Abstract
The alterations in collagen fibril diameter distribution, mean fibril diameter and the area occupied by collagen after posterior cruciate ligament reconstruction using a patellar tendon autograft were estimated 2, 6, 16, 26, 52 and 104 wk postoperatively. Patellar tendons and posterior cruciate ligaments from unoperated animals were used as control tissues. Collagen fibrils were divided into histograms according to their diameter in order to analyse distribution maxima. There was a significant decrease in mean fibril diameter of the grafts in comparison with the control tissues. At 104 wk it was only about 51% of that for control posterior cruciate ligaments. The total area occupied by collagen was significantly reduced at 6 wk postoperatively and was about 57% in comparison with normal posterior cruciate ligaments. A considerable increase of small diameter collagen fibrils together with a loss of large fibrils was responsible for these results. There was no evidence of reestablishment of large diameter fibrils, which are normally found in tendon and ligaments, up to 2 y after transplantation. The total area covered by collagen was still reduced at this stage although the number of fibrils had increased.
Full text
PDF

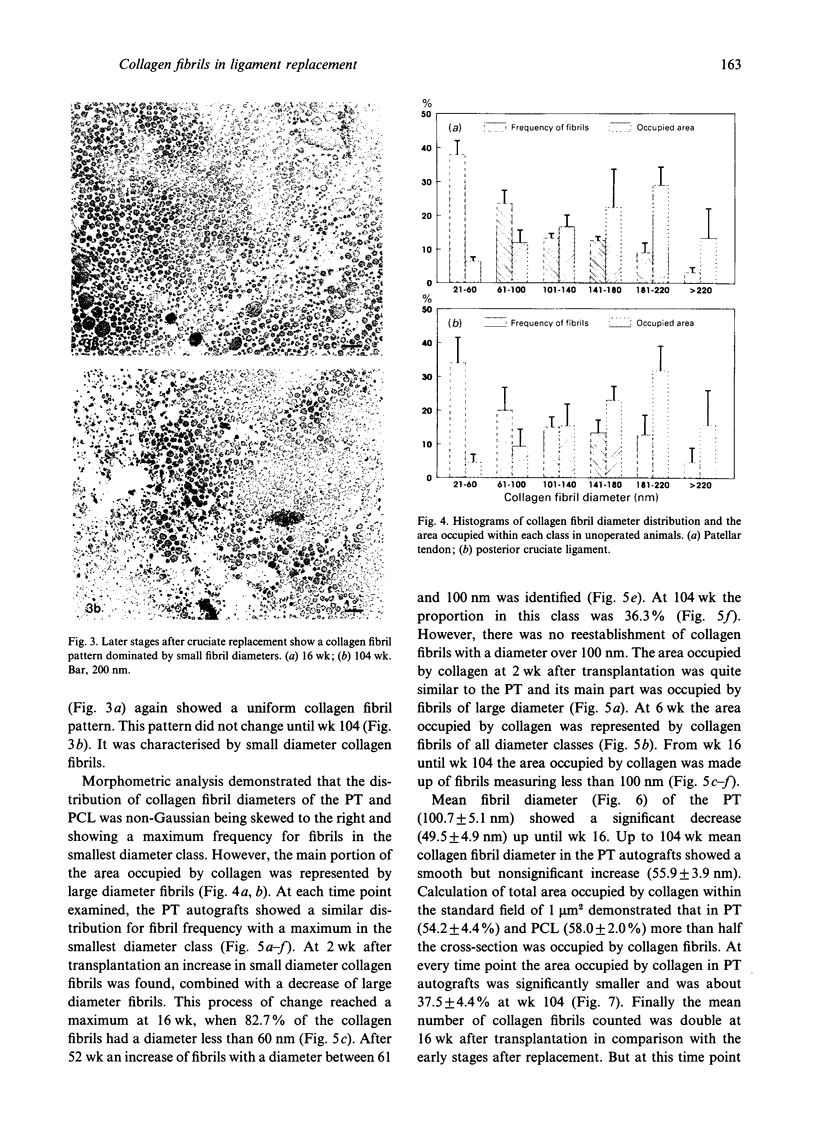
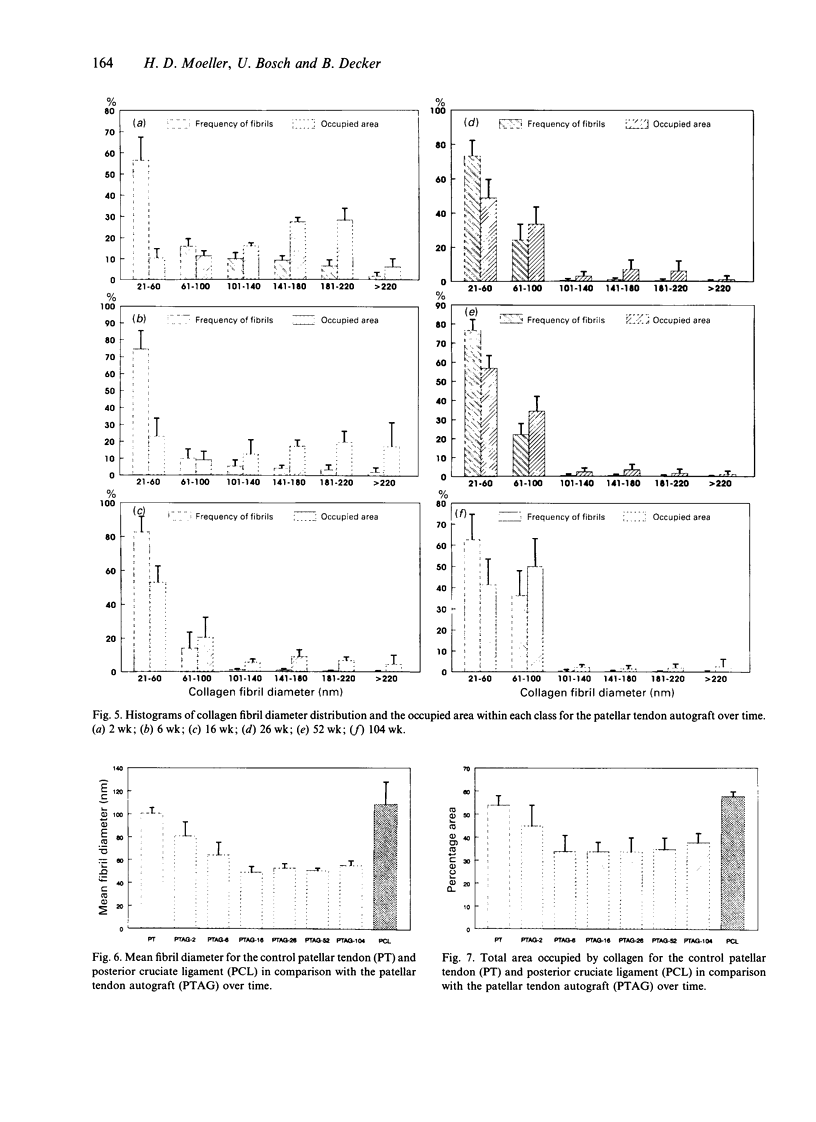



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi E., Hayashi T. In vitro formation of hybrid fibrils of type V collagen and type I collagen. Limited growth of type I collagen into thick fibrils by type V collagen. Connect Tissue Res. 1986;14(4):257–266. doi: 10.3109/03008208609017469. [DOI] [PubMed] [Google Scholar]
- Birk D. E., Zycband E. Assembly of the tendon extracellular matrix during development. J Anat. 1994 Jun;184(Pt 3):457–463. [PMC free article] [PubMed] [Google Scholar]
- Bosch U., Decker B., Kasperczyk W., Nerlich A., Oestern H. J., Tscherne H. The relationship of mechanical properties to morphology in patellar tendon autografts after posterior cruciate ligament replacement in sheep. J Biomech. 1992 Aug;25(8):821–830. doi: 10.1016/0021-9290(92)90222-m. [DOI] [PubMed] [Google Scholar]
- Bosch U., Kasperczyk W. J. Healing of the patellar tendon autograft after posterior cruciate ligament reconstruction--a process of ligamentization? An experimental study in a sheep model. Am J Sports Med. 1992 Sep-Oct;20(5):558–566. doi: 10.1177/036354659202000513. [DOI] [PubMed] [Google Scholar]
- Clancy W. G., Jr, Narechania R. G., Rosenberg T. D., Gmeiner J. G., Wisnefske D. D., Lange T. A. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981 Oct;63(8):1270–1284. [PubMed] [Google Scholar]
- Decker B., Bosch U., Gässler N., Tugtekin I., Kasperczyk W., Reale E. Histochemical aspects of the proteoglycans of patellar tendon autografts used to replace the posterior cruciate ligament. Matrix Biol. 1994 Jan;14(1):101–111. doi: 10.1016/0945-053x(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., Burgeson R. E., Shaikh-Bahai F., Timpl R. Type I and type III collagen interactions during fibrillogenesis. Ann N Y Acad Sci. 1990;580:161–175. doi: 10.1111/j.1749-6632.1990.tb17927.x. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Timpl R., Tuderman L., Raisher L., Wiestner M., Perlish J. S., Graves P. N. Ultrastructural identification of extension aminopropeptides of type I and III collagens in human skin. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7360–7364. doi: 10.1073/pnas.78.12.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank C., Bray D., Rademaker A., Chrusch C., Sabiston P., Bodie D., Rangayyan R. Electron microscopic quantification of collagen fibril diameters in the rabbit medial collateral ligament: a baseline for comparison. Connect Tissue Res. 1989;19(1):11–25. doi: 10.3109/03008208909016811. [DOI] [PubMed] [Google Scholar]
- Gay S., Martin G. R., Muller P. K., Timpl R., Kuhn K. Simultaneous synthesis of types I and III collagen by fibroblasts in culture. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4037–4040. doi: 10.1073/pnas.73.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gässler N., Tugtekin I., Decker B., Bosch U., Delbrück A. Changes in the extracellular matrix of the autogenous patellar tendon graft after posterior cruciate ligament reconstruction: a biochemical study in sheep. Matrix Biol. 1994 Jan;14(1):87–99. doi: 10.1016/0945-053x(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Keene D. R., Sakai L. Y., Bächinger H. P., Burgeson R. E. Type III collagen can be present on banded collagen fibrils regardless of fibril diameter. J Cell Biol. 1987 Nov;105(5):2393–2402. doi: 10.1083/jcb.105.5.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiere C. M., Nusgens B., Pierard G. E. Interaction between collagen type I and type III in conditioning bundles organization. Connect Tissue Res. 1977;5(1):21–29. doi: 10.3109/03008207709152608. [DOI] [PubMed] [Google Scholar]
- Matthew C. A., Moore M. J. Regeneration of rat extensor digitorum longus tendon: the effect of a sequential partial tenotomy on collagen fibril formation. Matrix. 1991 Aug;11(4):259–268. doi: 10.1016/s0934-8832(11)80233-7. [DOI] [PubMed] [Google Scholar]
- Michna H. Morphometric analysis of loading-induced changes in collagen-fibril populations in young tendons. Cell Tissue Res. 1984;236(2):465–470. doi: 10.1007/BF00214251. [DOI] [PubMed] [Google Scholar]
- Newton P. O., Woo S. L., Kitabayashi L. R., Lyon R. M., Anderson D. R., Akeson W. H. Ultrastructural changes in knee ligaments following immobilization. Matrix. 1990 Oct;10(5):314–319. doi: 10.1016/s0934-8832(11)80187-3. [DOI] [PubMed] [Google Scholar]
- Noyes F. R., Butler D. L., Grood E. S., Zernicke R. F., Hefzy M. S. Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am. 1984 Mar;66(3):344–352. [PubMed] [Google Scholar]
- Parry D. A., Barnes G. R., Craig A. S. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc R Soc Lond B Biol Sci. 1978 Dec 18;203(1152):305–321. doi: 10.1098/rspb.1978.0107. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Flint M. H., Gillard G. C., Craig A. S. A role for glycosaminoglycans in the development of collagen fibrils. FEBS Lett. 1982 Nov 22;149(1):1–7. doi: 10.1016/0014-5793(82)81060-0. [DOI] [PubMed] [Google Scholar]
- Viidik A. Functional properties of collagenous tissues. Int Rev Connect Tissue Res. 1973;6:127–215. doi: 10.1016/b978-0-12-363706-2.50010-6. [DOI] [PubMed] [Google Scholar]





