Abstract
Background
Thyroid disorders are common endocrine conditions impacting multiple organs, including the reproductive system and often lead to sexual dysfunction. These effects can vary by gender; for example, women with hypothyroidism frequently experience reduced libido. Low thyroid hormone levels are also linked to vaginal dryness, causing discomfort, especially during intercourse. This study aims to assess the global prevalence of sexual dysfunction in women with thyroid disorders.
Methods
Systematic searches were performed across electronic databases, including PubMed, Scopus, Web of Science, Embase, ScienceDirect and Google Scholar, to retrieve studies reporting the prevalence of sexual dysfunction in patients with thyroid disorders up to February 8, 2024. Inclusion criteria comprised studies that reported on the prevalence of female sexual dysfunction (FSD) in patients with thyroid disorders and studies published in English available full text. Exclusion criteria included case studies, intervention studies, studies with incomplete information, repeated studies and those not written in English. Cross-sectional studies were the primary study design included. Data were analyzed using the Comprehensive Meta-Analysis software (Version 2).
Results
Analysis of nine studies, involving a total sample size of 1013, found an overall prevalence of sexual dysfunction in women with thyroid disorders to be 44.8% (95% CI: 33.8–56.2). Given the substantial reporting of sexual dysfunction among women with either hypothyroidism or hyperthyroidism, subgroup analyses were conducted. The prevalence of sexual dysfunction was 41.8% (95% CI: 26.3–59) among women with hypothyroidism and 59.6% (95% CI: 50.5–68.1) among those with hyperthyroidism.
Conclusion
The notable prevalence of sexual dysfunction in women with thyroid disorders highlights the for increased awareness among this population. Targeted awareness initiatives may help mitigate the occurrence of sexual dysfunction and its adverse effects, improving overall quality of life for affected women.
Clinical trial number
Not applicable.
Keywords: Female sexual dysfunction, FSD, Thyroid, Meta-analysis
Background
Thyroid disorders are among the most common endocrine gland conditions, often stemming from iodine deficiency or other contributing factors [1]. These disorders encompass a range of conditions, including goiters, thyroid cancer, Hashimoto’s thyroiditis, Graves’ disease, hypothyroidism and hyperthyroidism [2]. Broadly, thyroid disorders can be categorized into hypothyroidism and hyperthyroidism, characterized by decreased or increased thyroid hormone levels, respectively [1, 3]. The thyroid gland produces hormones that influence nearly every cell, organ, and bodily system [2–5]. Consequently, thyroid disorders can significantly affect sexual health, with both men and women experiencing reduced sexual desire or impaired sexual function due to common symptoms of hypothyroidism, such as fatigue and depression [2–5]. In women, thyroid disorders often have unique manifestations. Hypothyroidism is commonly associated with decreased libido and low levels of thyroid hormone are linked to vaginal dryness, which can cause discomfort, particularly during intercourse [2–5].
Thyroid disorders are prevalent worldwide with significant variability in prevalence rates across different populations [4, 5]. One study reported the prevalence of thyroid disorders to range from 2 to 6% globally [6]. However, rates differ by region, with figures reaching 6.6% in European and American adults and up to 24.3% in a state in Sudan [7–9].
According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), sexual dysfunction is diagnosed when an individual experiences persistent distress or difficulties in sexual activity for at least six months, independent of substance use [10]. While sexual dysfunction can affect both genders, it is more commonly observed in women [11]. FSD encompasses a range of issues, including low sexual desire, arousal difficulties, orgasmic disorders, and pain or reduced sexual satisfaction [12]. Multiple factors can contribute to FSD, including religion, lifestyle, psychological issues and other medical conditions [11, 13].
Several studies have highlighted the association between thyroid disorders and FSD [14–16]. For instance, research conducted in Romania reported a 33.2% prevalence of FSD among women with autoimmune thyroid disorders, while a Spanish study found a 31.6% prevalence among women with hypothyroidism [14, 15]. In the Netherlands, the prevalence of FSD as 81% in women with hyperthyroidism due to Graves’ disease and 47% in women with hyperthyroidism associated with goiter [16, 17].
Given the significant impact of FSD on personal relationships and quality of life, as well as the varying prevalence rates of FSD in women with thyroid disorders reported across different studies, this study aims to systematically review existing literature to determine the global prevalence of FSD in women with thyroid disorders.
This review will address the inconsistencies in reported data and provide a clearer understanding of the burden of FSD among women affected by thyroid disorders, offering valuable insights for healthcare professionals and policymakers aiming to mitigate the consequences of these conditions.
Methods
This systematic review and meta-analysis were conducted following the PRISMA guidelines. Comprehensive searches were performed across multiple databases, including Web of Science, Scopus, ScienceDirect, PubMed, Embase and Google Scholar, using a combination relevant keywords such as “sexual disorders,” “sexual dysfunction,” “female sexual dysfunction,” “FSD,” “dyspareunia,” “orgasm disorder,” “females,” “women,” “thyroid,” “hyperthyroidism,” “hypothyroidism,” and “thyroiditis,” combined with logical operators (AND, OR). A manual search of bibliographies from relevant articles was also conducted to identify studies published up to February 8, 2024.
Search strategy in PubMed
(((((((((Prevalence[Title/Abstract]) AND (Sexual disorders[Title/Abstract])) OR (Sexual dysfunction[Title/Abstract])) OR (dyspareunia [Title/Abstract])) OR (orgasm disorder [Title/Abstract]))OR (Female sexual dysfunction[Title/Abstract])) OR (FSD [Title/Abstract])) OR (women sexual dysfunction[Title/Abstract])) AND (Thyroid disorders[Title/Abstract])) OR (Thyroid diseases[Title/Abstract])) OR (Hyperthyroidism[Title/Abstract] OR (hypothyroidism [Title/Abstract])))))))))
Inclusion criteria
Studies reporting the prevalence of FSD in patients with thyroid disorders.
Studies with full-text availability.
Studies published in English.
Cross-sectional studies.
Exclusion criteria
Case studies.
Interventional studies.
Studies with incomplete information.
Duplicate studies.
Studies not written in English.
Study selection
Articles were transferred to EndNote software for management. Two researchers independently screened the studies, removed duplicate articles and reviewed the titles and abstracts of the remaining articles. Irrelevant articles were excluded based on the exclusion criteria, while studies meeting the inclusion criteria proceeded to full-text review. Two researchers independently reviewed the full texts and discrepancies were resolved by a third researcher who provided guidance when consensus was not reached.
Qualitative evaluation of the studies
The STROB tool was employed to assess the quality of observational studies. This tool evaluates studies based on six domains: title, statement of the problem, study objectives, study type, study population, sampling method, sample size, and study variables. The checklist comprises 22 questions, some with subcategories, leading to a scoring range of 0 to 32. Articles scoring below 16 were classified as low quality, whereas those scoring 16 or higher were considered moderate to high quality [18].
Data extraction
A standardized checklist was designed to extract essential information, including authors’ names, year of publication, study type, study location, sample size, prevalence of FSD in women with thyroid disorders, participant age range and the assessment tool used for evaluating FSD.
Statistical analysis
Data analysis was performed using Comprehensive Meta-Analysis software (Version 2). Study heterogeneity was evaluated using the I2 test and publication bias was assessed with the Egger test at a significance level of 0.05. Additionally, a funnel plot was used to visually assess publication bias.
Results
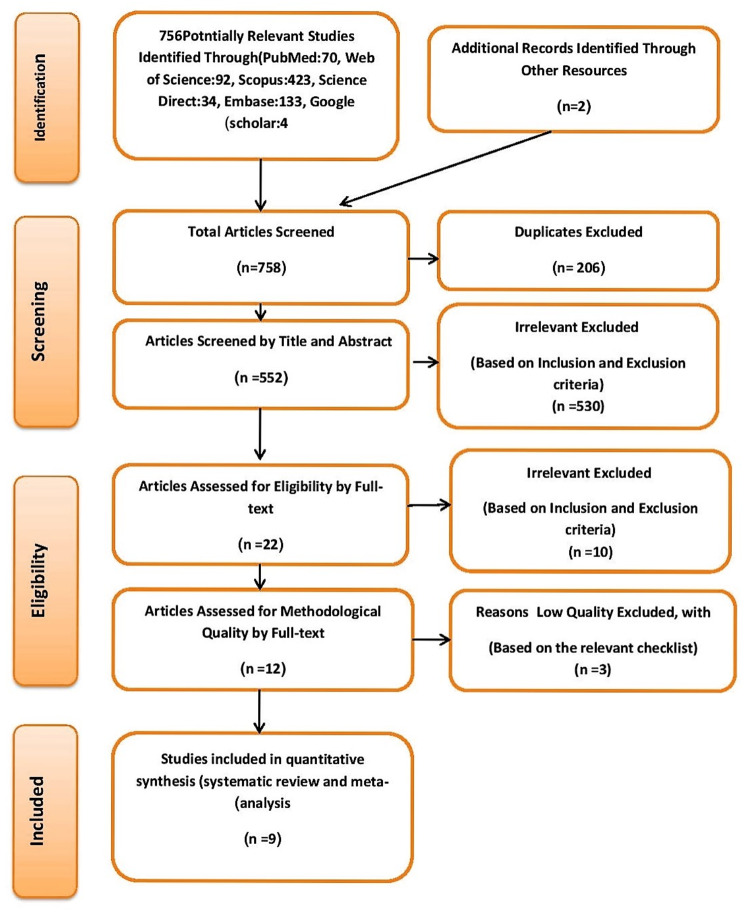
Based on the systematic search, 756 studies were initially identified as relevant to the research objective of determining the global prevalence of sexual dysfunction in women with thyroid disorders. An additional 2 studies were manually included. After removing duplicates, 552 studies remained. Following the screening of abstracts and titles and the application of the defined inclusion and exclusion criteria, 530 studies were excluded resulting in 22 studies for further examination. A detailed review of the full texts of these 22 articles led to the exclusion of 10 due to insufficient information, such as the absence of sample size details and prevalence reporting. Additionally, three studies were excluded during the quality assessment phase for scoring below 16. Ultimately, nine studies met the criteria for analysis (Fig. 1; Table 1).
Fig. 1.
The flowchart on the stages of including the studies in the systematic review and meta-analysis (PRISMA 2009)
Table 1.
Summary of characteristics of included studies of prevalence of FSD in patient with thyroid disorders
| Author | Year | Qualitative Evaluation | country | Sample Size | Prevalence of FSD in Patient (%) | Age | Instrument | Type of Disorder |
|---|---|---|---|---|---|---|---|---|
| Atis et al [22] | 2010 | Moderate | Turkey | 50 | 44% | 38.09 ± 6.20 | FSFI | Hypothyroidism |
| Atis et al [23] | 2011 | Moderate | Turkey | 40 | 60% | 37.3 ± 11.8 | FSFI | Hyperthyroidism |
| Pasquali et al [21] | 2013 | Moderate | Italy | 104 | 43.27% | 41.7 ± 10.3 | FSFI1 | hyperthyroidism, hypothyroidism, Hashimoto’s thyroiditis, nodular goiter |
| Hong et al [19] | 2015 | Moderate | Korea | 138 | 67.40% | 47 to 56 | FSFI | Hypothyroidism |
| Krysiak et al [24] | 2016 | Moderate | Poland | 50 | 46% | 30.33 ± 4.66 | FSFI | Hashimoto’s thyroiditis, nonautoimmune subclinical hypothyroidism, autoimmune subclinical hypothyroidism |
| Luo et al [20] | 2018 | High | China | 168 | 21.40% | 39.2 ± 7.6 | CVFSFI2 | hypothyroidism |
| Krysiak et al [16] | 2019 | High | Poland | 61 | 64% | 30.5 ± 6 | FSFI | overt hyperthyroidism induced by Graves’ disease, overt hyperthyroidism caused by toxic multinodular goiter or toxic adenoma |
| Romero-Gómez et al [14] | 2020 | High | Spain | 152 | 31.60% | 36.58 ± 9.96 | WSF3 | Hypothyroidism |
| Bortun et al [15] | 2021 | High | Romania | 250 | 33.20% | 20 to 45 | FSFI-6 | Thyroid Autoimmune Disease |
1 The Female Sexual Function Index
2 Chinese Version of the FSFI
3 Women’s Sexual Function
In total, these nine studies encompassed a sample size of 1013 individuals. Among them, the study by Luo et al. and the study by Hong et al. reported the lowest and highest prevalence of sexual dysfunction among women with thyroid disorders, respectively [19, 20]. Luo et al.’s study, conducted in China in 2018, reported a prevalence of 21.4% for sexual dysfunction among women with hypothyroidism [20]. In contrast, the cross-sectional study by Hong et al., conducted in Korea in 2015, found that 67.4% of Korean women with hypothyroidism were diagnosed with sexual dysfunction using the Female Sexual Function Index (FSFI) [19]. The most commonly used tools for assessing sexual dysfunction in women with thyroid disorders were the Female Sexual Function Index (FSFI) [16, 19, 21–24], Women’s Sexual Function (WSF) [14], FSFI-6 [15] and the Chinese version of the FSFI [20] (Table 1).
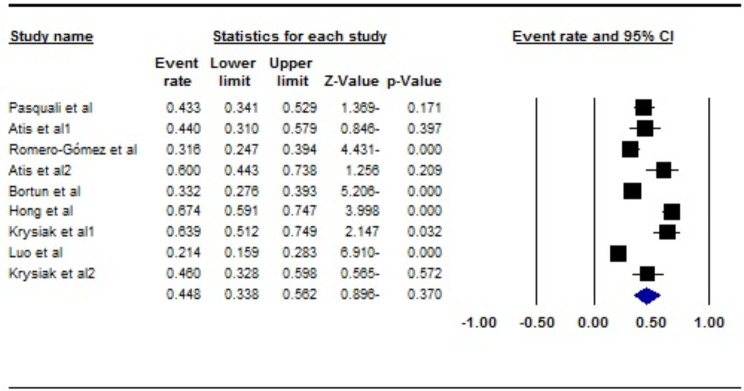
In the analysis of the nine studies, encompassing a total sample size of 1013 individuals, the heterogeneity test I2 indicated substantial heterogeneity (I2 = 91.2%). Therefore, a random-effects model was applied for the meta-analysis. The global prevalence of Female Sexual Dysfunction (FSD) in women with thyroid disorders was estimated to be 44.8% (95% CI: 33.8–56.2) (Fig. 2). Additionally, the assessment of publication bias using the Egger test showed no significant evidence of publication bias (p = 0.235) (Fig. 3).
Fig. 2.
Forest plot of the global prevalence of FSD in patients with thyroid disorders based on the random-effects model
Fig. 3.
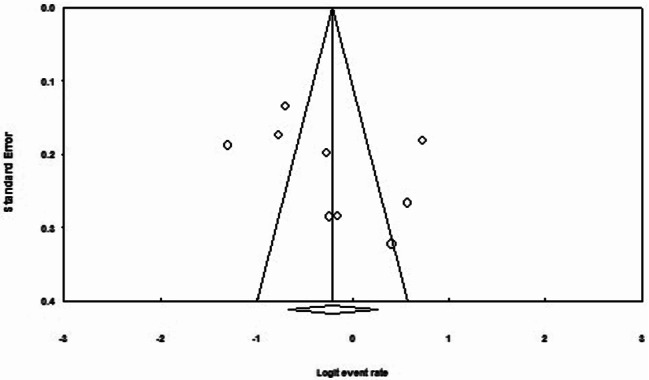
Funnel plot assessing publication bias in the reviewed studies
An analysis of the factors contributing to heterogeneity among the studies, including the effect of sample size, indicated that as a sample size increased, the reported prevalence of FSD in patients with thyroid disorders decreased (p < 0.05) (Fig. 4). Additionally, the prevalence of FSD in patients with thyroid disorders was found to decrease with more recent study years (p < 0.05) (Fig. 5).
Fig. 4.
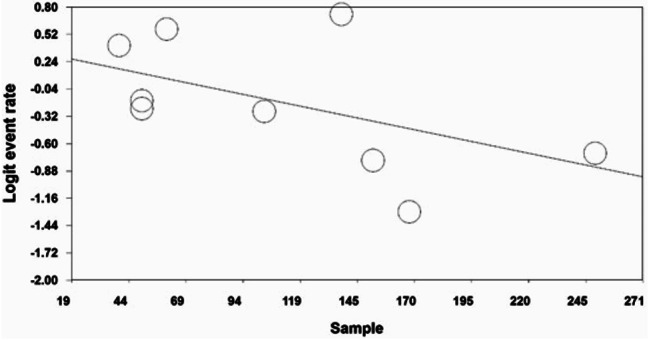
Meta-regression of the effect of sample size on the prevalence of FSD in patients with thyroid disorders
Fig. 5.
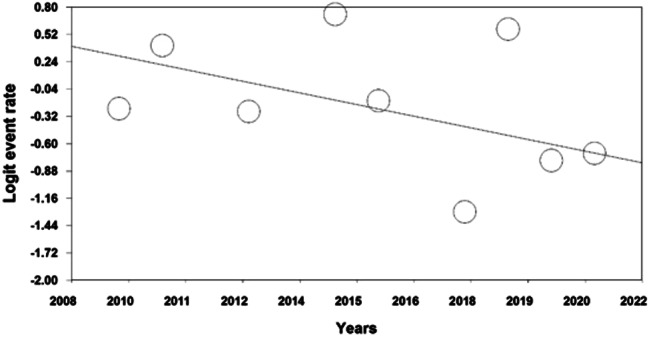
Meta-regression of the effect of study year on the prevalence of FSD in patients with thyroid disorders
Subgroup analysis based on thyroid disorder types
According to the subgroup analysis based on the type of thyroid disorder, the global prevalence of FSD in patients with hyperthyroidism was higher, reported at 59.6 (95% CI: 50.5–68.1) (Table 2).
Table 2.
Subgroup analysis based on types of thyroid disorders
| Thyroid disorders | N | Sample size | I2 | Egger test | Prevalence (95% CI) |
|---|---|---|---|---|---|
| Hyperthyroidism | 3 | 119 | 6.6 (Fixed effects method) | 0.052 | 59.6 (95%CI: 50.5–68.1) |
| Hypothyroidism | 6 | 564 | 92.5 | 0.842 | 41.8 (95%CI: 26.3–59) |
Discussion
Thyroid disorders are among the most prevalent diseases globally, with a higher incidence in women compared to men [4]. Early detection is common, often due to the availability of diagnostic tools [2]. Although not all thyroid disorders necessitate treatment, studies have demonstrated their potential impact on sexual function. This effect is likely due to thyroid hormones influencing sex hormone levels, thereby affecting sexual activity in individuals with thyroid disorders [2, 10]. Several studies have reported on the prevalence of sexual dysfunction among women with thyroid disorders [14, 16]. The objective of this study was to determine the global prevalence of Female Sexual Dysfunction (FSD) in patients with thyroid disorders.
The analysis conducted in this study revealed an overall prevalence of 44.8% for sexual dysfunction among women with thyroid disorders. While various thyroid disorders exist [2], evidence shows that hypothyroidism and hyperthyroidism are more common in iodine-rich regions [4]. Based on the findings from multiple studies [14, 16, 20], the prevalence of sexual dysfunction in women with hypothyroidism and hyperthyroidism was calculated separately, showing rates of 41.8% and 59.6%, respectively.
In a study conducted in Italy, the overall prevalence of sexual dysfunction in women with thyroid disorders was 46.1%, with a 41% prevalence among women with hypothyroidism [21]. Similarly, research from the Netherlands reported a 41% prevalence of sexual dysfunction among Dutch women with hypothyroidism [24]. A cross-sectional study conducted from Turkey found that 60% of women with hyperthyroidism met the diagnostic criteria for sexual dysfunction [23]. Another study indicated that 47% of Dutch women with hyperthyroidism due to toxic adenoma exhibited signs of sexual dysfunction [16]. These findings align with the prevalence reported in the present study.
Some studies have reported significantly higher or lower prevalence rates than those calculated in this study [16, 19, 20]. For example, a study among Korean women with hypothyroidism found that 67.4% experienced sexual dysfunction [19]. Conversely, only 21.4% of 168 Chinese women with hypothyroidism were reported to have sexual dysfunction [20]. Additionally, research from the Netherlands indicated that 81% of women with hyperthyroidism due to Graves’ disease experienced sexual dysfunction [21].
Sexual dysfunction is a multifaceted disorder influenced by various factors [25]. Biological factors, socioeconomic status, education, psychological disorders and chronic physical conditions can all impact sexual function [25, 26]. Cultural and social norms in certain regions may lead to underreporting of sexual dysfunction [27]. Moreover, insufficient information on sexual health and the presence of chronic illnesses in partners can negatively affect sexual function [27]. These contributing factors, along with thyroid disorders, may explain the variability in reported prevalence rates. The results of this study can guide healthcare providers in enhancing the quality of life for women with thyroid disorders. This study offers comprehensive information to inform policy decisions related to diagnostic and treatment strategies available to gynecologists and endocrinologists. Future research should focus on intervention measures, as observational studies have shown that women with thyroid diseases have a higher prevalence of FSD compared to control groups [21].
The primary limitations of this study include the limited number of articles available and the small sample size in some studies. Additionally, the variability in contributing factors across the examined studies may have impacted the measurement of FSD prevalence.
Conclusion
Given the relatively high prevalence of Female Sexual Dysfunction (FSD) in patients with thyroid disorders, particularly hyperthyroidism and the negative impact of sexual disorders on marital life and relationship continuity, it is imperative for policymakers to address the needs of this patient group.
Acknowledgements
By Student Research Committee of Kermanshah University of Medical Sciences.
Author contributions
NS and PH and MM contributed to the design, MM statistical analysis, and participated in most of the study steps. MM and PH and FB prepared the manuscript. MM and FM and FJ and SS and MN assisted in designing the study, and helped in the, interpretation of the study. All authors have read and approved the content of the manuscript.
Funding
By Deputy for Research and Technology, Kermanshah University of Medical Sciences (IR) (4030387). This deputy has no role in the study process.
Data availability
Datasets are available through the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Ethics approval was received from the ethics committee of deputy of research and technology, Kermanshah University of Medical Sciences (IR.KUMS.REC.1403.072).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sonuç E, editor. Thyroid disease classification using machine learning algorithms. Journal of Physics: Conference Series; 2021: IOP Publishing.
- 2.Uslar V, Becker C, Weyhe D, Tabriz N. Thyroid disease-specific quality of life questionnaires - a systematic review. Endocrinol Diabetes Metabolism. 2022;5(5):e357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coskuner ER, Ozkan B. Premature ejaculation and endocrine disorders: a Literature Review. World J men’s Health. 2022;40(1):38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khaleghzadeh-Ahangar H, Talebi A, Mohseni-Moghaddam P. Thyroid disorders and Development of Cognitive Impairment: a review study. Neuroendocrinology. 2022;112(9):835–44. [DOI] [PubMed] [Google Scholar]
- 5.Muñoz-Ortiz J, Sierra-Cote MC, Zapata-Bravo E, Valenzuela-Vallejo L, Marin-Noriega MA, Uribe-Reina P, et al. Prevalence of hyperthyroidism, hypothyroidism, and euthyroidism in thyroid eye disease: a systematic review of the literature. Syst Reviews. 2020;9(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wouters HJ, Slagter SN, Muller Kobold AC, van der Klauw MM, Wolffenbuttel BH. Epidemiology of thyroid disorders in the lifelines Cohort Study (the Netherlands). PLoS ONE. 2020;15(11):e0242795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biondi B, Kahaly GJ, Robertson RP. Thyroid dysfunction and diabetes Mellitus: two closely Associated disorders. Endocr Rev. 2019;40(3):789–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medani KE. Prevalence of thyroid disorders and reference range of thyroid hormones in Khartoum State, Sudan. J Res Med Dent Sci. 2020;8(1):158–61. [Google Scholar]
- 9.Taheriniya S, Arab A, Hadi A, Fadel A, Askari G. Vitamin D and thyroid disorders: a systematic review and Meta-analysis of observational studies. BMC Endocr Disorders. 2021;21(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bortun A-MC, Ivan V, Navolan D-B, Dehelean L, Borlea A, Stoian D. Thyroid autoimmune disease—impact on sexual function in young women. J Clin Med. 2021;10(2):369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chew PY, Choy CL, Sidi HB, Abdullah N, Che Roos NA, Salleh Sahimi HM, et al. The association between female sexual dysfunction and sexual dysfunction in the male partner: a systematic review and meta-analysis. J Sex Med. 2021;18(1):99–112. [DOI] [PubMed] [Google Scholar]
- 12.Linares-Gonzalez L, Lozano-Lozano I, Gutierrez-Rojas L, Lozano-Lozano M, Rodenas-Herranz T, Ruiz-Villaverde R. Sexual dysfunction and atopic dermatitis: a systematic review. Life. 2021;11(12):1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madbouly K, Al-Anazi M, Al-Anazi H, Aljarbou A, Almannie R, Habous M, et al. Prevalence and predictive factors of female sexual dysfunction in a sample of Saudi Women. Sex Med. 2020;9(1):100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero-Gómez B, Guerrero-Alonso P, Carmona-Torres JM, Laredo-Aguilera JA, Pozuelo-Carrascosa DP, Cobo-Cuenca AI. Sexual function in levothyroxine-treated hypothyroid women and women without hypothyroidism: a case-control. Int J Environ Res Public Health. 2020;17(12). [DOI] [PMC free article] [PubMed]
- 15.Bortun AC, Ivan V, Navolan DB, Dehelean L, Borlea A, Stoian D. Thyroid autoimmune disease-impact on sexual function in Young Women. J Clin Med. 2021;10(2). [DOI] [PMC free article] [PubMed]
- 16.Krysiak R, Kowalcze K, Okopień B. Sexual function and depressive symptoms in young women with overt hyperthyroidism. Eur J Obstet Gynecol Reproductive Biology. 2019;234:43–8. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz BA, Sonmez Y, Sezik M. Prevalence and risk factors for sexual dysfunction in reproductive-aged married women: a cross‐sectional epidemiological study. J Obstet Gynecol Res. 2020;46(3):507–16. [DOI] [PubMed] [Google Scholar]
- 18.Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong H, Lee HJ, Kim SM, Jeon MJ, Shin DW, Choi HC, et al. Subclinical hypothyroidism is not a risk factor for female sexual dysfunction in Korean middle-aged women. Thyroid: Official J Am Thyroid Association. 2015;25(7):784–8. [DOI] [PubMed] [Google Scholar]
- 20.Luo H, Zhao W, Yang H, Han Q, Zeng L, Tang H, et al. Subclinical hypothyroidism would not lead to female sexual dysfunction in Chinese women. BMC Womens Health. 2018;18(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasquali D, Maiorino MI, Renzullo A, Bellastella G, Accardo G, Esposito D, et al. Female sexual dysfunction in women with thyroid disorders. J Endocrinol Investig. 2013;36(9):729–33. [DOI] [PubMed] [Google Scholar]
- 22.Atis G, Dalkilinc A, Altuntas Y, Atis A, Caskurlu T, Ergenekon E. Sexual dysfunction in women with clinical hypothyroidism and subclinical hypothyroidism. J Sex Med. 2010;7(7):2583–90. [DOI] [PubMed] [Google Scholar]
- 23.Atis G, Dalkilinc A, Altuntas Y, Atis A, Gurbuz C, Ofluoglu Y, et al. Hyperthyroidism: a risk factor for female sexual dysfunction. J Sex Med. 2011;8(8):2327–33. [DOI] [PubMed] [Google Scholar]
- 24.Krysiak R, Drosdzol-Cop A, Skrzypulec-Plinta V, Okopien B. Sexual function and depressive symptoms in young women with thyroid autoimmunity and subclinical hypothyroidism. Clin Endocrinol. 2016;84(6):925–31. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Li S, Lu P, Chen H, Zhang Y, Cao Y, et al. Sexual dysfunction and health condition in Chinese doctor: prevalence and risk factors. Sci Rep. 2020;10(1):15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres-Ferrus M, López-Veloso AC, Gonzalez-Quintanilla V, González-García N, de Díaz J, Gago-Veiga A et al. The MIGREX study: prevalence and risk factors of sexual dysfunction among migraine patients. Neurologia (Engl Ed) . 2021:S0213-4853(21)00036-0. [DOI] [PubMed]
- 27.Yilmaz BA, Sonmez Y, Sezik M. Prevalence and risk factors for sexual dysfunction in reproductive-aged married women: a cross-sectional epidemiological study. J Obstet Gynaecol Res. 2020;46(3):507–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets are available through the corresponding author upon reasonable request.