Abstract
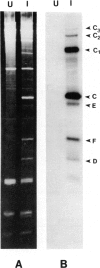
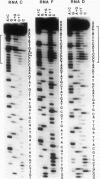
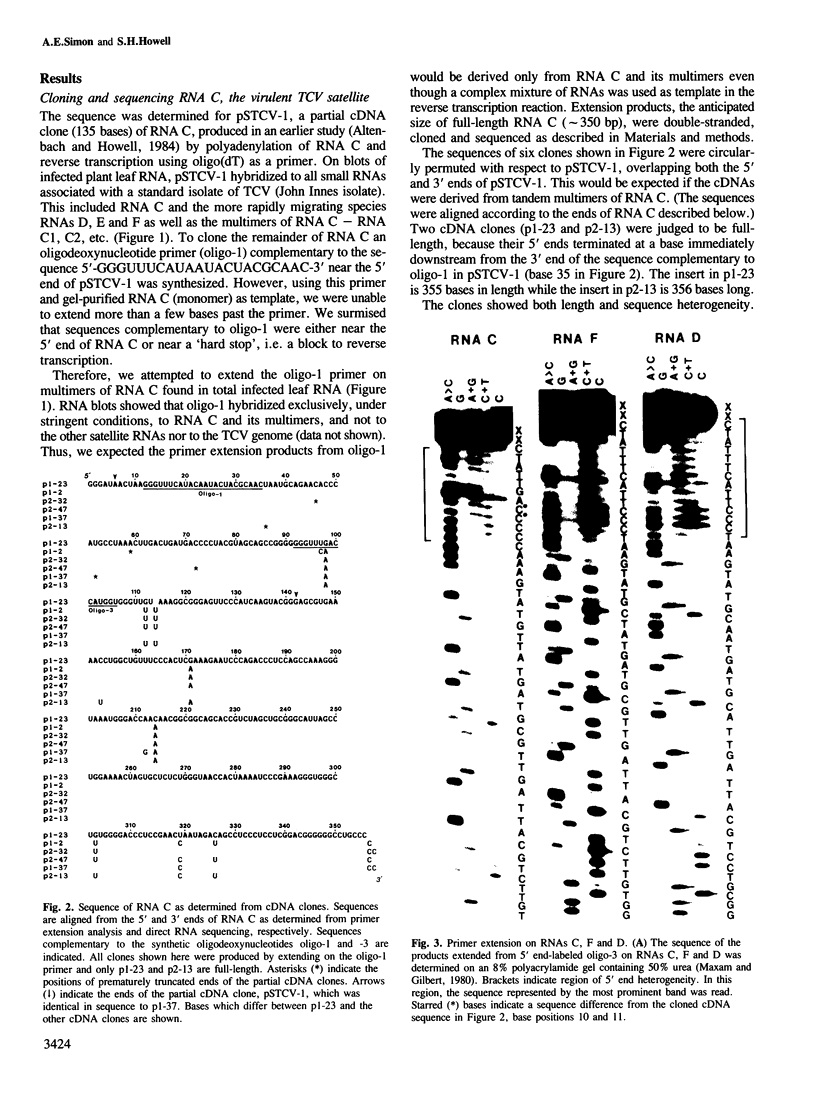
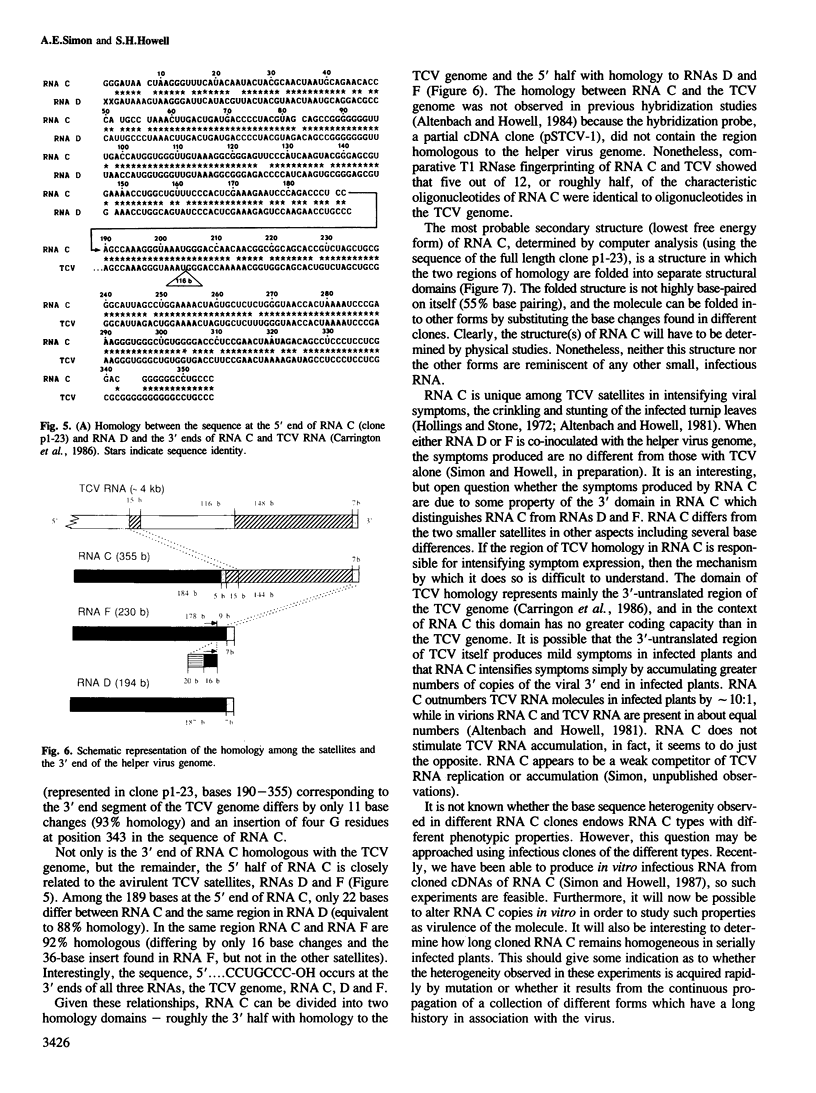
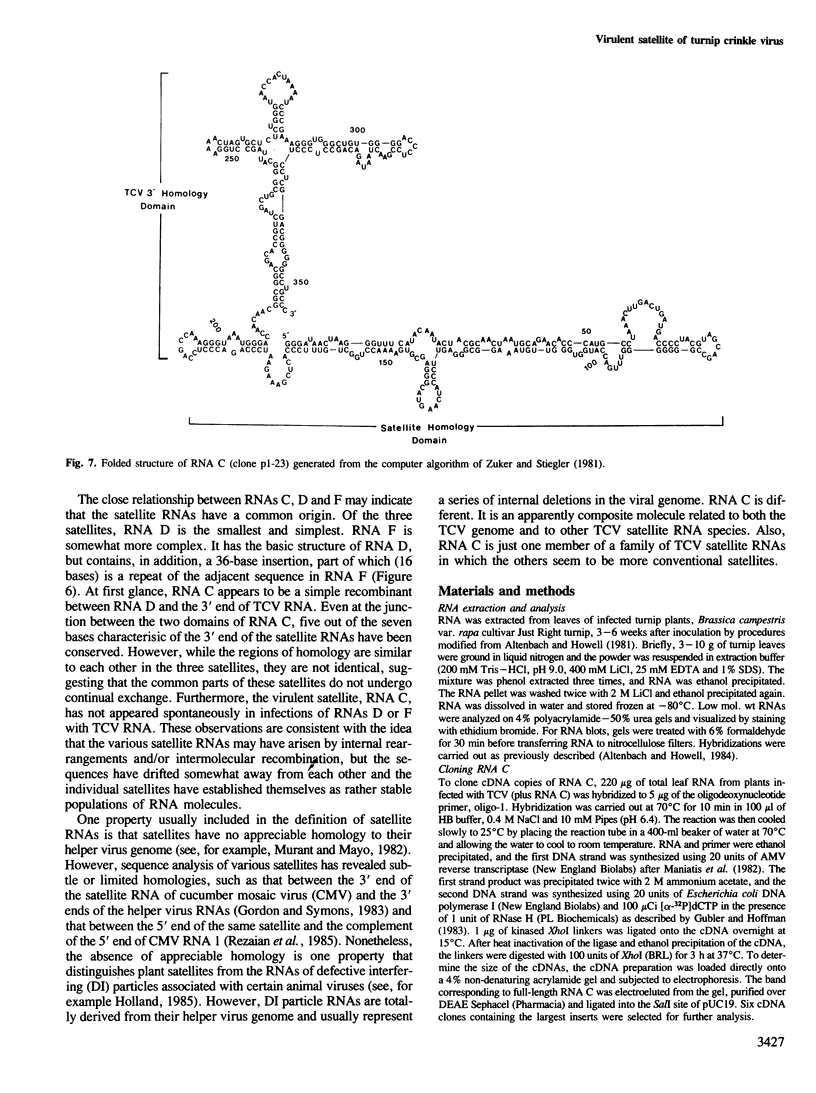
RNA C (355 bases), RNA D (194 bases) and RNA F (230 bases) are small, linear satellite RNAs of turnip crinkle virus (TCV) which have been cloned as cDNAs and sequenced in this study. These RNAs produce dramatically different disease symptoms in infected plants. RNA C is a virulent satellite that intensifies virus symptoms when co-inoculated with its helper virus in turnip plants, while RNA D and RNA F are avirulent. RNA D and RNA F, the avirulent satellites, are closely related to each other except that RNA F has a 36-base insert near its 3' end, not found in RNA D. The 189 bases at the 5' end of RNA C, the virulent satellite, are homologous to the entire sequence of RNA D. However, the 3' half of RNA C, is composed of 166 bases which are nearly identical to two regions at the 3' end of the TCV helper virus genome. Hence, the virulent satellite is a composite molecule with one domain at its 5' end homologous to the other avirulent satellites and another domain at its 3' end homologous to the helper virus genome. All four TCV RNAs, RNAs C, D and F and the helper virus genome have identical 7 bases at their 3' ends. The secondary structure of RNA C deduced from the sequence can be folded into two separate domains — the domain of helper virus genome homology and the domain homologous to other TCV satellite RNAs. Comparative sequences of several different RNA C clones reveal that this satellite is a population of molecules with sequence and length heterogeneity.
Keywords: plant virus, satellite RNAs, turnip crinkle virus, small RNAs
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gordon K. H., Symons R. H. Satellite RNA of cucumber mosaic virus forms a secondary structure with partial 3'-terminal homology to genomal RNAs. Nucleic Acids Res. 1983 Feb 25;11(4):947–960. doi: 10.1093/nar/11.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Comparative sequence and structure of viroid-like RNAs of two plant viruses. Nucleic Acids Res. 1982 Jun 25;10(12):3681–3691. doi: 10.1093/nar/10.12.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D. W., Gilbert C. W., Smith R. E., Sasavage N. L., Clark J. M., Jr Translation of satellite tobacco necrosis virus ribonucleic acid by an in vitro system from wheat germ. Biochemistry. 1976 Nov 2;15(22):4943–4950. doi: 10.1021/bi00667a030. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaian M. A., Williams R. H., Symons R. H. Nucleotide sequence of cucumber mosaic virus RNA. 1. Presence of a sequence complementary to part of the viral satellite RNA and homologies with other viral RNAs. Eur J Biochem. 1985 Jul 15;150(2):331–339. doi: 10.1111/j.1432-1033.1985.tb09025.x. [DOI] [PubMed] [Google Scholar]
- Salvato M. S., Fraenkel-Conrat H. Translation of tobacco necrosis virus and its satellite in a cell-free wheat germ system. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2288–2292. doi: 10.1073/pnas.74.6.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth H. E., Kaper J. M., Tousignant M. E. CARNA 5, the Small Cucumber Mosaic Virus--Dependent Replicating RNA, Regulates Disease Expression. Science. 1979 May 25;204(4395):845–847. doi: 10.1126/science.204.4395.845. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]