutaneous infusions, communication and continuity of care. Training is needed to improve incident descriptions so these pinpoint precise targets for safer care.
Keywords: Drug safety, infusions, subcutaneous, injection, subcutaneous, mixed methods analysis, palliative care, patient safety
What is already known about the topic?
The third World Health Organization Global Patient Safety Challenge ‘Medication without harm’, emphasises the need for improved infrastructure through better reporting practices and cross-organisational learning from adverse events and near misses.
Medication is implicated in one-fifth of serious palliative care patient safety incidents, with approximately 25% of these incidents involving continuous subcutaneous infusions.
Inadequate analysis of continuous subcutaneous infusions as safety-critical, risk-prone interventions dependent on complex structural and human factor issues is a lost opportunity for learning.
What this paper adds?
Continuous subcutaneous infusion incidents occur across all settings including the home, hospices and hospitals and particularly after the transfer of patients between settings with harm present in nearly three-quarters of reports.
Multiple points of system failure were identified in continuous subcutaneous infusion incident reports including monitoring and supply (405, 31%), administration (383, 29%) and prescribing (268, 20%); recurring contributory factors included discontinuity of care within and between care settings, inadequate time, inadequate staffing and unfamiliarity with protocols.
Narrative descriptions of psychological and social harm, alongside physical harm risk, are not being adequately recognised or responded to through existing approaches to measure harm in palliative care, hindering learning in practice
Implications for practice, theory or policy
The structural changes needed to minimise harm and maximise safety in palliative care are likely to be replicated in other parts of the world where patient safety reporting practices are less well established, for example, shifting from focussing on lack of experience and competency at an individual practitioner-level to addressing deficits in working environments and infrastructures for care provision.
When patients move between care locations, more attention should be given to the timeliness and effective transfer of medication management (e.g. if someone is discharged from hospital to a care home that rarely uses continuous subcutaneous infusions for palliative care, this needs to be preceded by refreshing staff skills and ensuring they can access further community support if needed).
Professional training and further research are needed to increase quality of reporting of psychological and social harms (including for families and other stakeholders involved) to facilitate organisational learning and pinpoint precise targets for further improvement.
Background
Unsafe healthcare contributes significantly to global morbidity and mortality.1,2 The third World Health Organization Global Patient Safety Challenge ‘Medication without harm’, aimed to halve severe, avoidable harms to patients due to medicines by 2022, emphasising the need for improved infrastructure through better reporting practices and cross-organisational learning from adverse events and near misses.
Medication is implicated in one-fifth of all serious patient safety incidents reported from palliative care, with patients at home disproportionately affected.3,4 Patients receiving palliative care often require considerable input at home5 –7 but incidents also occur in hospitals, hospices and during transfers between settings.3,8 Multiple professional roles in all settings3,8,9 make coordination essential for safe care. Prescribing is widely researched but is not necessarily the most incident-prone medication process.10 –16
Continuous subcutaneous infusions are commonly used in palliative care when the oral route is either ineffective (e.g. ongoing vomiting or other causes of poor absorption) or not possible (e.g. patient unable to swallow) and/or when subcutaneous delivery is least burdensome and most practical (i.e. when intravenous access is either difficult or not provided for, as is common in community settings). Approximately 25% of reported palliative medication incidents involve a continuous subcutaneous infusion.3,17 Medications including strong opioids and sedatives are routinely prescribed and administered by infusion when managing symptoms with oral medication ceases to be viable or effective (see Supplemental Files: Use and definition of continuous subcutaneous infusions).5,18 Infusion devices (syringe drivers/pumps) are used globally to optimise end-of-life care. 1 These battery-powered devices are usually replenished once every 24 h unless symptom control is inadequate, necessitating earlier changes.
The use of continuous subcutaneous infusions involves multistep, complex, risk-prone processes dependent on structural and human factors.4,19 For example, assessment of the patient’s fluctuating needs, agreeing (reviewing/revising) treatment plans, prescribing, dispensing, sourcing equipment, adequate staffing and skillsets, administration and disposal.4,19,20 Steps involved in monitoring patients’ needs and titrating medications are of particular concern18,21,22 but there also remain considerable gaps in evidence regarding how and where unsafe care can occur.15,8,19,21,22,23,24,25,26,27,28,29,30
Methods
The aims of this research were to:
RQ1: To understand continuous subcutaneous infusion safety incidents and their impact on patients and families.
RQ2: To identify targets for system improvements by learning from recurrent events and contributory factors.
| Definitions |
| • Primary incidents are incidents that occurred closest to the patient outcome, that is what happens. • Contributory incidents precede primary incidents within a trajectory of events, that is the X and Y, in the sequence X->Y->Z, where Z is the primary incident and X and Y are what happens beforehand. • Contributory factors are reasons (e.g. circumstances, actions or influences) underlying why the incident might have occurred. Often these factors are present more widely, not solely confined to a specific incident for example distractions and interruptions in an environment where medication tasks are being completed. |
Design
Incident reporting systems of healthcare-associated harms and near misses are intended to aid learning and inform strategies to reduce future harm. Utilising these data can enhance understanding and learning from reported continuous subcutaneous infusion incidents in palliative care.
The National Reporting and Learning System in England and Wales is a world leader in patient safety incident reporting. NHS England encourages reporting of all incidents and near misses without a specific threshold. No demographic data are included. We used this centralised database to identify patient safety incidents from January 2016 to December 2021. It contains structured information such as incident type, harm severity, outcome and location8,24,25 and unstructured free-text prompts to describe what happened, and why and how reoccurrence could be prevented. A systematic approach was developed and applied to identify and analyse incidents. 17
A cross-sectional quantitative descriptive analysis was undertaken using the PatIent SAfety (PISA) classification system.17,8,26 This operationalises key concepts from the World Health Organization International Classification for Patient Safety into coding frameworks describing incident type, contributory factors, harm outcomes and severity; it has been refined through multiple studies.26 –28 Incidents were screened for inclusion then coded. Free-text descriptions were further examined using qualitative narrative analysis. 31 The PISA approach of quantification involves drop list coding only what is explicitly found in the data, whilst the qualitative analysis includes interpretation of what is implied in the narrative.
Study dataset eligibility
Ethical approval was granted by Cardiff University School of Medicine Research Ethics Committee (Ref 19/28). Secure access to the most recently available anonymised palliative medication incidents (1st January 2016–31st December 2021) in the National Reporting and Learning System was granted by National Health Service Improvement. As individual people/parties are not identifiable, data were exempt from United Kingdom research regulations regarding informed consent.
Using previous approaches to studying incident datasets,3,8,26,29,30,32,33 we identified a sample of 7506 palliative medication incidents stratified by degree of reported harm, including those reported as no harm or harm unclear, as well as those reported as resulting in low/medium/severe harm and death (see Supplemental Files: Sample Stratification). A palliative medication was defined as a medication used for symptom control, that is with palliative intent. We used the Palliative Care Formulary alongside the standard British National Formulary.17,34,35 Incidents specific to continuous subcutaneous infusions were systematically identified using the search terms: syringe, pump, driver or continuous subcutaneous infusion; and McKinley or Graseby (the two commonest UK brands). A total of 1692 potentially relevant reports were manually screened using pre-defined exclusion criteria (Table 1) by AB; SY and BB double screened 20% and 5% respectively. Uncertainty regarding eligibility resulted in all three discussing to reach consensus.
Table 1.
Criteria used to identify reports containing continuous subcutaneous infusion palliative medication incidents.
| Palliative exclusion criteria |
| 1. Patient not in last phase of life that is, having a potentially life- limiting or progressive condition requiring general or specialist palliative care for symptom control, social, psychological and/or spiritual support (but not limited to last days of life). |
| 2. Not a medication process that is, no mention of a medication name, tablet, CSCI, continuous subcutaneous infusion or other medication delivery method. |
| 3. Medications used without palliative intent for example, anaesthetic procedures, incident solely related to disease-modifying treatment, for example, chemotherapy drug errors. |
| 4. Incident not related to patient care that is, the incident did not describe a patient safety incident. For example, reporting an expected death where no patient safety incident occurred. |
| 5. Not a syringe driver process that is, no mention of syringe driver, CSCI, continuous subcutaneous infusion, driver or giving set. |
Classification of reports: Coding
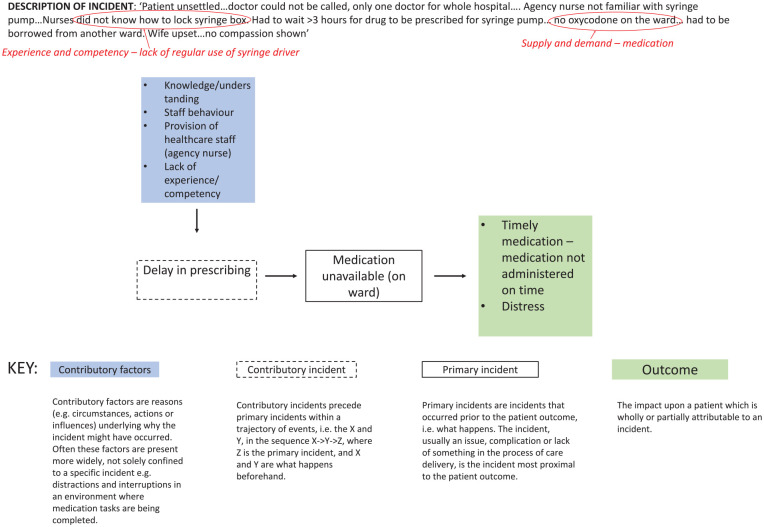
Each incident report was coded by AB using the PatIent SAfety (PISA) classification system into a sequence of events (primary and contributory incidents), contributory factors, resultant harm outcomes and severity, (see Figure 1). BB independently coded 5% and SY 10%. Consensus was reached through reflexive discussions that informed AB’s subsequent coding of remaining incidents. Where data were available, the medication process implicated in each incident was allocated according to primacy (i.e. which medication process step was most crucial for that incident to occur) from a coding list: prescribing, administration, monitoring and supply, decision-making, transition between care settings, stopping medications and other (e.g. reporting a fall when a syringe driver was in use). Where a report described multiple incidents or contributory factors, they were coded in reverse chronological order, working back from the primary incident, thereby providing a richer analysis of reported causal chains. This enabled the identification of prevalent patterns between codes for example ‘incident type’ and ‘contributory factor’. Where no existing outcome code was adequate for patients at their end of lives, we amended the classification system to include ‘uncontrolled symptoms, unspecified’ and ‘disturbed dying’.
Figure 1.
Example continuous subcutaneous infusion incident using the recursive model of incident analysis 27 and qualitative narrative analysis (plus coding/theme identification via qualitative narrative synthesis process highlighted in red.
Harm severity
Where reported harm severity clearly did not align with the free-text incident description, it was adjusted using World Health Organization International Classification of Patient Safety definitions (Table 1). 3 AB, SY, BB and ACS discussed this when information was ambiguous in individual reports. Regardless of our classification (unclear, no/low/medium/severe harm or death) all incidents were included in our full analysis to explore the learning potential (e.g. themes in contributory factors).
Qualitative narrative analysis
To identify a purposive sample for more in-depth qualitative analysis of the most salient semantic relationships between primary incident type, contributory incident type and contributory factors, a cross-tabulation (using a median frequency of 4 as a cut off) was created between:
(1) the 14 most common primary incidents and 11 recurring contributory factors;
(2) 5 contributory incidents and 9 recurring contributory factors.
This created a subsample of 129 incidents, of which 111 reports contained adequate free-text descriptions to enable a detailed thematic analysis of incident narratives (see Figure 1 for an example of this process).
AB re-read incident descriptions to iteratively identify and describe recurring patterns and themes. Further thematic analysis was carried out by SY. 36 Themes and their implications for practice were refined through critical reflection meetings of AB, SY, BB and ACS, plus weekly wider research team discussion meetings.36,37
Results
1317 reports were included in the quantitative analysis and 111 in the thematic analysis (see Supplemental Files: PRISMA diagram summarising incident handling and selection of continuous subcutaneous infusion incidents for quantitative and qualitative analysis). Double/triple screening for inclusion produced consensus without discussion in 150/158 (95%); 8 reports were discussed to reach consensus on harm severity. Abridged individual unattributed examples are provided to contextualise and illustrate the analysis, whilst retaining anonymity.
Quantitative results
Harm severity and outcomes
About 924/1317 (70%) of included reports were characterised by the initial reporters of incidents as resulting in harm, the other 30% as no harm. Where harm was recognised by reporters it was most commonly characterised as low. The code ‘unclear’ harm was not used by initial incident reporters and thus 39 incidents were changed to an unclear harm outcome by the research team when in our analysis (Table 2), 118 incidents (9%) were reclassified to align with World Health Organization International Classification for Patient Safety definitions. Overall reclassification led to 77 reports being upgraded, and 41 reports downgraded. Following reclassification, 924/1317 (70%) reports were now characterised as resulting in harm (including death); the other 393 (30%) were now characterised as no or unclear harm (including no harm due to mitigating action).
Table 2.
Number (%) of continuous subcutaneous infusion incidents by reclassified severity of harm (n = 1317).
| Severity of harm | Description | Example individual incident description | Number of incidents | Percentage of total incidents (rounded to nearest 0.5) |
|---|---|---|---|---|
| No harm | Patient outcome is not symptomatic, or no symptoms detected and no treatment is required | . . .visited to set the driver up and Metoclopramide not available. . .Stat dose given. . .family struggled to get medication. . . still awaiting GP to prescribe/get hold of alternative. . .managed patients comfort by giving stat doses. . .resolved following day by OOH GP [out of hours general practitioner] who changed prescription for syringe driver [CSCI] so all required medications were readily available. . . – unknown reporter role | 273 | 21 |
| No harm due to mitigating action | Patient outcome is not symptomatic or no symptoms detected and no treatment is required due to an intervention | . . .prescription. . .written. . .prescribed hyoscine hydrobromide 60mg over 24 hours [via a CSCI] when they had intended to prescribe hyoscine butylbromide 60mg over 24 hours. . .nurse realised. . .mistake and put hyoscine butylbromide in the syringe driver. . .doctor noted their mistake within two hours and confirmed. . .hyoscine hydrobromide was never administered – unknown reporter role | 81 | 6 |
| Low harm | Patient outcome is symptomatic, symptoms are mild, loss of function or harm is minimal or intermediate but short term, and no or minimal intervention (e.g extra observation, investigation, review or minor treatment is required) | Patient unsettled. . .doctor could not be called, only one doctor for whole hospital. . . . Agency nurse not familiar with syringe pump [CSCI pump]. . . Nurses did not know how to lock syringe box. Had to wait >3 hours for drug to be prescribed for syringe pump. . . no oxycodone on the ward. . . had to be borrowed from another ward. Wife upset. . .no compassion shown. – unknown reporter role | 656 | 50 |
| Moderate harm | Patient outcome is symptomatic, requiring intervention (e.g. additional operative procedure or additional therapeutic treatment), an increased length of stay or causing permanent or long-term harm or loss of function | . . .visit patient to administer stat doses. . .agitated and pain. . .only ampoule of Oxycodone had been opened and re-taped . . .patient extremely agitated and complaining of pain. . .patient had Oxycodone in the driver [CSCI pump] with 2.5mg Midazolam but sticker attached stated Diamorphine and Midazolam. . .No Diamorphine written up and no Diamorphine ampoules in home. . .administer from opened used ampoule which is against policy . . .GP to visit urgently and administer stat doses but patient died before GP could visit. . . – unknown reporter role | 218 | 16.5 |
| Severe harm | Patient outcome is symptomatic, requiring life-saving intervention or major surgical/ medical intervention, shortening life expectancy or causing major permanent or long-term harm or loss of function | patient discharged. . . no time intervals, no diamorphine or syringe driver [CSCI] issued/prescribed. Midazolam sent out unsuitable for stat use. Not temp[orarily] registered with GP. . . Dr refused visit, ref[erral] to prescribe driver or diamorphine . . .drugs and directive not obtained. . .gave max doses of drugs as prescribed/advice. Went to surgery to get max doses changed/prescribed. Consultant took 30 mins to return phone call to GP and would not prescribe from conversation. . .returned to house 45 minutes later. . . patient had died 10 minutes earlier – unknown reporter role | 42 | 3 |
| Death | On balance of probabilities, death was caused or brought forward in the short term by the incident | Nurse read medication chart. . . identified drug within dispensing box. . .chart states 0.1mg to 0.2 mg and box range states 0.5 mg - 1.5mg. . .nurse administered 1mg. . .patient passed away shortly after visit. . . –unknown reporter role | 8 | 0.5 |
| Unclear | It is unclear from the free-text description what level of harm has occurred | Pt [patient] had syringe driver [CSCI] with Midazolam 20mg, Haloperidol 5mg and Morphine 10mg. . .over last 24hrs patient required x4 prn doses of Midazolam; 3 x 5mg doses & 1x10mg dose. . .totalling 25mg of PRN [as needed] doses given over 24hrs, additional 20mg in syringe driver. . . now asking if DN [district nurse] can visit patient and increase drugs to: 50mg Midazolam, 10mg Haloperidol and 20mg Morphine over 24hr. . .set up an additional syringe driver. . .with 150mg Levomepromazine. . .explained. . . no decision to set up anticipatory syringe drivers. . .concerned regarding dose. . . –unknown reporter role | 39 | 3 |
Most incidents occurred in acute hospitals or in patients’ homes (Table 3). An outcome was described in 1092 (83%) incidents, most frequently this was stated as the patient experiencing pain (195 incidents, 15%). Incidents resulting in severe harm or death in the community (the most frequent location of these levels of harm) were reviewed for common themes. This identified that most of these incidents occurred out of hours. Frequently the clinicians involved were the out of hours GP and the district nursing service, and communication between these services was impaired in many of these incidents. Discharging from the hospital frequently resulted in uncontrolled symptoms due to the lack of medications and failure to hand over the patient to the district nursing service in a timely manner.
Table 3.
The frequency and harm severity of reported continuous subcutaneous infusion incidents by care setting.
| Setting of CSCI incident | Harm severity rating | Total | Percentage of total incidents (%) | |||
|---|---|---|---|---|---|---|
| Low | Moderate | Severe harm/death | Other | |||
| Acute hospital | 382 | 109 | 18 | 200 | 709 | 54 |
| Own home – usual place of residence | 190 | 64 | 20 | 114 | 388 | 29.5 |
| Nursing care – usual place of residence | 23 | 13 | 4 | 13 | 53 | 4 |
| General practice surgery | 16 | 9 | 2 | 15 | 41 | 3 |
| Hospice | 9 | 1 | 0 | 20 | 30 | 2 |
| Residential care – usual place of residence | 6 | 6 | 3 | 4 | 19 | 1.5 |
| Other institutional setting | 3 | 2 | 0 | 4 | 9 | 1 |
| Unknown (not stated) | 27 | 14 | 3 | 23 | 67 | 5 |
| Total | 656 | 218 | 50 | 393 | 1317 | 100 |
Process breakdowns
Three medication processes were identified as those most prone to breakdowns and resulting in a wrong dose being administered, that is, a different medication and/or giving too much or too little medication (Table 4). These medication processes were:
Table 4.
Number of incidents by main medication process involved.
| Medication process | Own home | Residential care | Nursing care | Other institutional setting | Hospice | Acute hospital | General practice surgery | Unknown | Number by harm severity (%) | Number (%) n = 1317 |
|---|---|---|---|---|---|---|---|---|---|---|
| Monitoring and supply | 130 | 6 | 15 | 3 | 2 | 229 | 5 | 14 | Low - 216 Moderate - 74 Severe harm/death - 13 Other - 102 |
405 (31%) |
| Administration | 132 | 8 | 20 | 2 | 10 | 176 | 3 | 32 | Low - 216 Moderate - 67 Severe harm/death - 11 Other - 108 |
383 (29%) |
| Prescribing | 66 | 4 | 4 | 2 | 11 | 142 | 27 | 102 | Low - 126 Moderate - 29 Severe harm/death - 9 Other - 104 |
268 (20%) |
| Transition between care settings | 20 | 0 | 1 | 0 | 4 | 98 | 2 | 2 | Low - 49 Moderate - 26 Severe harm/death - 12 Other - 40 |
127 (10%) |
| Decision making a | 24 | 1 | 7 | 1 | 0 | 34 | 5 | 4 | Low - 40 Moderate - 16 Severe harm/death - 5 Other - 15 |
76 (6%) |
| Stopping medications | 7 | 0 | 1 | 0 | 0 | 30 | 1 | 2 | Low - 22 Moderate - 5 Severe harm/death - 0 Other - 14 |
41 (3%) |
| Other | 6 | 0 | 3 | 0 | 1 | 3 | 1 | 3 | Low – 6 Moderate – 1 Severe harm/ death – 0 Other - 10 |
17 (1%) |
Decision-making includes clinician decisions to prescribe and choice of medication.
• administration (383 incidents of wrong doses administered in 115 cases);
• monitoring and supply (405 incidents with wrong doses subsequently administered in 117 cases) and;
• prescribing (268 incidents with wrong doses subsequently administered in 5 cases).
Commonly more than one medication process was involved as illustrated in this example:
. . .syringe driver had already been commenced with Morphine sulphate and cyclizine. . .diluent used was normal saline which was indicated on prescription sheet. . .cyclizine not compatible with normal saline. Patient’s leg became red and syringe driver needed to be re-sited
Medication processes were broken down into harm severity. Monitoring and supply incidents commonly resulted in low harm in 216 incidents (53%), administration resulted in low harm in 197 incidents (51%) and prescribing incidents resulted in low harm in 125 (47%) of incidents (Table 4). In all three of the major medication processes (monitoring and supply, administration and prescribing), the harm level was nil or it was difficult to ascertain the level of harm. This category of other was 102 (25%) of incidents in monitoring and supply, 108 (28%) in administration and 104 (39%) in prescribing process incidents.
Identifying a single point of failure was often challenging due to insufficient detail in reports, although interacting contributory factors and multiple weak points increasing risk were clear. Nevertheless, the wrong dose administered (248 incidents, 19%); medication timeliness (233 incidents, 18%); prescribing error (114 incidents, 9%); and drug omission (107 incidents, 8%) were identified most frequently as the primary incident.
Two fifths of reports (540 incidents, 41%) contained one or more contributory factor (i.e. at least one perceived reason why the incident occurred ) and a similar proportion (537 incidents, 41%) had contributory incidents (i.e. events leading up to the incident that resulted in the harmful outcome). Most commonly, an inadequate skill set or knowledge were cited by reporters, including limited prior experience of continuous subcutaneous infusions (84 incidents), and inadequate education or up-to-date training (69 incidents). Other contributory factors included lack of equipment (56 incidents) or continuity of care (56 incidents). Often when a drug omission (not administered to the patient in error) was reported as the primary incident, no contributory factors were mentioned (67/107 incidents, 63%).
Qualitative narrative analysis: Themes
Four major themes were identified in free-text narratives: continuity of care; communication and collaboration; supply and demand; and experience and competency. Intersections between themes were common. For example, incidents were often caused or exacerbated by poor communication; and a lack of awareness of receiving teams’ processes and procedures for using continuous subcutaneous infusions was notable in incidents involving patient transfers between teams and settings.
Continuity of care: Coordination and transitions
Incidents arising in transfers between settings featured in 127 (10%) reports, with inadequate exchange of patient information frequently leading to reported harm. About 84% (26/31) of discharge-related incidents reported harm, frequently coinciding with other incidents, such as delayed prescribing. Valid community medication authorisation and administration records, equipment and diluents (e.g. water for injection) were frequently missing:
. . .end-of-life anticipatory medications. . .would be coming home with patient. . .found to be in a great deal of pain. . .no anticipatory medications in place. . .did have analgesic patch in situ but this had not been handed over by hospital staff and was not effective
Several incidents reported no medication or incorrect medications being supplied by hospitals on discharge:
Patient discharged to care home with anticipatory medication and drug chart, but drug chart not signed by doctor. . . Hyoscine prescribed but the patient had been discharged with Glycopyrronium. . .delay in district nurses being able to set up the syringe driver
Hospital admissions and discharges also often lacked coordination with community-based services:
. . .neither DNs [district nurses] or SPCT [specialist palliative care team] had received direct verbal or written referral. . .found distressed patient and family. . .patient symptomatic. . .unable to swallow. . .no anticipatory medication sent home. . .
In one case an error in transfer-related communication included a 10-fold increase in medication:
. . .Syringe and prescription both stated 187.5mg of levomepromazine, this has been administered for previous 4 days. . .site in the patient left leg appeared very red. . .checked through patient notes. . .handwritten GP referral to AMU [acute medical unit] stated 187.5mg levomepromazine. . .this dose seemed too high. . .Community Nursing Team who had been doing the syringe driver in the community. . .confirmed that the dose they were administering had been 18.75mg. . .
Mismatches in perceptions of respective responsibilities resulted in patients not receiving planned and timely care. Cultural working practices varied, and consequently insufficient communication was reported:
. . .advised ward doctors to start syringe driver with midazolam and oxycodone spoke to the doctor left written instructions in the medical notes and asked nursing staff if there was a driver available. . .as they would be able to obtain one from our office until 5pm. . .colleague reviewed the patient the next day and found that the driver had not been started. . .This resulted in the patient being agitated and in pain. . .
Variations occurred both within (as shown above) and between healthcare teams/settings:
. . .Staff commenced syringe driver at 16:20 with Oxycodone 20mg at 0.67 mls/hour to run over 24 hours. . .he already had a syringe driver in situ from previous facility which staff had not been made aware of.
Communication and collaboration
Collaboration between healthcare professionals, family carers and other staff members was reported as preventing or mitigating adverse patient outcomes. Collective decisions to commence infusions were reported as important for preventing harm and distress. In some cases, a breakdown in the multiple steps of initiating a syringe driver subsequently caused further incidents. For example, the absence of a continuous subcutaneous infusion contributed to a family carer using medications with unintended consequences:
. . .Syringe driver medication not written on correct prescription sheet. . .daughter instructed verbally and written on discharge letter to give 1/4 a tablet 6.25mg every 2 hours prn [as needed] nausea and vomiting. . .proceeded to give a total of 25mg of levomepromazine in 4 x 6.25mg doses as prescribed. . .Patient heavily sedated by drug and barely rousable
Supply and demand
Inadequate staff numbers or skill mix, and lack of medication or equipment, led to delays in starting and replenishing and/or incorrect setting up of infusions and subsequently led to inadequate symptom control in hospital and in the community:
. . .imminently dying admitted from A&E. . .required continuous subcutaneous infusion to improve comfort. . .included midazolam, glycopyrronium. . .neither drugs kept as stock. . . delays in obtaining supply which delayed administration and control of symptoms. . .additionally CDU [clinical decision unit] does not stock basic equipment needed for syringe driver medication. . .many of the staff unfamiliar and not skilled/experienced in the use of subcutaneous infusions. . .
The decision was made to commence a syringe driver. Patient also taking oral dexamethasone so needing to convert this to a subcutaneous route. . .Those pharmacies that I called were also unable to order in as item saying out of stock.
Many reports identified that the reporter knew equipment was needed, but they were unsure of procedures to access these or experienced barriers to following established protocols. Equipment malfunctions (operator or technical failures) were recurring. Ongoing shortages of syringe drivers in hospital settings were frequently highlighted:
No syringe drivers available in the Trust. . . she required numerous separate subcutaneous injections, into cachexic tissue, which was very painful. . .died 24 hours after.
Several incidents could not be mitigated until equipment became available from another ward or until another patient had died.
There were cases when supply was sufficient, but equipment was not utilised correctly, set at incorrect rate or ran at a different rate than expected:
When changing patient’s syringe driver I noticed. . .end of the giving set still had the sheaf on it. . .no medication was being administered. . .patient had been complaining of a lot of pain.
. . .I checked the syringe driver at start of my shift and initially had no concerns. . .as the night went on, I noticed the infusion was running quicker than it should be and consequently realised that the patient had been overdosed. . .the syringe driver had ran for 17.5 hours instead of 24 hours.
Medication and equipment supply issues were more pronounced out of hours, when fewer staff were working with limited access to specialist advice in both hospital and community settings:
Contacted 111 [UK urgent care phone number] just before 9am on Saturday still no response or returned call by 3.30pm, patient had no meds for syringe driver at home so eventually FP10 [prescription document] completed from inpatient unit so that community nurses could refill the driver. . .On Sunday. . .patient in pain dropped oramorph bottle and it smashed. . .he had no extra doses at home unable to get hold of doctor.
Experience and competency: Systematic gaps in infrastructure
Poor decision making (76/1317 incidents, 6%) around dose ranges and titrating medication occurred, as did incidents because prescribers were unwilling or uncertain in prescribing infusions for patients with whom they were unfamiliar:
Locum GP asked to prescribe medication for syringe driver. . .As locum did not know patient this request was declined and advised the family to call out of hours GP if syringe driver was required. . .
Reporters commonly attributed these incidents to lack of experience/incompetency without considering contextual/infrastructure factors, suggesting there was a problematic tendency to view incidents as individual-level human errors rather than seek to identify and address structural deficiencies. For example, lone working, practical barriers to following protocols, reliance on agency staff and lack of timely access to guidelines and specialist advice may all have contributed to incidents but were rarely considered:
. . .On reviewing the prescription charts it appears the drop in conscious level is more likely to be related to the addition of midazolam. . .for which the antidote is flumazenil - this drug was not considered for use. . .200mcg dose of naloxone caused the patient to have a pain crisis. . .I asked [name] why flumazenil was not considered - he is unfamiliar with its use and therefore did not consider it.
Other examples attributed to ‘simple’ staff error included administration errors:
. . .new syringe driver prescribed containing 45mg morphine over 24 hours (ten times the dose he should have had). . .also 90mg cyclizine prescribed. . .which is incorrect dosing. . .
duplicate prescriptions:
patient has two prescriptions. . .patient had syringe driver changed containing Hyoscine Butylbromide 60mg. Correct prescription as per Palliative care advice should have contained Hyoscine Butylbromide 60mg, Midazolam 10mg, Morphine Sulphate 10mg to volume of 24mls over 24-hours. . .
and dose miscalculations:
instead of using 2 [x] 30mg ampules[sic], used 2 [x] 10mg ampules. . .patient received 18.3mg instead of 55mg via syringe driver over 24hr period.
as well as knowledge deficiencies such as failure to appropriately discontinue alternative opioids once an infusion commenced:
Twilight service commenced a syringe driver to deliver pain relief overnight and dosage to be reviewed next day. . .nurse informed patient’s wife to still give oral medication including the slow release Oxycodone. . .wife also gave drug next morning and patient became semiconscious
Similar sounding medication names, were commonly confused at the prescribing, dispensing and administration level:
. . .patient had been discharged from our hospital with Hyoscine hydrobromide although Hyoscine Butylbromide was prescribed.
Inadequate knowledge and understanding of prescribing appropriate medications and doses, and using infusion equipment, underpinned over half the common incident/contributory factor reporting patterns; the lack of identifiable protocols, equipment or prescribing guidance compounded these issues. Of the 44 incidents that reflected this theme, 30 (68%) involved nursing staff not being trained in using, or even if they had received training, not being confident to use syringe drivers.
Lack of peer support and blame were notable in many report narratives:
4am nurse contacted OOHs GP for advice on what dose to commence syringe driver on. . . . . .verbal advice by GP to start with Diamorphine 40mg and Midazolam 40mg. . .night staff commenced. . .stat doses over past 24 hours indicated a total of midazolam 12.5mg and Diamorphine 7.5mg. . .
Additional learning derived from researcher-led qualitative narrative analysis
Commonly, psychological and social harms to patients and their families were identified or implied in free-text reports but these were difficult to quantify due to insufficient description. In addition to family involvement in some of the communication examples above, one incident described the involvement of a daughter in a subcutaneous medication error:
According to [patient] the OOH GP asked her [daughter] to pass him Midazolam 2.5mg which he administered s/c. . .then the daughter realised it was actually Haloperidol. . .daughter felt she was to blame.
While numerically rare, the identification of incidents where family carers’ actions and opinions diverged significantly from the healthcare professionals involved is concerning. Three instances were reported where a continuous subcutaneous infusion was found ‘switched off’ by family carers, and professionals were unable to appropriately use a continuous subcutaneous infusion for another patient because of family disagreement:
. . .syringe driver commenced previous day for symptom management, but district nurses found it discontinued. . .wife had stated she had stopped it through the night as she felt drugs were killing him. . .she did not want her husband to receive any further stat doses to relieve symptoms. . .
Despite this patient’s desire to remain at home, he was instead transferred to hospital as the professionals felt it would not be possible to negotiate symptom control at home.
Patients’ and families’ perspectives in these reports were reframed in what the healthcare professional reporter deemed appropriate. Professionals also appeared to default to the use of additional authority to impose their views when faced with challenges from family carers for example, safeguarding referrals, seeking input from the primary responsible clinician.
Discussion
Harm was present in nearly three-quarters of continuous subcutaneous infusion incident reports, often resulting in uncontrolled symptoms and significant distress for patients and family carers. We found reports solely indicating physical harm when it was beyond reasonable doubt that broader psychological and social harm occurred. This suggests that these harms, though prevalent, are frequently overlooked in incident reporting, highlighting a need for systematic recognition of the impact of care deficiencies. More focus also needs to be given to assuring continuity and quality of palliative care at interfaces between different care providers, particularly during hospital admission and discharge processes. 38
Inadequate continuity when patients were transferred between settings and providers frequently resulted in safety incidents.38,39 Effective communication between multiple clinical teams as well as between professionals, patients and families is vital for safe use of palliative medications to achieve symptom control.6,9,16,18,39 Mechanisms embedded within cross-organisational working patterns are needed to provide time-sensitive training for professionals irregularly involved with continuous subcutaneous infusions, accompanied by the support of professionals who regularly use these regardless of patient location. Other issues are more complex, requiring the weighing of different risks. For example, the use of a single system-wide continuous subcutaneous infusion model reduces risks associated with confusion of pumps with different functions, whereas using different models in healthcare systems mitigates supply chain issues and product recalls.
While existing literature acknowledges individual-level issues, this research emphasises that multiple points of system failure are contributing to incidents with continuous subcutaneous infusions. It underscores the importance of ensuring timely access to medications when needed while addressing infrastructure and communication deficits to improve patient safety effectively.23,40 A focus in reports on lack of experience and competency at an individual practitioner-level also demonstrates the need to consider how working environments and infrastructure deficits place undue reliance on human mitigation in care provision.
Tools like the Systems Engineering Initiative for Patient Safety (SEIPS) could aid healthcare professionals in considering broader contributory factors and implementing systemic changes.41,42 SEIPS is a well-established healthcare improvement and patient safety framework that integrates evaluation of the structural, organisational and human factors that typically interact in care outcomes 43 .
Strengths and limitations
This study’s strengths lie in identifying what is overlooked in incident reporting, such as psychological and social harms, and highlighting the need for systemic solutions. We also identified that the existing taxonomy 27 for grading harm is structured to be systematically biased to focus on physical functional outcomes, and on the patient in isolation. This is at odds with the goals of palliative care, and with what is known about the lasting psychosocial impact of, for example, disturbed dying with respect to complex bereavement for families.9,43,44 Undertaking a narrative analysis of the incidents was important for maximising system learning, and would be further enhanced with better reporting of psychological and social harms.
Limitations include the self-selecting nature of incident reporting. Incident databases are inherently dependent on reporting culture and training for reporters; not all incidents are reported, nor all contributing factors and outcomes detailed. 24 Despite these limitations, our analysis highlights what is perceived important by reporters and, through considering gaps in free-text reporting, key factors that may be overlooked. Our study design initially sought to identify isolated incidents in the multi-step processes of continuous subcutaneous infusion use. In practice, we identified that reporters commonly fail to provide information that exactly pinpoints system breakdowns in process steps, and, when they do, incidents can be seen to involve several inter-related steps in medication processes.
Recommendations for policy, practice and future research
Examples of recommendations from our results synthesis are provided in Table 5.
Table 5.
Recommendations.
| Incident type | Commonly applied human-based solutions: | Potential structural solutions: |
|---|---|---|
| Incidents involving prescribing errors and clarity of prescriptions | Handwriting ‘micrograms’ as opposed to the abbreviated form ‘µg’. | Electronic systems to minimise ambiguity in prescriptions, and to highlight when an ‘out of range’ dose has been prescribed.40,45 |
| Wrong drug administered | Double checking with other staff members. | Drug manufacturers to ensure packaging, labelling and naming of drugs are unique and distinguishable, as recommended. 46 |
| Variation in practice/ cultural working practices across different healthcare teams | Local staff training on syringe driver practice from professionals who frequently administer syringe drivers and palliative medications. GP surgeries can discuss and agree as a practice the key skills and staff needed for different palliative care roles as discussed in the Primary Care Daffodil standards 47 Reflect with staff within GP practices on different cases (within a practice meeting, for example), ensuring team learning. Ensure mix of agency/ non-agency staff on shift. This could include mentoring opportunities as discussed in the Daffodil Standards. 47 |
National best practice guidance needs to attend to improving consistency pre-, post- and during patient transfers between locations as well as when different types of providers are contributing to care. This should go beyond standards for specific roles/settings. Ensure accessibility to out of hours helplines. 47 National / local strategies to reduce variability of syringe driver models across different manufacturers. |
| Lack of stock of key end-of life medications | Encourage timely and individualised anticipatory prescribing of end-of-life medication when needed. Agreeing on a protocol for identifying people with an Advanced Serious Illness or EOLC needs as discussed in the Daffodil Standards. 47 This could include reviewing medications and prescribing appropriate ‘Just in Case’ medications. |
Incentivisation/funding for more pharmacies to take part in schemes like the NHS Pharmacy Quality scheme, Marie Curie Daffodil Standards adapted for pharmacies, and others.47
–49 Such may encourage more independent pharmacies to become designated holders of a stock list of palliative medications. This is not currently consistently commissioned or funded, both of which will need to be addressed to provide an equitable system. Additional recommendations as to how policymakers can address national shortages have been described by Vogler and Fischer. 50 These recommendations included: registers to support shortages and facilitated regulatory procedures.47,51 |
Palliative care services are structured differently in different countries but in England and Wales these services are free to access, well-established and reporting systems are relatively integrated in comparison to most international provision. 52 Consequently, shortfalls in care identified in our study are likely to also apply elsewhere. Organisations must embrace the complexity of palliative care and discuss locally their experiences and challenges of generating meaningful learning from incident reporting. 53 Many of the reported incidents were not specific to the subcutaneous route or a palliative care patient population but could equally occur with other routes and in other populations. This highlights both the importance of a whole systems analysis of incidents and the wider potential relevance of our findings.
In future incident analyses, research methods are needed to allow analysis of multiple interacting processes to understand the realities of practice and target solutions that account for these complexities. Our team are currently conducting this research. Further research is also warranted to explore how best to address medication-related psychological and social harms, including through incident reporting (e.g. new frameworks, taxonomies and alternative reporting systems) as these are not limited to the patient alone in the context of palliative care. This is recognised as being problematic worldwide. NHS England have more recently advocated reporting of psychological harms, and some countries make reporting severe psychological trauma mandatory, but neither of these actions alone will address the harms identified within this study.53 –56.
Research into changes in UK community palliative care before the COVID-19 pandemic identified changes in palliative prescribing pathways and clinical contacts; these were accelerated during the pandemic.20,38,39,56,58 Increasing use of remote patient consultations in community settings alongside the accepted (but under-researched) norms of practice with respect to prescribing of continuous subcutaneous infusions in advance of clinical need38,39,56,57,59 require monitoring for new safety risks alongside exacerbation of the risks identified in this study given that combining these practices may introduce additional systemic vulnerabilities into complex and nuanced care-critical clinical processes. 60
Conclusion
Continuous subcutaneous infusion incidents often involve multiple breakdowns across steps in key clinical processes, compromising patient safety. Narrative descriptions of psychological and social harm alongside physical harm risk are not being adequately recognised through existing approaches to measure harm in palliative care, hindering learning in practice. Structural changes are needed to minimise harm and maximise safety in palliative care.
Supplemental Material
Supplemental material, sj-docx-1-pmj-10.1177_02692163241287639 for Multiple points of system failure underpin continuous subcutaneous infusion safety incidents in palliative care: A mixed methods analysis by Amy Brown, Sarah Yardley, Ben Bowers, Sally-Anne Francis, Lucy Bemand-Qureshi, Stuart Hellard, Antony Chuter and Andrew Carson-Stevens in Palliative Medicine
Acknowledgments
The views expressed are those of the authors and not necessarily those of the funders, sponsors, National Health Service, the NIHR or the Department of Health and Social Care.
Footnotes
Author contributions: All authors have made a substantial contribution to the research design and approved the final version of this paper. AB conducted the analysis supervised by ACS and SY. A sample of reports were double and triple coded by BB and SY. Clinical recommendations were composed by AB, SY, BB and ACS. AB drafted the article with all authors critically revising the intellectual content.
Data management and sharing: The data for this study is held by National Health Service Improvement who considers applications to use National Reporting and Learning System data on a case-by-case basis. Permission was granted to Cardiff University through a data sharing agreement for the duration of the study.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Central and North West London NHS Foundation Trust Starter Grant Scheme. ACS’s and SH’s contribution to the study was funded by PRIME Centre Wales. BB is supported by Wellcome Trust [225577/Z/22/Z]. Cardiff University is the sponsor of this study.
Research ethics and patient consent: Ethical approval was granted by Cardiff University School of Medicine Research Ethics Committee (Ref 19/28). A data sharing agreement exists between Cardiff University and National Health Service Improvement. Data released to the research team was anonymised based on in-house data cleaning processes led by the National Health Service Improvement team. Should identifiable content have been recognised in a report, the research team would adhere to strict Information Governance procedures which includes notifying National Health Service Improvement.
ORCID iDs: Sarah Yardley  https://orcid.org/0000-0002-1645-642X
https://orcid.org/0000-0002-1645-642X
Ben Bowers  https://orcid.org/0000-0001-6772-2620
https://orcid.org/0000-0001-6772-2620
Sally-Anne Francis  https://orcid.org/0000-0003-2384-1518
https://orcid.org/0000-0003-2384-1518
Supplemental material: Supplemental material for this article is available online.
References
- 1. Landrigan C. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med 2010; 363(26): 2573–2134. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Universal health coverage. https://www.who.int/health-topics/universal-health-coverage#tab=tab_1 (2017, accessed 31 March 2022).
- 3. Yardley I, Yardley S, Williams H, et al. Patient safety in palliative care: a mixed-methods study of reports to a national database of serious incidents. Palliat Med 2018; 32(8): 1353–1362. [DOI] [PubMed] [Google Scholar]
- 4. Yardley S, Francis SA, Dean Franklin B, et al. Getting palliative medications right across the contexts of homes, hospitals and hospices: protocol to synthesise scoping review and ethnographic methods in an activity theory analysis. BMJ Open 2022; 12(3): e061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowers B, Pollock K, Barclay S. Unwelcome memento mori or best clinical practice? Community end-of-life anticipatory medication prescribing practice: a mixed methods observational study. Palliat Med 2022; 36(1): 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pollock K, Wilson E, Caswell G, et al. Family and health-care professionals managing medicines for patients with serious and terminal illness at home: a qualitative study. NIHR J Lib 2021. https://www.ncbi.nlm.nih.gov/books/NBK573001/ DOI: 10.3310/hsdr09140 [PubMed] [Google Scholar]
- 7. Lindqvist O, Lundquist G, Dickman A, et al. Four essential drugs needed for quality care of the dying: a delphi-study based international expert consensus opinion. J Palliat Med 2013; 16(1): 38–43. [DOI] [PubMed] [Google Scholar]
- 8. Carson-Stevens A, Hibbert P, Williams H, et al. Characterising the nature of primary care patient safety incident reports in the England and Wales National Reporting and Learning System: a mixed-methods agenda-setting study for general practice. HSDR 2016. [PubMed] [Google Scholar]
- 9. National Institute for Health and Care Excellence. Care of dying adults in the last days of life. National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/ng31/resources/care-of-dying-adults-in-the-last-days-of-life-pdf-1837387324357 (2015, accessed 7 October 2024). [PubMed] [Google Scholar]
- 10. Woodward HI, Mytton OT, Lemer C, et al. What have we learned about interventions to reduce medical errors? Ann Rev Public Health 2010; 31(1): 479–497. [DOI] [PubMed] [Google Scholar]
- 11. Lewis PJ, Dornan T, Taylor D, et al. Prevalence, incidence and nature of prescribing errors in hospital inpatients: a systematic review. Drug Saf 2009; 32(5): 379–389. [DOI] [PubMed] [Google Scholar]
- 12. Ross S, Hamilton L, Ryan C, et al. Who makes prescribing decisions in hospital inpatients? An observational study. Postgrad Med J 2012; 88(1043): 507–510. [DOI] [PubMed] [Google Scholar]
- 13. Ross S, Ryan C, Duncan EM, et al. Perceived causes of prescribing errors by junior doctors in hospital inpatients: a study from the PROTECT programme. BMJ Qual Saf 2013; 22(2): 97–102. [DOI] [PubMed] [Google Scholar]
- 14. Tully MP, Ashcroft DM, Dornan T, et al. The causes of and factors associated with prescribing errors in hospital inpatients: a systematic review. Drug Saf 2009; 32(10): 819–836. [DOI] [PubMed] [Google Scholar]
- 15. Brennan N, Mattick K. A systematic review of educational interventions to change behaviour of prescribers in hospital settings, with a particular emphasis on new prescribers: Systematic review of behaviour change interventions. Br J Clin Pharmacol 2013; 75(2): 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dean B, Schachter M, Vincent C, et al. Causes of prescribing errors in hospital inpatients: a prospective study. Lancet 2002; 359(9315): 1373–1378. [DOI] [PubMed] [Google Scholar]
- 17. Yardley S, Francis S-A, Chuter A, et al. Mixed-methods study protocol: do national reporting and learning system medication incidents in palliative care reflect patient and carer concerns about medication management and safety? BMJ Open 2021; 11(9): e048696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cruickshank S, Adamson E, Logan J, et al. Using syringe drivers in palliative care within a rural, community setting: capturing the whole experience. Int J Palliat Nurs 2010; 6(3): 126–132. [DOI] [PubMed] [Google Scholar]
- 19. Bowers B, Ryan R, Hoare S, et al. Anticipatory syringe drivers: a step too far. BMJ Support Palliat Care 2019; 9: 149–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pollock K, Wilson E, Caswell G, et al. Family and health-care professionals managing medicines for patients with serious and terminal illness at home: a qualitative study. Health Serv Deliv Res 2021; 9(14): 194. [PubMed] [Google Scholar]
- 21. Thomas T, Barclay S. Continuous subcutaneous infusion in palliative care: a review of current practice. Int J Palliat Nurs 2015; 21(2): 60–64. [DOI] [PubMed] [Google Scholar]
- 22. Mitten T. Subcutaneous drug infusions: a review of problems and solutions. Int J Palliat Nurs 2001; 7(2): 75–85. [DOI] [PubMed] [Google Scholar]
- 23. Payne S, Turner M, Seamark D, et al. Managing end-of-life medications at home—accounts of bereaved family carers: a qualitative interview study. BMJ Support Palliat Care 2015; 5(2): 181–188. [DOI] [PubMed] [Google Scholar]
- 24. Carson-Stevens A, Hibbert P, Avery A, et al. A cross-sectional mixed methods study protocol to generate learning from patient safety incidents reported from general practice. BMJ Open 2015; 5(12): e009079-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Donaldson LJ, Panesar SS, Darzi A. Patient-safety-related hospital deaths in England: thematic analysis of incidents reported to a national database, 2010–2012. PLoS Med 2014; 11(6): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams H, Donaldson SL, Noble S, et al. Quality improvement priorities for safer out-of-hours palliative care: lessons from a mixed-methods analysis of a national incident-reporting database. Palliat Med 2019; 33(3): 346–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization. Conceptual framework for the International classification for patient safety. Version 1.1. https://apps.who.int/iris/handle/10665/70882 (2009, accessed 31 March 2022).
- 28. Dinnen T, Williams H, Yardley S, et al. Patient safety incidents in advance care planning for serious illness: a mixed-methods analysis. BMJ Support Palliat Care 2019; 12: e403–e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rees P, Edwards A, Panesar S, et al. Safety incidents in the primary care office setting. Pediatrics 2015; 135(6): 1027–1035. [DOI] [PubMed] [Google Scholar]
- 30. Rees P, Edwards A, Powell C, et al. Pediatric immunization-related safety incidents in primary care: a mixed methods analysis of a national database. Vaccine 2015; 33(32): 3873–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Braun V, Clarke V. Successful qualitative research: a practical guide for beginners. London: Sage, 2013. [Google Scholar]
- 32. Carson-Stevens A, Edwards A, Panesar S, et al. Reducing the burden of iatrogenic harm in children. Lancet 2015; 385(9978): 1593–1594. [DOI] [PubMed] [Google Scholar]
- 33. Rees P, Edwards A, Powell C, et al. Patient safety incidents involving sick children in primary care in England and Wales: a mixed methods analysis. PLoS Med 14(1): e100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Institute for Health and Care Excellence. National Institute for Health and Care Excellence, https://bnf.nice.org.uk/ (2024, accessed 7 October 2024).
- 35. Wilcock A, Howard P and Charlesworth S (eds.). Palliative Care Formulary. London: Pharmaceutical Press. https://www.pharmaceuticalpress.com/products/palliative-care-formulary/ (accessed 7 October 2024).
- 36. Urquhart A, Yardley S, Thomas E, et al. Learning from patient safety incidents involving acutely sick adults in hospital assessment units in England and Wales: a mixed methods analysis for quality improvement. J R Soc Med 2021; 114(12): 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mauthner NS, Doucet A. Reflexive accounts and accounts of reflexivity in qualitative data analysis. Sociology 2003; 37(3): 413–431. [Google Scholar]
- 38. Williams H, Edwards A, Hibbert P, et al. Harms from discharge to primary care: mixed methods analysis of incident reports. Br J Gen Pract 2015; 65(641): e829–e837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bowers B, Pollock K, Barclay S. Simultaneously reassuring and unsettling: a longitudinal qualitative study of community anticipatory medication prescribing for older patients. Age Ageing 2022; 51(12): afac293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. UK Gov. One chance to get it right. Leadership Alliance for the Care of Dying People, https://assets.publishing.service.gov.uk/media/5a7e301ced915d74e33f09ee/One_chance_to_get_it_right.pdf (2014, accessed 7 October 2024). [Google Scholar]
- 41. Heath Education England. Human factors. Health Education England, https://www.hee.nhs.uk/our-work/human-factors (2023, accessed 7 October 2024). [Google Scholar]
- 42. Yardley S, Williams H, Bowie P, et al. Which human factors design issues are influencing system performance in out- of-hours community palliative care? Integration of realist approaches with an established systems analysis framework to develop mid-range programme theory. BMJ Open 2022; 12: e048045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holden RJ, Carayon P. SEIPS 101 and seven simple SEIPS tools. BMJ Qual Saf 2021; 30(11): 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harrop E, Morgan F, Byrne A, et al. “It still haunts me whether we did the right thing”: a qualitative analysis of free-text survey data on the bereavement experiences and support needs of family caregivers. BMC Palliat Care 2016; 15(1): 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Au YK, Baker L, Hindmarsh J. The impact of an electronic prescribing template with decision support upon the prescribing of subcutaneous infusions at the end of life in a community setting: a future vision for community palliative care. Pharmacy 2022; 10(5): 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Emmerton LM, Rizk MFS. Look-alike and sound-alike medicines: risks and ‘solutions’. Int J Clin Pharm 2011; 34(1): 4–8. [DOI] [PubMed] [Google Scholar]
- 47. Royal College of General Practitioners. The Daffodil Standards: A breakdown by standard. Royal College of General Practitioners, https://www.rcgp.org.uk/learning-resources/daffodil-standards/breakdown#full (2023, accessed 7 October 2024). [Google Scholar]
- 48. Mason B, Carduff E, Laidlaw S, et al. Integrating lived experiences of out-of-hours health services for people with palliative and end-of-life care needs with national datasets for people dying in Scotland in 2016: a mixed methods, multi-stage design. Palliat Med 2022; 36(3): 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. National Health Service England. Pharmacy Quality Scheme. NHS England, https://www.england.nhs.uk/primary-care/pharmacy/pharmacy-quality-payments-scheme/ (2023/4, accessed 7 October 2024). [Google Scholar]
- 50. Vogler S, Fischer S. How to address medicines shortages: Findings from a cross-sectional study of 24 countries. Health Policy 2020; 124(12): 1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Royal Pharmaceutical Society & Marie Curie. Daffodil Standards for Community Pharmacy. RPS, https://www.rpharms.com/recognition/setting-professional-standards/daffodil-standards/the-standards (2024, accessed 7 October 2024). [Google Scholar]
- 52. Etkind SN, Bone AE, Gomes B, et al. How many people will need palliative care in 2040? Past trends, future projections and implications for services. BMC Med 2017; 15(1): 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yardley S, Williams H, Bowie P, et al. Which human factors design issues are influencing system performance in out-of-hours community palliative care? Integration of realist approaches with an established systems analysis framework to develop mid-range programme theory BMJ Open 2022; 12: e048045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cooper J, Williams H, Hibbert P, et al. Classification of patient-safety incidents in primary care. Bull World Health Organ 2018; 96(7): 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. NHS England. Policy guidance on recording patient safety events and levels of harm. NHS England. https://www.england.nhs.uk/long-read/policy-guidance-on-recording-patient-safety-events-and-levels-of-harm/ (2023, accessed 7 October 2024). [Google Scholar]
- 56. World Health Organization. WHO reporting and learning guide. World Health Organization, https://iris.who.int/bitstream/handle/10665/334323/9789240010338-eng.pdf?sequence=1 (2020, accessed 7 October 2024). [Google Scholar]
- 57. Hultman T, Reder EAK, Dahlin CM. Improving psychological and psychiatric aspects of palliative care: the national consensus project and the national quality forum preferred practices for palliative and hospice care. J Death Dying 2008; 57(4): 323–339. [DOI] [PubMed] [Google Scholar]
- 58. Caswell G, Wilson E, Turner N, et al. ‘It’s not like in the films’: bereaved people’s experiences of the deathbed vigil. Omega. Epub ahead of print 14 October 2022. DOI: 10.1177/00302228221133413. [DOI] [PubMed] [Google Scholar]
- 59. Coyle S, Sinden R, Wignall-Coyle J, et al. Continuous subcutaneous infusion: is community anticipatory prescribing and administration safe? BMJ Support Palliat Care 2024; 13: e1373–e1378. [DOI] [PubMed] [Google Scholar]
- 60. Bowers B. Understanding community end of life anticipatory medication care. University of Cambridge Repository, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-pmj-10.1177_02692163241287639 for Multiple points of system failure underpin continuous subcutaneous infusion safety incidents in palliative care: A mixed methods analysis by Amy Brown, Sarah Yardley, Ben Bowers, Sally-Anne Francis, Lucy Bemand-Qureshi, Stuart Hellard, Antony Chuter and Andrew Carson-Stevens in Palliative Medicine