Abstract
Background
Vegetable soybean is an important legume vegetable. High sucrose content is a significant quality characteristic of vegetable soybean that influences consumers’ taste. However, the genetic basis of sucrose content in vegetable soybean is currently unclear.
Results
In this study, the genome-wide association study (GWAS) of sucrose content in vegetable soybean was performed using Chinese soybean mini-core collection. The results showed a wide genetic variation for the sucrose content in the mini-core collection. The sucrose content of genotypes from HHR (Huanghuai region) and SR (Southern region) was higher than that of genotypes from NER (Northeast region) and NR (Northern region). Furthermore, 82,187 high quality SNPs (Single nucleotide polymorphism) were used for GWAS of sucrose content. Based on SNPs detected in multiple environments, the chromosome 8 19,496,314–19,698,413 bp interval was identified as the candidate interval. And Glyma.08g234100 was most likely to affect the sucrose content of vegetable soybean seeds.
Conclusions
This study has created new details to be used for breeding for high sucrose content in vegetable soybean.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-06006-3.
Keywords: Vegetable soybean, Taste quality, Sucrose content, GWAS
Background
Soybean at the R6 (full seed) stage [1], commonly referred to as vegetable soybean, has been documented to have been cultivated in eastern China as early as the 2nd century BC [2]. At present, vegetable soybean cultivation has become popular in Asian countries, including Japan, Thailand, and India, as well as in the continents of the Americas, Europe, and Africa [2–4]. As a legume crop, vegetable soybean has a short maturity period, which can bring great economic benefits to farmers [2]. Meanwhile, vegetable soybean has gained popularity among consumers for its unique taste and rich nutritional value [5–7].
Despite the high level of vegetable soybean production, there is a need to improve the quality of vegetable soybean to meet the demand for consumers and breeders [5]. The quality traits of vegetable soybean can be divided into three main aspects: appearance quality, taste quality, and nutritional quality [6]. The taste quality of vegetable soybean, including sweetness, freshness, texture, flavor, and wax, has a critical impact on consumer purchase intention [8–10]. Among them, sweetness is the most important influencing factor.
The sweetness of vegetable soybean is mainly influenced by the soluble sugar content [2]. In accordance with the most recent standard set by the Ministry of Agriculture of China (NY-T 3705 − 2020), the soluble sugar content in the seeds should not be less than 2.2%. Sucrose is the predominant soluble sugar in vegetable soybean, accounting for more than 80% of the total content, with fructose and glucose being the subsequent components [11]. Li et al. (2012) determined the quality traits of 30 vegetable soybeans in Northeast China and found that the sucrose content varied widely, ranging from 21.1 to 93.7 mg/g [12]. Song et al. (2013) determined the sucrose content of vegetable soybean varieties in Jiangsu Province, China and found a similar range of variation [13]. Therefore, the sucrose content of vegetable soybean is controlled by quantitative genes, and it can be genetically improved.
Previous studies have mainly focused on genetic analysis of sucrose content in bi-parental populations. Maughan et al. (2000) used RIL populations derived from a cross between ‘V71-370’ and ‘PI407162’ to identify four QTLs associated with sucrose content [14]. Wang et al. (2014) used F2:3 populations derived from ‘V97-3000’ × ‘V99-5089’ and identified 3 QTL on chromosomes 7, 11 and 20 [15]. Compared with the conventional method of QTL mapping, genome-wide association study (GWAS) provides a broader detection range [16]. At present, several studies have conducted GWAS to investigate candidate genes associated with sucrose content in grain soybean mature seeds [17–19]. Ficht et al. (2022) provided a comprehensive analysis of the QTL associated with sucrose content in 266 soybean genotypes by GWAS and identified seven potential QTL located on chromosomes 1, 6, 8, 9, 10, 13 and 14 [17]. However, only a few studies have detected QTLs associated with sucrose or total soluble sugar content in vegetable soybean. Xu et al. (2022) identified two loci associated with the soluble sugar content in vegetable soybeans in two different environments, located on chromosomes 6 and 15, respectively [20]. Lu et al. (2023) identified thirteen QTL associated with soluble sugar content in vegetable soybean under different environmental [21].
So, the main purpose of this study was: To identify genetic intervals involved in sucrose content in vegetable soybeans through GWAS and predict candidate genes. This research will help to effectively improve the taste quality of vegetable soybean in breeding programs.
Materials and methods
Plant materials
A total of 133 soybean genotypes were obtained from the Chinese soybean mini-core collection, which was derived from a larger pool of 23,587 soybean genotypes stored in the Chinese National Soybean Genebank (Table S1) [22]. The SSR loci, sample composition, sampling rate, and low-frequency allele parameters all demonstrate the effectiveness of this collection [23]. It represents 94.5% of the phenotypic diversity and 63.5% of the genetic diversity of the entire germplasm. These 133 germplasms were collected from four ecological regions of China, including 19 from the Northeast Region (NER), 18 from the North Region (NR), 32 from the Huanghuai Region (HHR), and 64 from the Southern Region (SR).
Field trials
The seeds were planted in two specific geographical locations: (1) Nanjing (32°03’N, 118°88’E), located in Jiangsu Province, during the summer of 2021; and (2) Dangtu (31°58’N, 118°50’E), located in Anhui Province, during the summer of 2022. All the test soybean germplasms were planted in a single hill plot (2 × 0.5 m) in a randomized complete block design with 3 replications in each environment. Fertilization and pest control were implemented in a manner consistent with standard field management. Soybean pods at the R6 stage were collected [1], and a total of 50 pods from a row were sampled from each plant and rapidly frozen in liquid nitrogen.
Soluble sugar extraction
Samples were ground using an IKA A11 grinder (IKA, Staufen, Germany). The extraction of soluble sugars from R6 soybean seeds followed the procedure described by Hou et al. (2009) [24], with minor modifications. In brief, 0.1 g of finely ground powder was measured and transferred to a 2 ml (millilitre) centrifuge tube. Subsequently, 0.75 ml of 80% ethanol was added to the tube. The mixture was immersed in an ultrasonic water bath for duration of 10 min, while maintaining a room temperature. The samples were then centrifuged at 8000 rpm (revolutions per minute) for 10 min. The resulting supernatant was then carefully transferred to a fresh centrifuge tube. Finally, the procedure was repeated twice, adjusting the volume to 2 ml.
Ultra performance liquid chromatography system
Sugar identification and quantification were conducted using a Waters Acquity UPLC system (Waters, Milford, MA, USA) that was equipped with a binary pump solvent management system, an online degasser, an auto-sampler, and a column temperature maintainer set at 55 ℃. Chromatographic separation was performed using a UPLC Acquity BEH Amide column (2.1 mm × 100 mm, 1.7 μm). The mobile phase consisted of a mixture of solvent A (acetonitrile, v/v) and solvent B (2% triethylamine in water, v/v), both sourced from Macklin in Shanghai, China. The duration of the activity was 8 min.
Three standard sugars including glucose, fructose, and sucrose, were acquired from Solarbio Life Science Co (Beijing, China). Sugars present in the extracts were identified by comparing their retention times with those of the standard sugars. To establish regression curves for the quantification of the three sugars, sugar standards were prepared at contents of 0.8, 0.6, 0.4, 0.2, 0.1, and 0.05 mg·ml− 1. In this study, the retention times for the fructose standard, glucose standard, and sucrose standard were determined to be 3.391 min, 4.172 min, and 6.499 min, respectively (Fig. S1).
Phenotypic data analysis
IBM SPSS v19.0 was used for analysis of variance (ANOVA) and correlation analysis. Based on the average of sucrose content in different environments, the heritability was calculated. The formula for calculating heritability of this study was:
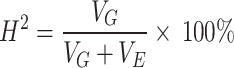 |
where H2 represents heritability, VG represents genetic variance, and VE represents environmental variance. Genotypes were grouped into ecological regions (NER, NR, HR, and SR) to evaluate their effect on sucrose content via ANOVA. And Dunnett’s test was used for statistical test.
Genotypic data
The 180 K AXIOM Soya SNP array was employed for genotyping 133 soybean genotypes, encompassing a cumulative count of 180,961 SNP markers. The comparison of the above SNP markers with the reference genome (Wm82.a2.v1) was conducted. After removing unmatched SNP markers, a total of 169,028 markers were retained. To enhance the precision of the association analysis, TASSEL v5.2 was used to eliminate markers with deletion and heterozygosity rates rate exceeding 10% and minor allelic frequency (MAF) below 0.05. Then, nupte-lnx64 software was used to fill in the missing SNPs [25]. The final set of 82,187 SNPs was utilized for the subsequent association analysis. Linkage disequilibrium (LD) parameters (r2) for estimating the degree of LD between pairwise SNPs (MAF > 0.2) were calculated using PLINK V1.07, and the diagram showing average LD decay was drawn with R [22]. The LD decay of this collection was 119.07 kb [22].
GWAS analysis
The GWAS analysis was performed for the 133 genotypes of Chinese soybean mini-core collection using the most statistically valid model, Bayesian-information and Linkage disequilibrium Iteratively Nested Keyway (BLINK) in GAPIT 3(https://zzlab.net/GAPIT/index.html) [26]. The markers above the significant association threshold of –log10(P) ≥ 3.5 was considered significantly associated with sucrose content. This threshold has been used to detect SNP markers associated with soybean architecture-related traits [27].
Results
Genetic variation of sucrose content in vegetable soybean seeds
The sucrose content of 133 genotypes at the R6 stage of seed was determined for both 2021NJ and 2022DT environments. In any of the environments, the sucrose content showed a normal distribution (Fig. 1). However, the correlation coefficient of sucrose content between the two environments was 0.44 and significantly different (Fig. S2). Statistical analyses including minimum, maximum, mean, standard deviation (SD), coefficient of variation (CV), and heritability of each environment were presented in Table 1. In environment 2021NJ, the minimum sucrose content was observed at 5.73 mg/g, while the maximum content reached 30.76 mg/g. Similarly, in environment 2022DT, the minimum sucrose content was observed at 3.49 mg/g, while the maximum content reached 24.78 mg/g. On average, the sucrose content of 2022DT was slightly higher than that of 2021NJ. The coefficient of variation (CV) in the two environments showed relatively high values of 34.78% and 26.88%, respectively. The combined heritability in different environments was 59.79% (Table 1).
Fig. 1.
The variation of sucrose content in 2021NJ and 2022DT. Pink represents 2021NJ, blue represents 2022DT
Table 1.
Descriptive study on the sucrose content
| Environment | Min(mg / g) | Max(mg / g) | Mean(mg / g) | SD | CV(%) | h2(%) |
|---|---|---|---|---|---|---|
| 2021NJ | 5.73 | 30.76 | 14.98 | 5.21 | 34.78 | 59.79 |
| 2022DT | 3.49 | 24.78 | 15.44 | 4.15 | 26.88 | |
| Mean | 7.52 | 26.60 | 15.21 | 3.87 | 25.44 |
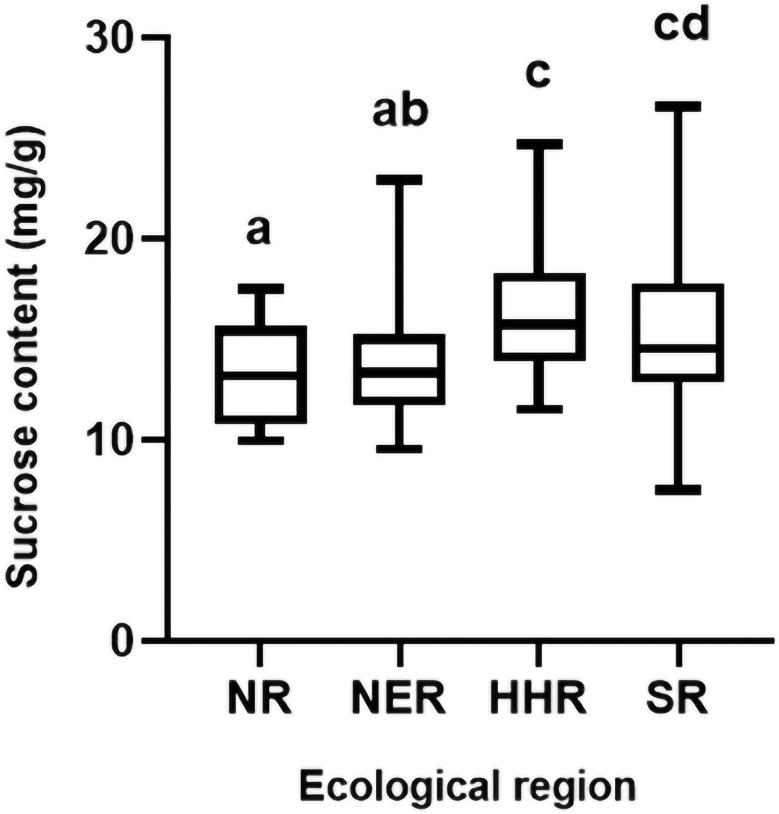
The differences in sucrose content during the R6 stage of genotypes from different ecological regions were compared based on the mean values of 2021NJ and 2022DT. Wide variations in sucrose content were observed in the four ecological regions, and the germplasm from SR exhibited the widest range of variation. Interestingly, the sucrose content of germplasm derived from HHR and SR was higher than that from NR and NER (Fig. 2).
Fig. 2.
Distribution of sucrose content among different ecological regions. The Y-axis represents the sucrose content, and the X-axis represents different ecological regions. Different lowercase letters indicate statistical differences at p < 0.05
Correlation analysis between soluble sugars
The relationship between sucrose content and other soluble sugar (fructose and glucose) was also studied. The analysis revealed significant positive relationships between fructose and glucose in all environments (Table 2). In 2021NJ and Mean, sucrose content was positively correlated with glucose content (p < 0.01) (Table 2). In 2021NJ, sucrose content was positively correlated with fructose content (r = 0.33, p < 0.01), however, it was negatively correlated in 2022DT (r = -0.26, p < 0.01) (Table 2).
Table 2.
Pearson’s correlation between sucrose, fructose, and glucose content
| Environment | Trait | Fructose | Glucose |
|---|---|---|---|
| 2021NJ | Sucrose | 0.33** | 0.30** |
| Fructose | 0.55** | ||
| 2022DT | Sucrose | -0.26** | -0.02 |
| Fructose | 0.20* | ||
| Mean | Sucrose | -0.01 | 0.24** |
| Fructose | 0.65** |
* Significant at p < 0.05. ** Significant at p < 0.01
GWAS of seed sucrose content
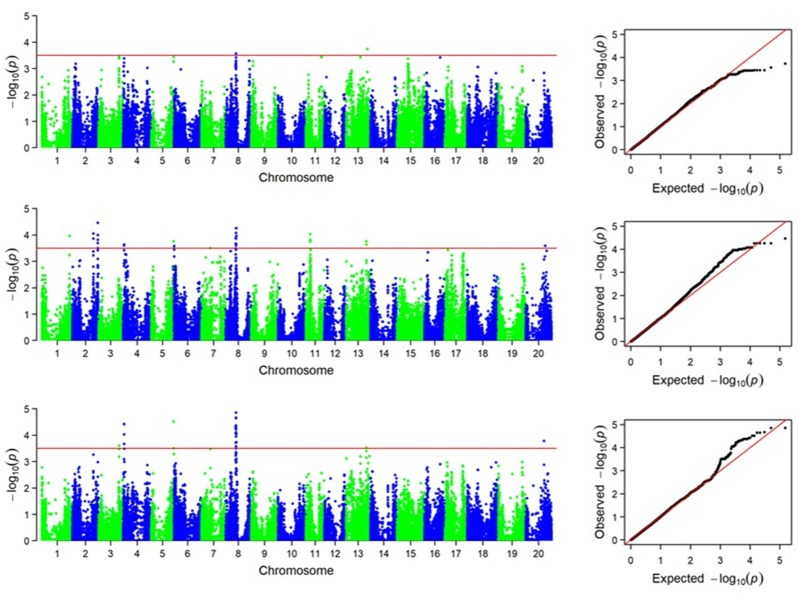
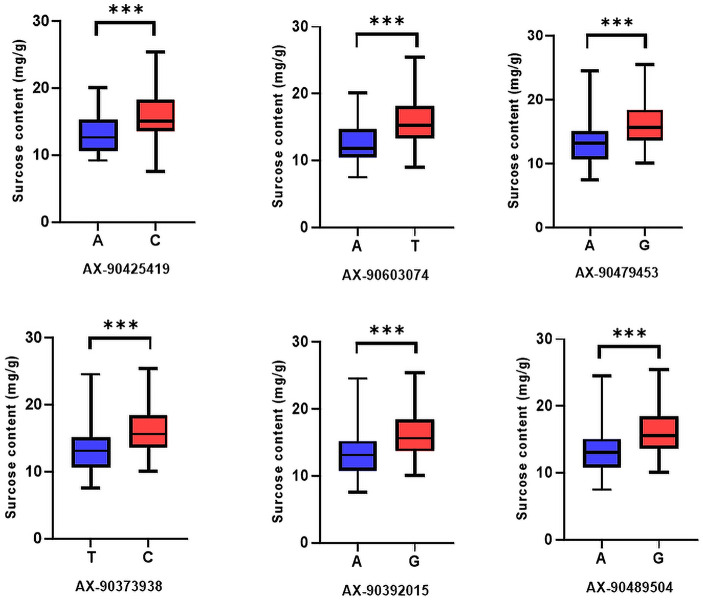
Sucrose content of 2021NJ, 2022DT and the mean value (Mean) of two years of data were used for GWAS analysis. Only two SNP markers associated with sucrose content were identified in the 2021NJ environment, and a larger number of SNPs were identified in the other environments (Fig. 3). Interestingly, similarly positioned SNPs were identified on chromosome 8 in all environments (Table 3). Based on the LD decay distance, the candidate interval was identified as 19,496,314–19,698,413 bp on chromosome 8 (Table 3). In order to verify the validity of the candidate interval, the effectiveness of all SNPs within the interval was counted. There were six SNPs within the candidate interval, and there were significant differences in sucrose content of vegetable soybean carrying different alleles (Fig. 4). Therefore, this candidate interval was critical for the sucrose content of vegetable soybeans.
Fig. 3.
Manhattan plot and Q-Q plot of GWAS of sucrose content in vegetable soybean. A: 2021NJ. B: 2022DT. C: Mean
Table 3.
Significance SNP markers and candidate intervals associated with sucrose content on chromosome 8
| Environment | Chromosome | Marker | Position(bp) | Allele | -log10(P) | Candidate interval (bp) |
|---|---|---|---|---|---|---|
| 2021NJ | 8 | AX-90,425,419 | 19,615,384 | C/A | 3.56 | 19,496,314–19,698,413 |
| 2022DT | AX-90,504,763 | 19,356,110 | A/T | 3.88 | ||
| AX-90,315,111 | 19,410,649 | T/C | 3.48 | |||
| AX-90,458,454 | 19,425,573 | G/A | 3.48 | |||
| AX-90,363,074 | 19,515,808 | A/T | 4.07 | |||
| AX-90,479,453 | 19,543,430 | G/A | 3.62 | |||
| AX-90,373,938 | 19,579,343 | C/T | 3.62 | |||
| AX-90,460,930 | 19,853,041 | G/A | 3.67 | |||
| AX-90,470,529 | 19,973,842 | T/C | 4.25 | |||
| AX-90,373,249 | 20,069,462 | T/C | 4.25 | |||
| AX-90,422,251 | 20,212,806 | G/T | 3.96 | |||
| Mean | AX-90,315,111 | 19,410,649 | T/C | 4.33 | ||
| AX-90,458,454 | 19,425,573 | G/A | 4.33 | |||
| AX-90,411,790 | 19,492,544 | C/T | 4.11 | |||
| AX-90,363,074 | 19,515,808 | A/T | 4.86 | |||
| AX-90,373,938 | 19,579,343 | C/T | 4.37 | |||
| AX-90,425,419 | 19,615,384 | C/A | 3.95 | |||
| AX-90,489,504 | 19,677,651 | G/A | 4.22 | |||
| AX-90,460,930 | 19,853,041 | G/A | 4.67 | |||
| AX-90,373,249 | 20,069,462 | T/C | 4.05 | |||
| AX-90,436,132 | 20,130,437 | G/A | 3.73 | |||
| AX-90,422,251 | 20,212,806 | G/T | 3.73 | |||
| AX-90,425,640 | 20,295,654 | A/C | 4.05 |
Fig. 4.
Comparison of the sucrose content in germplasm with different alleles of SNPs in candidate interval by boxplot. Blue represents low sugar content. Red represents high sugar content. ***, p < 0.001
Prediction of candidate genes
The soybean public database (Soybase) and soybean reference genome (Williams 82) annotation (Wm82.a2.v1) were used to identify candidate genes for sucrose content of vegetable soybean. There were nine genes in the candidate interval, of which genes Glyma.08g234200 and Glyma.08g234400 encode the Ca2+-dependent phospholipid-binding protein (Table 4). We compared the expression of these nine genes in nine tissues of 26 soybean varieties (3 wild-types, 9 landrace-types, and 14 cultivated-types) and found that gene Glyma.08g234100 was specifically expressed in stages G (4 weeks stage pod and seed) and H (6 weeks stage seed) (Table S2) [28]. Therefore, we predicted that gene Glyma.08g234100 is the most important candidate gene affecting the sucrose content of vegetable soybean.
Table 4.
Candidate genes related to sucrose content
| Gene ID | Physical Position (bp) | Annotation |
|---|---|---|
| Glyma.08g233900 | Chr08:19514763.19525357 | Pentatricopeptide repeat (PPR) superfamily protein |
| Glyma.08g234000 | Chr08:19536056.19537966 | RNA recognition motif |
| Glyma.08g234100 | Chr08:19551922.19552439 | 2OG-Fe II oxygenase family protein |
| Glyma.08g234200 | Chr08:19572589.19580249 | Ca2+-dependent phospholipid-binding protein (Copine) family |
| Glyma.08g234300 | Chr08:19604791.19605475 | Pathogenesis-related protein Bet v I family |
| Glyma.08g234400 | Chr08:19607852.19617474 | Ca2+-dependent phospholipid-binding protein (Copine) family |
| Glyma.08g234500 | Chr08:19639161.19648401 | Nuclear pore anchor |
| Glyma.08g234600 | Chr08:19676789.19683763 | LEM3 (ligand-effect modulator 3) family protein |
| Glyma.08g234700 | Chr08:19684178.19708129 | S-adenosyl-L-methionine-dependent methyltransferases superfamily protein |
Discussion
For consumption, soybeans can be divided into two types: grain soybeans and vegetable soybeans [29]. However, from an evolutionary perspective, soybeans can be divided into wild-type, landrace-type, and cultivated-type [30]. GWAS requires the selection of genotypes with rich genetic resources. The seeds of wild soybeans are small and hard, and fresh seeds are not suitable for consumption. In addition, most cultivated soybeans are for grain use, and during the selection process, they have lost their dominant traits in the fresh consumption period [31]. Therefore, landraces with abundant genetic resources were selected for this study. In the previous study, Li et al. conducted GWAS analysis on the yield traits of vegetable soybean using this landrace collection [22].
In the present study, soluble sugars (fructose, glucose, and sucrose) of R6 stage soybeans were determined. Among them, sucrose had the highest percentage, followed by fructose and glucose [11]. For any of the sugars, there was a tendency for a normal distribution, which was consistent with the previous conclusions [24]. Furthermore, we found that sucrose content was higher in the HHR and SR than in the NR and NER. Qi et al. (2022) determined the content of soluble sugars in 1164 soybeans from China and found that the sucrose content was higher in the SR than in the NR [32]. The SR has long been an important area for vegetable soybean cultivation, and genotypes from this region may have undergone some degree of directional selection, in addition to the lower latitudes and higher temperatures in the southern region, which favor the accumulation of sugar substances [32, 33].
Strong and positive correlations were reported previously between fructose and glucose (r = 0.9887), while negative correlations were found between fructose and sucrose (r = − 0.68), and glucose and sucrose (r = − 0.68) [24]. In another study, Wang et al. (2014) obtained similar results [15]. The above researches all focused on the soluble sugar of mature soybean. However, in the present study, the correlation between sucrose and other sugars was weak and unstable. This may be due to different seed developmental status of soybeans. During the R6 to R8 stage of soybeans, the soluble sugar content in soybean seed changes. Surprisingly, this means that an increase in sucrose content does not lead to a significant decrease in the content of other soluble sugars. This finding provides theoretical support for the idea of simultaneously increasing the total soluble sugar content.
The significant association threshold between SNP markers and target traits (log10(P) = 3.5) was used in this study. No SNP loci were detected at the strict threshold (log10(P) = 4.91). This may be due to the fact that there are many genes responsible for the sucrose content of vegetable soybean. Despite the low threshold used in this study, clear association signals were still valid [16]. In all three environments, an interval of approximately 200,000 bp on chromosome 8 was repeatedly identified. In the previous study, this interval has been identified as correlating with soybean serine and proline [34]. Panthee et al. (2004) found that this interval correlated with soybean R6 stage N content [35]. This interval was also associated with the 100-seed fresh weight of vegetable soybean [36]. Within the candidate interval, Glyma.08g234100 encoding the 2OG-Fe (II) oxygenase superfamily protein is specifically expressed in developing pods and seeds (Table S2). In peanut, this family of genes played a key role in pod development [37]. Therefore, Glyma.08g234100 was identified as a candidate gene for this study, but its function in vegetable soybeans needs to be further investigated.
Currently, there is limited research on the flavor quality of vegetable soybeans. However, with the increasing emphasis on vegetable soybeans, sucrose content is becoming an important trait. After thousands of years of directional selection for oil and protein, the variation in sucrose content in vegetable soybean may have been reduced. The identified QTL will lay the foundation for the development of high-quality vegetable soybean.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We extend the thanks to Dr. Lijuan Qiu (Chinese Academy of Agricultural Sciences) for germplasm provision.
Abbreviations
- GWAS Genome
wide association study
- HHR
Huanghuai region
- SR
Southern region
- NER
Northeast region
- NR
Northern region
- SNP
Single nucleotide polymorphism
- RIL
Recombinant inbred lines
- QTL
Quantitative-trait loci
- NJ
Nanjing
- DT
Dangtu
- K
Kinship
- ANOVA
Analysis of variance
- MAF
Minor allelic frequency
- LD
Linkage disequilibrium
- BLINK
Bayesian-information and Linkage disequilibrium Iteratively Nested Keyway
- UPLC
Ultra performance liquid chromatography
- SD
Standard deviation
- CV
Coefficient of variation
Author contributions
P. W. W., J. M. Z., H. X., and N. G. conceived and designed research. P. W. W., D. D. L., and D. Q. N. conducted experiments. C. C. X. and X. C. provided experimental fields. P. W. W, S. G. and Y. P. H. analyzed data. P. W. W. drafted the manuscript, P. W. W., J. M. Z., H. X., and N. G. reviewed and revised the manuscript. All authors read and approved the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2021YFD1201605), the Zhongshan Biological Breeding Laboratory (ZSBBL-KY2023-03), the “JBGS” Project of Seed Industry Revitalization in Jiangsu Province (JBGS〔2021〕059), China Agriculture Research System of MOF and MARA (CARS-04), the earmarked fund for Jiangsu Agricultural Industry Technology System (JATS[2023]442), the Fundamental Research Funds for the Central Universities (XUEKEN2023022), Jiangsu Collaborative Innovation Center for Modern Crop Production and Cyrus Tang Innovation Center for Seed Industry.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jinming Zhao, Email: jmz3000@126.com.
Han Xing, Email: hanx@njau.edu.cn.
Na Guo, Email: guona@njau.edu.cn.
References
- 1.Fehr W, Caviness C. Stages of soybean development. Holland: Extension and Experiment Station; 1977. [Google Scholar]
- 2.Zhang Q, Li Y, Chin KL, Qi Y. Vegetable soybean: seed composition and production research. Italian J Agron. 2017;12(3). 10.4081/ija.2017.872.
- 3.Nair RM, Boddepalli VN, Yan MR, Kumar V, Gill B, Pan RS, Somta P. (2023). Global status of vegetable soybean. Plants, 12(3), 609. 10.3390/plants12030609 [DOI] [PMC free article] [PubMed]
- 4.Zeipiņa S, Vågen IM, Lepse L. (2022). Possibility of vegetable soybean cultivation in North Europe. Horticulturae, 8(7), 593. 10.3390/horticulturae8070593
- 5.Young G, Mebrahtu T, Johnson J. Acceptability of green soybeans as a vegetable entity. Plant Foods Hum Nutr. 2000;55:323–33. 10.1023/A:1008164925103. [DOI] [PubMed] [Google Scholar]
- 6.Hu R, Lin G. Dynamic metabolic profiling in vegetable soybean seed development. Emirates J Food Agric. 2018;90–8. 10.9755/ejfa.2018.v30.i1.1594.
- 7.Carneiro R, Adie K, Yu D, Beverly M, Neill C, Zhang B, Duncan S. Understanding the role of overall appearance and Color in consumers’ acceptability of Edamame. Front Sustainable Food Syst. 2022;6:738453. 10.3389/fsufs.2022.738453. [Google Scholar]
- 8.Aggarwal A, Rehm CD, Monsivais P, Drewnowski A. Importance of taste, nutrition, cost and convenience in relation to diet quality: evidence of nutrition resilience among US adults using National Health and Nutrition Examination Survey (NHANES) 2007–2010. Prev Med. 2016;90:184–92. 10.1016/j.ypmed.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Li Y, Tu B, Wang X, Tian B, Zhang Q, Liu X. Seed nutritional quality comparison of vegetable soybean genotypes at fresh pod and mature stage. Emirates J Food Agric. 2019;405–14. 10.9755/ejfa.2019.v31.i6.1960.
- 10.Spendrup S, Hovmalm HP. Consumer attitudes and beliefs towards plant-based food in different degrees of processing–the case of Sweden. Food Qual Prefer. 2022;102:104673. 10.1016/j.foodqual.2022.104673. [Google Scholar]
- 11.Agyenim-Boateng, K. G., Zhang, S., Zhang, S., Khattak, A. N., Shaibu, A., Abdelghany,A. M., … Sun, J. (2023). The nutritional composition of the vegetable soybean (Maodou)and its potential in combatting malnutrition.Frontiers in Nutrition,9, 1034115. 10.3389/fnut.2022.1034115. [DOI] [PMC free article] [PubMed]
- 12.Li YS, Du M, Zhang QY, Wang GH, Hashemi M, Liu XB. Greater differences exist in seed protein, oil, total soluble sugar and sucrose content of vegetable soybean genotypes [‘Glycine max‘(L.) Merrill] in Northeast China. Aust J Crop Sci. 2012;6(12):1681–6. 10.13140/2.1.2841.2163. [Google Scholar]
- 13.Song J, Wu G, Liu C, Li D. Effect of exogenous spermine on chilling injury and antioxidant defense system of immature vegetable soybean during cold storage. J Food Sci Technol. 2018;55:4297–303. 10.1007/s13197-018-3372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maughan PJ, Maroof MS, Buss GR. Identification of quantitative trait loci controlling sucrose content in soybean (Glycine max). Mol Breeding. 2000;6:105–11. 10.1023/A:1009628614988. [Google Scholar]
- 15.Wang Y, Chen P, Zhang B. Quantitative trait loci analysis of soluble sugar contents in soybean. Plant Breeding. 2014;133(4):493–8. 10.1111/pbr.12178. [Google Scholar]
- 16.Fang, C., Ma, Y., Wu, S., Liu, Z., Wang, Z., Yang, R., … Tian, Z. (2017). Genome-wide association studies dissect the genetic networks underlying agronomical traits in soybean.Genome Biology,18(1), 1–14. 10.1186/s13059-017-1289-9. [DOI] [PMC free article] [PubMed]
- 17.Ficht A, Bruce R, Torkamaneh D, Grainger CM, Eskandari M, Rajcan I. Genetic analysis of sucrose concentration in soybean seeds using a historical soybean genomic panel. Theor Appl Genet. 2022;135(4):1375–83. 10.1007/s00122-022-04040-z. [DOI] [PubMed] [Google Scholar]
- 18.Salari MW, Ongom PO, Thapa R, Nguyen HT, Vuong TD, Rainey KM. (2021). Mapping QTL controlling soybean seed sucrose and oligosaccharides in a single family of soybean nested association map (SoyNAM) population. Plant breeding, 140(1), 110–22. 10.1111/pbr.12883
- 19.Sui, M., Wang, Y., Bao, Y., Wang, X., Li, R., Lv, Y., … Han, Y. (2020). Genome-wide association analysis of sucrose concentration in soybean (Glycine max L.) seed based on high‐throughput sequencing.The Plant Genome,13(3), e20059. 10.1002/tpg2.20059. [DOI] [PubMed]
- 20.Xu, W., Liu, H., Li, S., Zhang, W., Wang, Q., Zhang, H., … Chen, H. (2022). GWAS and identification of candidate genes associated with seed soluble sugar content in vegetable soybean.Agronomy,12(6), 1470. 10.3390/agronomy12061470.
- 21.Lu W, Sui M, Zhao X, Jia H, Han D, Yan X, Han Y. Genome-wide identification of candidate genes underlying soluble sugar content in vegetable soybean (Glycine max L.) via association and expression analysis. Front Plant Sci. 2022;13:930639. 10.3389/fpls.2022.930639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, X., Zhang, X., Zhu, L., Bu, Y., Wang, X., Zhang, X., … Xing, H. (2019). Genome-wide association study of four yield-related traits at the R6 stage in soybean.BMC genetics,20(1), 1–15. 10.1186/s12863-019-0737-9. [DOI] [PMC free article] [PubMed]
- 23.Qiu L, Li Y, Guan R, Liu Z, Wang L, Chang R. Establishment, Representative Testing and Research Progress of Soybean Core Collection and Mini Core Collection. Acta Agron Sinica. 2009;35(4):571–9. 10.3724/SP.J.1006.2009.00571. [Google Scholar]
- 24.Hou A, Chen P, Alloatti J, Li D, Mozzoni L, Zhang B, Shi A. Genetic variability of seed sugar content in worldwide soybean germplasm collections. Crop Sci. 2009;49(3):903–12. 10.2135/cropsci2008.05.0256. [Google Scholar]
- 25.Roberts A, McMillan L, Wang W, Parker J, Rusyn I, Threadgill D. Inferring missing genotypes in large SNP panels using fast nearest-neighbor searches over sliding windows. Bioinformatics. 2007;23(13):i401–7. 10.1093/bioinformatics/btm220. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Zhang Z. GAPIT version 3: boosting power and accuracy for genomic association and prediction. Genomics Proteom Bioinf. 2021;19(4):629–40. 10.1016/j.gpb.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, X., Ding, W., Xue, D., Li, X., Zhou, Y., Shen, J., … Zhao, J. (2021). Genome-wide association studies of plant architecture-related traits and 100-seed weight in soybean landraces. BMC Genomic Data, 22, 1–14. 10.1186/s12863-021-00964-5. [DOI] [PMC free article] [PubMed]
- 28.Yang, Z., Luo, C., Pei, X., Wang, S., Huang, Y., Li, J., … Fang, C. (2024). SoyMD:a platform combining multi-omics data with various tools for soybean research and breeding. Nucleic Acids Research, 52(D1), D1639-D1650. 10.1093/nar/gkad786. [DOI] [PMC free article] [PubMed]
- 29.Jiang, G. L., Chen, P., Zhang, J., Florez-Palacios, L., Zeng, A., Wang, X., … Berry,H. (2018). Genetic analysis of sugar composition and its relationship with protein,oil, and fiber in soybean.Crop Science,58(6), 2413–2421. 10.2135/cropsci2018.03.0173.
- 30.Wen Z, Zhao T, Ding Y, Gai J. Genetic diversity, geographic differentiation and evolutionary relationship among ecotypes of Glycine max and G. Soja in China. Chin Sci Bull. 2009;54(23):4393–403. 10.1007/s11434-009-0696-z. [Google Scholar]
- 31.Liu, N., Niu, Y., Zhang, G., Feng, Z., Bo, Y., Lian, J., … Gong, Y. (2022). Genome sequencing and population resequencing provide insights into the genetic basis of domestication and diversity of vegetable soybean.Horticulture Research,9, uhab052. 10.1093/hr/uhab052. [DOI] [PMC free article] [PubMed]
- 32.Qi, J., Zhang, S., Azam, M., Shaibu, A. S., Abdelghany, A. M., Feng, Y., … Sun, J.(2022). Profiling seed soluble sugar compositions in 1164 Chinese soybean accessions from major growing ecoregions. The Crop Journal, 10(6), 1825–1831. 10.1016/j.cj.2022.04.015.
- 33.Zhang QY, Gao QL, Herbert SJ, Li YS, Hashemi AM. Influence of sowing date on phenological stages, seed growth and marketable yield of four vegetable soybean cultivars in North-eastern USA. Afr J Agric Res. 2010;5(18):2556–62. [Google Scholar]
- 34.Panthee DR, Pantalone VR, Saxton AM, West DR, Sams CE. Genomic regions associated with amino acid composition in soybean. Mol Breeding. 2006;17:79–89. 10.1007/s11032-005-2519-5. [Google Scholar]
- 35.Panthee DR, Pantalone VR, Sams CE, Saxton AM, West DR, Rayford WE. Genomic regions governing soybean seed nitrogen accumulation. J Am Oil Chem Soc. 2004;81:77–81. 10.1007/s11746-004-0860-4. [Google Scholar]
- 36.Hou, J., Wang, C., Hong, X., Zhao, J., Xue, C., Guo, N., … Xing, H. (2011). Association analysis of vegetable soybean quality traits with SSR markers. Plant Breeding, 130(4),444–449. 10.1111/j.1439-0523.2011.01852.x.
- 37.Sun, J., Zhang, X., Fu, C., Ahmad, N., Zhao, C., Hou, L., … Zhao, S. (2023). Genome-wide identification and expression analysis of GA20ox and GA3ox genes during pod development in peanut. PeerJ, 11, e16279. 10.7717/peerj.16279. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.