Abstract
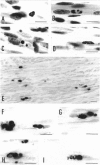
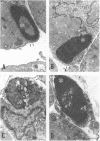
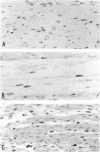
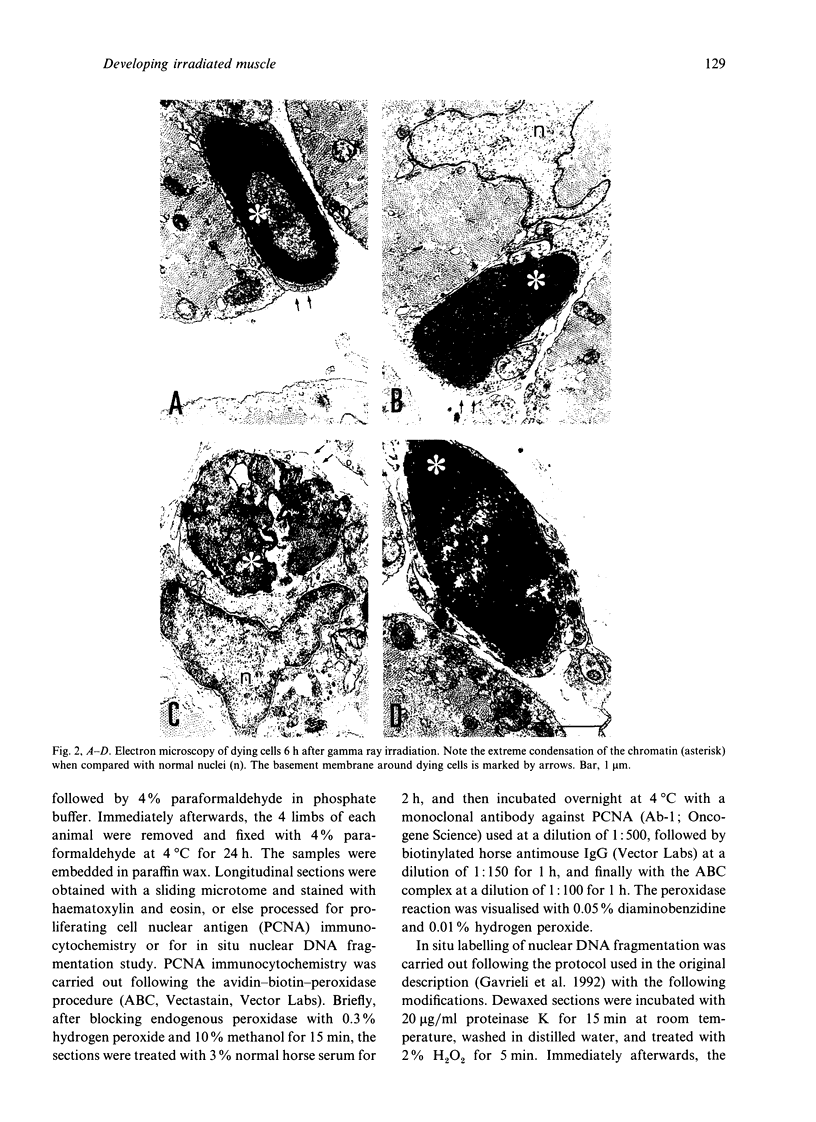
Newborn Sprague-Dawley rats were exposed to a single dose of 2 Gy gamma rays and killed from 6 h to 5 d later. Increased numbers of dying cells, characterised by their extreme chromatin condensation and often nuclear fragmentation were seen in skeletal muscle 6 h after irradiation. Dying cells decreased to nearly normal values 48 h later. In situ labelling of nuclear DNA fragmentation identified individual cells bearing fragmented DNA. The effects of gamma rays were suppressed following cycloheximide i.p. at a dose of 1 microgram/g body weight given at the time of irradiation. Taken together, the present morphological and pharmacological results suggest that gamma ray induced cell death in skeletal muscle is apoptotic, and that the process is associated with protein synthesis. Finally, proliferating cell nuclear antigen-immunoreactive cells, which were abundant in control rats, decreased in number 48 h after irradiation. However, a marked increase significantly above normal age values was observed at the 5th day, thus suggesting that regeneration occurs following irradiation-induced cell death in developing muscle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Celis J. E., Madsen P., Celis A., Nielsen H. V., Gesser B. Cyclin (PCNA, auxiliary protein of DNA polymerase delta) is a central component of the pathway(s) leading to DNA replication and cell division. FEBS Lett. 1987 Aug 10;220(1):1–7. doi: 10.1016/0014-5793(87)80865-7. [DOI] [PubMed] [Google Scholar]
- Clarke P. G. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990;181(3):195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- Cotter T. G., Lennon S. V., Glynn J. G., Martin S. J. Cell death via apoptosis and its relationship to growth, development and differentiation of both tumour and normal cells. Anticancer Res. 1990 Sep-Oct;10(5A):1153–1159. [PubMed] [Google Scholar]
- Ferrer I., Serrano T., Rivera R., Olivé M., Zújar M. J., Graus F. Radiosensitive populations and recovery in X-ray-induced apoptosis in the developing cerebellum. Acta Neuropathol. 1993;86(5):491–500. doi: 10.1007/BF00228585. [DOI] [PubMed] [Google Scholar]
- Ferrer I. The effect of cycloheximide on natural and X-ray-induced cell death in the developing cerebral cortex. Brain Res. 1992 Aug 21;588(2):351–357. doi: 10.1016/0006-8993(92)91599-a. [DOI] [PubMed] [Google Scholar]
- Fidziańska A., Goebel H. H. Human ontogenesis. 3. Cell death in fetal muscle. Acta Neuropathol. 1991;81(5):572–577. doi: 10.1007/BF00310140. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenson L. E., Rotello R. J. Apoptosis: a different type of cell death. FASEB J. 1992 Apr;6(7):2450–2455. doi: 10.1096/fasebj.6.7.1563596. [DOI] [PubMed] [Google Scholar]
- Gulati A. K. The effect of X-irradiation on skeletal muscle regeneration in the adult rat. J Neurol Sci. 1987 Mar;78(1):111–120. doi: 10.1016/0022-510x(87)90083-9. [DOI] [PubMed] [Google Scholar]
- Khan M. Y. Radiation-induced changes in skeletal muscle. An electron microscopic study. J Neuropathol Exp Neurol. 1974 Jan;33(1):42–57. doi: 10.1097/00005072-197401000-00004. [DOI] [PubMed] [Google Scholar]
- Kurki P., Ogata K., Tan E. M. Monoclonal antibodies to proliferating cell nuclear antigen (PCNA)/cyclin as probes for proliferating cells by immunofluorescence microscopy and flow cytometry. J Immunol Methods. 1988 Apr 22;109(1):49–59. doi: 10.1016/0022-1759(88)90441-3. [DOI] [PubMed] [Google Scholar]
- LEWIS R. B. Changes in striated muscle following single intense doses of x-rays. Lab Invest. 1954 Jan-Feb;3(1):48–55. [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M., Franza B. R., Jr, Garrels J. I. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984 May 24;309(5966):374–376. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- Ogata K., Kurki P., Celis J. E., Nakamura R. M., Tan E. M. Monoclonal antibodies to a nuclear protein (PCNA/cyclin) associated with DNA replication. Exp Cell Res. 1987 Feb;168(2):475–486. doi: 10.1016/0014-4827(87)90020-6. [DOI] [PubMed] [Google Scholar]
- Wakeford S., Watt D. J., Partridge T. A. X-irradiation improves mdx mouse muscle as a model of myofiber loss in DMD. Muscle Nerve. 1991 Jan;14(1):42–50. doi: 10.1002/mus.880140108. [DOI] [PubMed] [Google Scholar]
- Webb J. N. Cell death in developing skeletal muscle: histochemistry and ultrastructure. J Pathol. 1977 Nov;123(3):175–180. doi: 10.1002/path.1711230307. [DOI] [PubMed] [Google Scholar]
- Webb J. N. The development of human skeletal muscle with particular reference to muscle cell death. J Pathol. 1972 Apr;106(4):221–228. doi: 10.1002/path.1711060403. [DOI] [PubMed] [Google Scholar]
- Wirtz P., Loermans H., Rutten E. Effects of irradiation on regeneration in dystrophic mouse leg muscles. Br J Exp Pathol. 1982 Dec;63(6):671–679. [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]