Abstract
Background
Optimal selection of anastomosis technique is crucial in colectomy surgeries to ensure success and minimize postoperative complications. Various methods, both manual and stapler-assisted, are employed for intestinal anastomosis. This study aims to compare two surgical methods of intestinal anastomosis through macroscopic and microscopic examination.
Methods
Twenty-five albino Wistar rats were randomly divided into two groups: the first group (n = 10) underwent Side-to-End anastomosis, while the second group (n = 15) underwent End-to-End anastomosis. After a 5-day observation period under uniform laboratory conditions, both groups underwent a second surgery. Anastomoses were assessed for adhesion and leakage, followed by histopathological examination of excised samples using the oxygenal method. Data were analyzed using the Mann-Whitney statistical method with a significance level of p < 0.05.
Results
Following the initial surgery, the second group exhibited a higher mortality rate compared to the first group. Based on our data, the mortality of the rats was unrelated to the type of anastomosis or the surgical procedure. The higher mortality rate in one group was due to other factors. Additionally, the second group demonstrated significantly greater adhesion formation. Histopathological examination revealed no significant difference between the groups, although neovascularization and collagen accumulation appeared more pronounced in the Side-to-End group.
Conclusion
Histopathologically, Side-to-End anastomosis showed superior repair conditions compared to End-to-End anastomosis. However, due to the limited sample size, statistical significance was not achieved. Conversely, Side-to-End anastomosis was associated with increased adhesion formation. These findings suggest the need for further comprehensive studies with larger sample sizes conducted in well-equipped centers to ascertain the preferred distal colon anastomosis technique and to achieve statistically significant results that can be more reliably generalized.
Keywords: Rat, Anastomosis, Colon, Leakage
Introduction
While colorectal surgery is generally not considered to be particularly challenging from a technical standpoint, it does carry inherent risks of complications such as the formation of fistulas, bleeding, strictures at the site of anastomosis, and leakage from the anastomotic site. Research has compared various anastomosis techniques to identify the optimal approach associated with the lowest complication rates. Among these complications, anastomotic dysfunction stands out as one of the most concerning after bowel surgery [1, 2].
Advancements in surgical techniques facilitated by technological progress and modern equipment, including laparoscopic and robotic surgery, alongside the adoption of mechanical suturing using circular staples, have significantly evolved over the past fifty years. These innovations, coupled with antibiotic prophylaxis and paradigm shifts in preoperative and postoperative care strategies, have notably enhanced the safety of colorectal cancer surgery. Despite these advancements, complications following colorectal surgery remain unavoidable and can vary from mild cases with limited patient impact to severe instances with potentially life-threatening outcomes, such as anastomotic leakage [3, 4].
Anastomotic leakage stands out as the most severe and devastating complication arising from colorectal surgery. It continues to pose significant challenges, contributing to serious complications and mortality rates among patients undergoing such procedures. Notably difficult to manage, anastomotic leakage may necessitate repeat laparotomy. Its occurrence not only profoundly impacts patients clinically but also places surgeons at a critical juncture where prevention, diagnosis, and treatment intersect. Despite advancements, many aspects surrounding colorectal anastomotic leakage, including its etiology, remain unclear. However, contemporary practice should prioritize risk assessment during surgery, with surgical techniques adapted as necessary [5, 6].
The reported incidence of anastomotic leakage following colorectal cancer surgery averages around 11%, with figures ranging widely from 3 to 30% depending on various factors such as diagnostic criteria, patient’s clinical condition at admission, tumor characteristics, operative variables, surgical approach, and duration of postoperative monitoring [4].
The leakage rate for rectal anastomosis ranges from 12 to 19%, while for colon anastomosis, it is approximately 11%, as indicated by studies [7–9].
The location of the anastomosis emerges as the most consistent predictor of anastomotic leakage. Generally, the more distal the anastomosis, the higher the likelihood of failure. For instance, in cases involving distal rectal cancer resection, the risk of anastomotic leakage is approximately five times greater compared to colon cancer resection. Additional risk factors include malnutrition, immunosuppression, diabetes, use of nonsteroidal anti-inflammatory drugs (NSAIDs), and radiation therapy [10].
Following bowel resection, the establishment of an anastomosis is crucial to maintain bowel continuity. There are three primary methods of anastomosis commonly employed post-colectomy: Side-to-Side, Side-to-End, and End-to-End anastomosis.
While many surgeons may prefer one method over another, definitive superiority remains unproven. All reconstruction techniques share similar principles aimed at optimizing the structural integrity of the anastomosis to reduce postoperative complications, particularly anastomotic leakage [11].
In this study, postoperative complications including anastomotic leakage, adhesion formation, inflammation, neovascularization, and collagen accumulation were compared between Side-to-End and End-to-End anastomosis techniques in Wistar rats.
Methods
Animals population
The research adhered to ethical standards at all stages. Approval for the study was obtained from the Animal Experiments Local Ethics Committee of the Army University of Medical Sciences, with the assigned study code (approval no: IR.AJAUMS.REC.1402.234).
This research involved 25 male albino Wistar rats with an average weight of 250 g. They were obtained from Tarbiat Modares University (Tehran, Iran). They were accommodated in groups of 5 within cages under typical laboratory conditions. The rats experienced a natural light cycle alternating between 12 h of darkness and 12 h of daylight, while maintaining room temperature within the range of 20–22 °C.
Throughout the study, the rats were provided with unrestricted access to standard food and water. Following random assignment, the rats were divided into two groups: one comprising 10 rats and the other consisting of 15 rats.
Study group allocation
The implementation of the study plan involved the allocation of 25 healthy rats into two distinct groups based on the techniques used for colonic anastomosis, as outlined below:
The study plan was executed by assigning 25 healthy rats to two separate groups according to the methods of colonic anastomosis, as detailed below:
Group 1 (n = 15): End-to-End Anastomosis - Rats in this group underwent surgery in which the distal colon was incised and subsequently anastomosed using the End-to-End method.
Group 2 (n = 10): Side-to-End Anastomosis - Conversely, rats in this group underwent a comparable surgical procedure, but the anastomosis was performed using the Side-to-End method.
The primary endpoint of our study was anastomosis leakage. The secondary endpoints included adhesions, abscess formation, and histological findings. The primary objective of the first surgery was to perform two different types of anastomosis methods, while the second surgery focused on histopathological and macroscopic examinations.
Implementation methodology and surgery
The animals received general anesthesia via intraperitoneal injections, consisting of a mixture containing 10 mg/kg xylazine HCl and 100 mg/kg ketamine HCl.
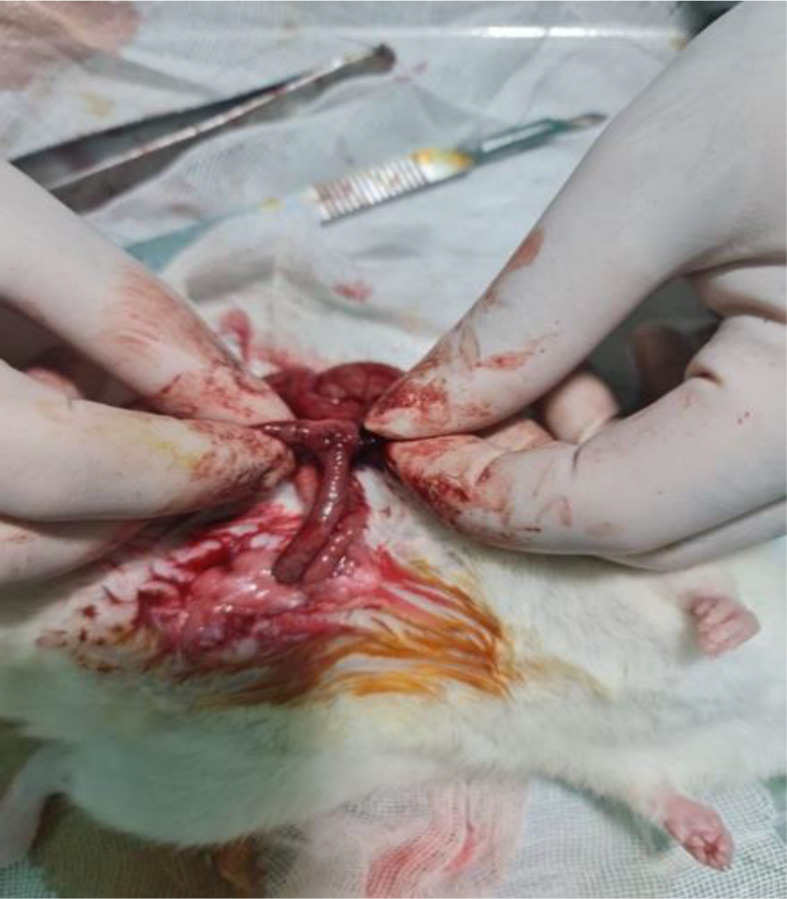
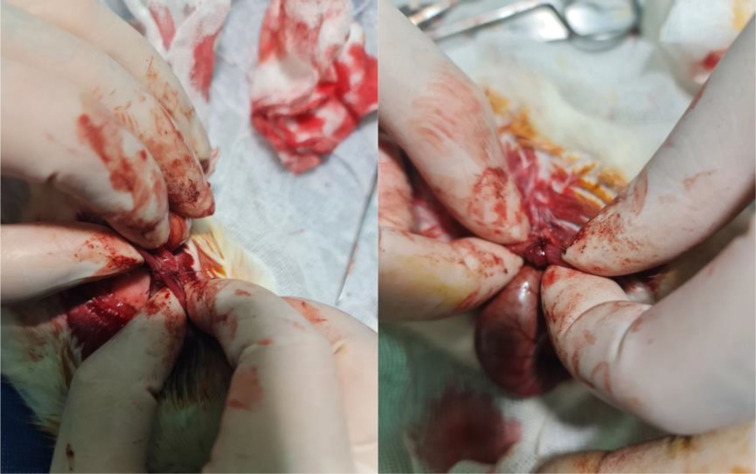
After a preoperative fasting period of 4 h and induction of anesthesia, the abdominal area was prepared by shaving and disinfecting with povidone iodine. Following the standard opening of the area, access to the colon was obtained, and complete layered resection of the left colon (descending colon) was performed across all groups. Group 1 underwent Colonic End-to-End anastomosis (Fig. 1), while Group 2 underwent Side-to-End anastomosis (Fig. 2). Surgery and anastomoses were performed using interrupted sutures, placed with a distance of 2–3 mm from each other and 2 mm from the edge of the colon. A simple suture technique using 4/0 Vicryl was employed as the suturing material for all anastomoses. The surgical procedure was concluded by closure of the area using standard techniques. This technique was chosen to ensure secure anastomosis and to minimize the risk of leakage. The use of interrupted sutures allowed for better tension distribution along the anastomotic line, and the specified distances between sutures and from the edge of the colon were optimized to provide adequate tissue apposition while preserving blood supply.
Fig. 1.
In this image, the proximal side of the colon is anastomosed with the distal end of the colon
Fig. 2.
In this image, the proximal end of the colon is anastomosed with the distal end of the colon
The surgery was performed by Dr. Mahyar Tahmasian, a general surgeon, under the supervision of Dr. Azita Shihegar, also an experienced surgeon.
Following a 5-day observation period in consistent laboratory conditions, both groups underwent a second surgical procedure via laparotomy. Initial assessment involved evaluating the appearance of both anastomoses for indications of adhesion and leakage. Subsequently, under controlled oxygen conditions, the anastomotic sites with a 2 cm margin were excised using surgical techniques. The excised samples were then forwarded for pathological examination to evaluate parameters such as collagen accumulation, neovascularization, and inflammation. On average, three slides were prepared from each tissue sample for thorough analysis.
Macroscopic examination
On the fifth day of the study, euthanasia was induced using a high dose of pentobarbital sodium administered intraperitoneally. Following this, standard procedures were followed to access the anastomotic colon. A macroscopic assessment of the anastomosis line was then conducted, and the colon was resected to include the anastomosis line. Macroscopic examination tests were carried out to evaluate anastomotic leakage and adhesion formation in sections of the anastomosis line.
Histopathological examination
Upon completion of the study, samples of tissue encompassing the anastomotic area were obtained from both experimental groups for histopathological analysis.
The tissue samples were immersed in a formaldehyde (10%) for fixation and then processed following standard histological tissue processing procedures. They were subsequently embedded in paraffin blocks. Following sectioning, the tissue samples were subjected to staining using the Masson trichrome and Hematoxylin and eosin (H&E) staining techniques to enable histological examination. Histological assessment of the tissues was conducted, and images were captured to document the findings. The evaluation involved assigning scores ranging from 0 to 3 based on the presence and severity of inflammatory cells, collagen accumulation, neovascularization, and other relevant factors (0 indicating absence, 1 indicating mild, 2 indicating moderate, and 3 indicating marked).
Data analysis method
The collected data underwent analysis using SPSS 23 software. Statistical analysis comparing the groups was performed using the Mann-Whitney test, with a significance level established at p < 0.05. The results were then illustrated using tables to facilitate understanding and interpretation.
Results
Clinical observations
Throughout the study period, there were no observed complications concerning the anastomosis line. However, by postoperative day 5, it became evident that out of the initial 15 rats in the first group undergoing End-to-End anastomosis, only 5 rats survived. Similarly, in the second group where Side-to-End anastomosis was performed on 10 rats, only 6 rats survived by the end of the observation period.
The mortality of rat was non-significantly higher in the End-to-End method (67%) than in the Side-to-End method (40%) (P = 0.183).
Based on our data, we can confirm that the mortality of the rats was unrelated to the type of anastomosis or the surgical procedure itself. The higher mortality rate in one group was due to factors not associated with the anastomosis techniques.
Macroscopic findings
Macroscopic examination conducted during re-laparotomy 5 days after surgery revealed no observable signs of leakage, infection, necrosis, or abscess at the anastomosis line in any of the groups. Nevertheless, a notable difference was observed between the Side-to-End and End-to-End groups, with the Side-to-End group exhibiting a visibly higher intensity of adhesion compared to the End-to-End group.
Histopathological findings
As per the pathologist’s assessment, the intensity of inflammation, neovascularization, and collagen accumulation was graded on a scale from 0 (indicating absence) to 3 (indicating high) for tissue samples collected from the anastomosis site (Figs. 3, 4).
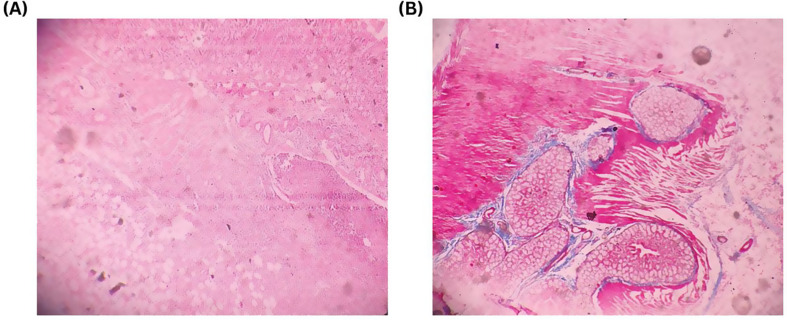
Fig. 3.
Trichrome and H&E staining technique are used to evaluate presence and severity of inflammatory cells, collagen accumulation, neovascularization, and other relevant factors. The evaluation involved assigning scores ranging from 0 to 3 (0 indicating absence, 1 indicating mild, 2 indicating moderate, and 3 indicating marked). (A) Hematoxylin stains nucleic acids a purple/blue color. Eosin stains components of the extracellular matrix/cytoplasm a pink color. In this slide H&E stain showing marked (score 3) degree of adhesion factors in Side-to-End anastomosis. (B) Trichrome stain showing characteristic pattern of histopathological findings and it stains collagen blue. In this slide Trichrome stain showing marked (score 3) degree of adhesion factors in Side-to-End anastomosis
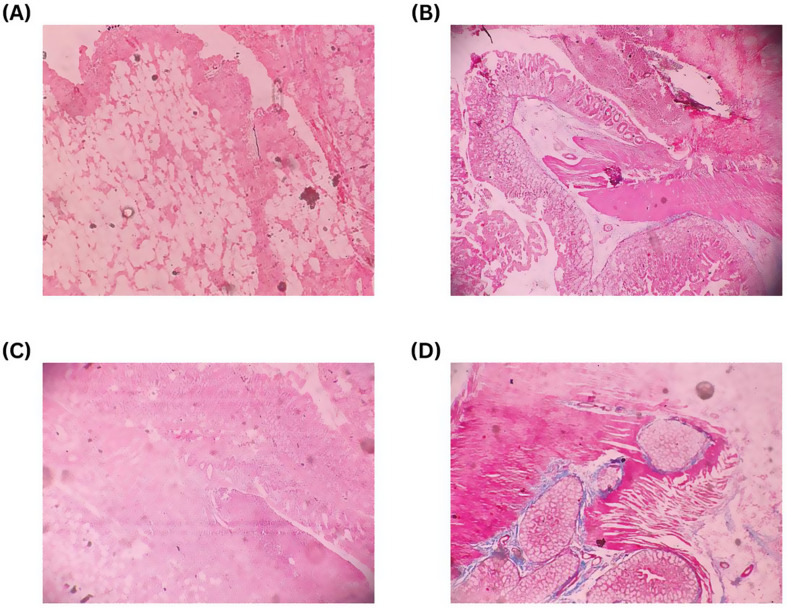
Fig. 4.
Trichrome and H&E staining technique are used to evaluate presence and severity of inflammatory cells, collagen accumulation, neovascularization, and other relevant factors. The evaluation involved assigning scores ranging from 0 to 3 (0 indicating absence, 1 indicating mild, 2 indicating moderate, and 3 indicating marked). (A) Hematoxylin stains nucleic acids a purple/blue color. Eosin stains components of the extracellular matrix/cytoplasm a pink color. In this slide H&E stain showing mild (score 1) degree of adhesion factors in End-to-End anastomosis. (B) Trichrome stain showing characteristic pattern of histopathological findings and it stains collagen blue. In this slide Trichrome stain showing mild (score 1) degree of adhesion factors in End-to-End anastomosis. (C) In this slide H&E stain showing moderate (score 2) degree of adhesion factors in End-to-End anastomosis. (D) In this slide Trichrome stain showing moderate (score 2) degree of adhesion factors in End-to-End anastomosis
The results obtained from multiple slides for each rat were averaged for consistency (Tables 1, 2 and 3). The results of the Mann-Whitney statistical analysis indicated no statistically significant difference between the median intensity of inflammation in the two groups (P = 0.792) (Table 3).There was no significant difference in angiogenesis and collagen accumulation intensity between the two groups (P = 0.662) (Table 3).
Table 1.
Frequency of inflammation intensity score in intestinal anastomosis surgery using side-to-end and end-to-end methods in rats
| The score of inflammation severity | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 2.5 | 2.75 | 3 | Total | |
| End to End (n) | 2 | 1 | 0 | 0 | 2 | 5 |
| Side to End (n) | 1 | 1 | 1 | 1 | 2 | 6 |
Table 2.
Frequency of angiogenesis and collagen accumulation intensity in intestinal anastomosis surgery using side-to-end and end-to-end methods in rats
| Angiogenesis Intensity score | ||||||
|---|---|---|---|---|---|---|
| 1 | 1.3 | 1.5 | 2 | 2.25 | Total | |
| End to End (n) | 2 | 0 | 0 | 2 | 1 | 5 |
| Side to End (n) | 2 | 1 | 1 | 1 | 1 | 6 |
| Collagen Accumulation Intensity score | ||||||
| 1 | 1.3 | 1.5 | 2 | 2.25 | Total | |
| End to End (n) | 2 | 0 | 0 | 2 | 1 | 5 |
| Side to End (n) | 2 | 1 | 1 | 1 | 1 | 6 |
Table 3.
Histopathological effects of intestinal anastomosis surgery using side-to-end and end-to-end methods in rats
| Side to End | End to End | P value | |
|---|---|---|---|
| Intensity of Inflammation (score) | 1.1 ± 1.4 | 2.0 ± 1.1 | 0.792 |
| Intensity of Angiogenesis and Collagen Accumulation (score) | 2.6 ± 1.3 | 2.0 ± 2.0 | 0.662 |
Data are expressed as median ± IQR and analyzed using Mann-Whitney statistical analysis, with P < 0.05 considered significant
Discussion
In the field of colon cancer surgery, a wide range of intestinal anastomosis techniques has been developed following colon resection. However, the occurrence of anastomotic leakage subsequent to surgery has become a notable concern, as it not only prolongs hospital stays but also escalates mortality rates [12].
Anastomotic leakage represents a critical challenge in colorectal surgery, entailing considerable risks for postoperative mortality and recurrence. The reported incidence of anastomotic leakage varies significantly. Analysis focusing on predictors for anastomotic leakage has underscored clear associations with patient factors such as malnutrition, surgical expertise, and the chosen surgical technique, including hand-sewn anastomosis and open approaches [13].
Despite advancements in colorectal surgery, there remains a scarcity of reports concerning anastomosis in left and sigmoid colon surgeries. Recent innovations have introduced techniques such as side-to-side anastomosis (e.g., left hemicolectomy), Side-to-End anastomosis, and mechanical End-to-End anastomosis.
In our research, we compared postoperative complications—including anastomotic leakage, adhesion, inflammation, neovascularization, and collagen accumulation—between the Side-to-End and End-to-End methods in 11 Wistar rats. Notably, during laparotomy conducted five days post-surgery in 11 Wistar rats, we observed no instances of anastomotic leakage in either group. This absence of leakage was consistent across both the End-to-End (5 rats) and Side-to-End (6 rats) anastomosis methods. Consequently, our findings indicate no significant difference in the leakage rates between End-to-End and Side-to-End anastomosis methods.
Adhesions are a natural part of the body’s healing process, and it’s estimated that intestinal adhesions occur in ninety to ninety-five% of patients [14]. However, concerns persist regarding the severity of adhesion formation, the increased risk of complications during reoperation, and the potential for long-term obstruction.
Indeed, assessing the success of colon anastomosis involves more than just ensuring physical strength and absence of leakage. In our study, we employed inflammation, neovascularization, and collagen accumulation as indirect measures to evaluate the integrity of the anastomosis.
The healing process of anastomosed intestinal tissue follows a pattern similar to other tissues, progressing through phases of inflammation, proliferation, and remodeling. However, intestinal healing presents unique characteristics, including its duration and interaction with the gastrointestinal environment. As the anastomosis advances, primary inflammatory cells are substituted by collagen- producing fibroblasts, strengthening the site. This intricate process is regulated by a network of cell-to-cell signaling, which includes cytokines and growth factors like platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), and vascular endothelial growth factor (VEGF) [15].
Given the potential risks associated with anastomotic failure, monitoring inflammatory markers and cytokines becomes crucial for early detection of leakage [16]. C-reactive protein (CRP), calprotectin, and procalcitonin are among the most reliable biological markers of anastomotic leakage after intestinal surgery. Elevated levels of CRP and calprotectin indicate intestinal inflammation, while procalcitonin serves as an indicator of bacterial infection, both common features of anastomotic leakage [17].
In our study, the severity of inflammation was macroscopically assessed by the pathologist, scoring tissue samples from the anastomosis site on a scale from 0 (no inflammation) to 3 (high). The results were averaged across multiple slides for each rat. Statistical analysis revealed no significant difference in inflammation intensity between the two groups: 5 rats with End-to-End anastomosis and 6 rats with Side-to-End anastomosis. Additionally, CRP levels were reported as negative in all rats from both groups.
The healing process of intestinal anastomosis involves distinct phases, including acute inflammation, proliferation, and regeneration. Collagen, crucial for intestinal wall strength, undergoes significant metabolism during this process, shaping our understanding of anastomosis treatment. Halsted’s 1887 discovery emphasized the submucosa’s role in providing gastrointestinal tract tensile strength, primarily composed of collagen, blood vessels, lymph, and nerve fibers [15].
Collagen type I dominates (68%), followed by types III (20%) and V (12%) in this layer. Proper placement of the serosa layer during anastomosis minimizes leakage risk [18]. In the initial postoperative days, anastomotic strength is limited, heightening the risk of wound failure due to collagen breakdown. The primary anastomosis’ strength relies on suture capacity or maintaining existing collagen until sufficient new collagen synthesis occurs from fibroblasts and smooth muscle cells. Consequently, anastomosis remains vulnerable for a brief period post-surgery [19].
In our study, we investigated collagen accumulation after 5 days in 11 Wistar rats: 5 with End-to-End anastomosis and 6 with Side-to-End anastomosis. Slide examinations suggested relatively higher collagen accumulation in the Side-to-End group. However, statistical analysis did not deem this difference significant. The reduction in rat numbers from 25 to 11 due to postoperative deaths within the initial 5 days likely influenced this statistical observation.
According to Schwartz’s Principles of Surgery (11th Edition), collagenase activity peaks in the colon after anastomosis on the fifth postoperative day, which corresponds to the highest probability of anastomosis leakage. We recognize that collagen deposition, which begins around the fifth day, might not have significantly increased to show notable changes during our follow-up. Considering that the highest incidence of leaks and complications occurs within this time frame, our pilot study focused on early leakage detection. However, the necessity of removing a portion of the anastomosis for histopathological analysis limited our ability to extend the observation period. Future studies should consider longer follow-up periods to investigate delayed leakage. This is indeed a major limitation of our study, along with the small sample size, which impacts the statistical significance and generalizability of our findings. The time for both anastomoses was the same, and a comparison was made. Future studies with larger sample sizes and extended follow-up periods are necessary to validate these preliminary findings and provide more robust conclusions.
Despite the lack of strong evidence on the advantages of Side-to-End anastomosis compared to End-to-End anastomosis, the theoretical improvement of Side-to-End anastomosis vascularity and neovascularization in line with each other can improve the intestinal healing process and reduce the risk of leakage and microleakage.
Although in our research, no significant difference was observed in the histological parameters for neovascularization between 5 rats with End-to-End colon anastomosis and 6 rats with Side-to-End colon anastomosis in the examination of the slides after 5 days, but the non-statistical and visual trend of significant superiority for Neovascularization values were present in the Side-to-End anastomosis group.
This study serves as a pilot and preliminary investigation into the potential benefits of the Side-to-End colonic anastomosis technique. The observed superiority of the Side-to-End colonic anastomosis group in terms of neovascularization was based on visual and individual observations by the surgeon. However, due to the small sample size, these observations were not statistically significant. This limitation highlights the study’s preliminary nature and suggests potential sources of bias, such as observer bias and the lack of objective outcome measures.
Recognizing this limitation, future studies with a larger sample size are essential to improve the statistical power and reliability of the results, and to conclusively determine the significance of these observations.
Additionally, to enhance the comprehensiveness of our findings, future research will incorporate more comprehensive outcome measures, including functional outcomes and long-term complications. This will provide a more understanding of the clinical implications and overall efficacy of the Side-to-End colonic anastomosis technique, ensuring that the findings are both robust and clinically meaningful.
Moreover, to improve the reproducibility and transparency of our study, we acknowledge the need for more detailed descriptions of the surgical techniques, postoperative care, and criteria for scoring used in this study. In future research, thorough documentation of the surgical procedures, including the specific steps taken during the Side-to-End colonic anastomosis, as well as detailed postoperative care protocols, will be provided. The criteria for scoring neovascularization and other relevant outcomes will also be clearly defined and standardized to ensure consistency and reproducibility. These enhancements will not only address the limitations and potential sources of bias identified in this pilot study but will also strengthen the design of further complementary studies, contributing valuable insights to the body of evidence supporting the use of this technique.
A retrospective rectal study was consistent with our conclusion, showing that Side-to-End anastomosis outperformed rectal End-to-End anastomosis in vascular perfusion and neovascularization [20].
The choice of anastomotic technique plays a pivotal role in mitigating anastomotic leakage risks. Studies indicate superior blood flow at the anti-mesenteric border compared to the colon’s end, a factor closely linked to anastomotic site integrity. Consequently, Side-to-End anastomosis may yield more favorable outcomes than End-to-End anastomosis. Moreover, the principle of Side-to-End anastomosis is a standard practice in various gastrointestinal surgeries, including esophageal-jejunal anastomosis [21].
Conclusion
In summary, while no statistically significant differences were noted in inflammation, collagen accumulation, and neovascularization between the two types of anastomoses, Side-to-End anastomosis exhibited potential superiority in wound healing based on histopathological assessments. Macroscopic examination revealed a greater degree of adhesion in Side-to-End anastomosis compared to End-to-End anastomosis, with no notable difference in anastomotic leakage between the two techniques.
However, due to the limited sample size, statistical significance was not achieved.These findings suggest the need for further comprehensive studies with larger sample sizes conducted in well-equipped centers to ascertain the preferred distal colon anastomosis technique and to achieve statistically significant results that can be more reliably generalized.
Acknowledgements
The authors would like to thank Dr. Saeideh Kavousi, from Tarbiat Modares University, for her assistance in providing animals for this study. We extend our gratitude to the dedicated staff of the animal laboratory for their valuable assistance during the surgical procedures conducted in this study.
Author contributions
Study conception and design: M.T, A.S. and A.A. Analysis and interpretation of data: I.M. and E.S. Critical revision of manuscript: A.S., A.A., I.M. and E.S. All authors approved the manuscript.
Funding
No financial support was received in the design and interpretation of data and in writing the manuscript.
Data availability
The data and materials generated in this study are included in this paper. For any further questions, contact the corresponding author.
Declarations
Ethics approval and consent to participate
The research adhered to ethical standards at all stages. Approval for the study was obtained from the Animal Experiments Local Ethics Committee of the Army University of Medical Sciences, with the assigned study code (approval no: IR.AJAUMS.REC.1402.234).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Karaca G, et al. The effects of scalpel, harmonic scalpel and monopolar electrocautery on the healing of colonic anastomosis after colonic resection. Ann Surg Treat Res. 2016;90:315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uysal E, Dokur M. Comparison of effects of the tacrolimus and cyclosporine A on the colon anastomosis recovery of rats. Ann Surg Treat Res. 2017;92:402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiarello MM, et al. Anastomotic leakage in colorectal cancer surgery. Surg Oncol. 2022;40:101708. [DOI] [PubMed] [Google Scholar]
- 4.Wallace B et al. Evidence according to Cochrane Systematic Reviews on Alterable Risk Factors for Anastomotic Leakage in Colorectal Surgery. Gastroenterol Res Pract 2020, 9057963 (2020). [DOI] [PMC free article] [PubMed]
- 5.Daglioglu YK, Duzgun O, Sarici IS, Ulutas KT. Comparison of platelet rich plasma versus fibrin glue on colonic anastomoses in rats. Acta Cir Bras. 2018;33:333–40. [DOI] [PubMed] [Google Scholar]
- 6.Strunden MS, Heckel K, Goetz AE, Reuter DA. Perioperative fluid and volume management: physiological basis, tools and strategies. Ann Intensive Care. 2011;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthiessen P, Hallböök O, Andersson M, Rutegård J, Sjödahl R. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis. 2004;6:462–9. [DOI] [PubMed] [Google Scholar]
- 8.Fielding LP, Stewart-Brown S, Blesovsky L, Kearney G. Anastomotic integrity after operations for large-bowel cancer: a multicentre study. Br Med J. 1980;281:411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pakkastie TE, Luukkonen PE, Järvinen HJ. Anastomotic leakage after anterior resection of the rectum. Eur J Surg. 1994;160:293–7. discussion 299–300. [PubMed] [Google Scholar]
- 10.Boccola MA, et al. Risk factors and outcomes for anastomotic leakage in colorectal surgery: a single-institution analysis of 1576 patients. World J Surg. 2011;35:186–95. [DOI] [PubMed] [Google Scholar]
- 11.Thomas MS, Margolin DA. Management of colorectal Anastomotic Leak. Clin Colon Rectal Surg. 2016;29:138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kube R, et al. Anastomotic leakage after colon cancer surgery: a predictor of significant morbidity and hospital mortality, and diminished tumour-free survival. Eur J Surg Oncol. 2010;36:120–4. [DOI] [PubMed] [Google Scholar]
- 13.2015 European Society of Coloproctology Collaborating Group. Predictors for anastomotic leak, postoperative complications, and Mortality after Right Colectomy for Cancer: results from an International Snapshot Audit. Dis Colon Rectum. 2020;63:606–18. [DOI] [PubMed] [Google Scholar]
- 14.Ellis, H. The clinical significance of adhesions: focus on intestinal obstruction. Eur J Surg Suppl := Acta chirurgica 1997;557:5–9. [PubMed]
- 15.Ballantyne GH. The experimental basis of intestinal suturing. Effect of surgical technique, inflammation, and infection on enteric wound healing. Dis Colon Rectum. 1984;27:61–71. [DOI] [PubMed] [Google Scholar]
- 16.Morgan RB, Shogan BD. The Science of Anastomotic Healing. Semin Colon Rectal Surg. 2022;33:100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamade B, Huang DT, Procalcitonin. Where are we now? Crit Care Clin. 2020;36:23–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martens MF, Hendriks T. Postoperative changes in collagen synthesis in intestinal anastomoses of the rat: differences between small and large bowel. Gut. 1991;32:1482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bissett IP. Ileocolic anastomosis. Br J Surg. 2007;94:1447–8. [DOI] [PubMed] [Google Scholar]
- 20.Kato H, et al. Efficacy of side-to-end anastomosis to prevent anastomotic leakage after anterior resection for rectal cancer. Mol Clin Oncol. 2022;16:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qu H, Liu Y, Bi D. Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc. 2015;29:3608–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials generated in this study are included in this paper. For any further questions, contact the corresponding author.