Abstract
Background
Coronary artery bypass grafting (CABG) remains the preferred treatment for complex multi-vessel coronary artery disease, offering substantial long-term benefits. Non-cardiac comorbidities such as frailty may significantly affect the outcomes of this procedure. However, the exact impact of frailty on CABG outcomes remains unclear, particularly given its exclusion from many pivotal revascularization trials. This systematic review and meta-analysis aimed to consolidate existing data to evaluate the impact of frailty on short- and long-term outcomes following CABG.
Methods
Searches across PubMed, Cochrane Library, Embase, and Scopus were done to identify studies that were published up to March 31, 2024, had detailed preoperative frailty assessments and compared frail versus non-frail adult patients undergoing CABG. Primary outcomes were all-cause mortality and major adverse cardiac events within one year. Secondary outcomes included hospital readmission rates and length of stay. A random-effects model was used to account for heterogeneity. Results were reported as odds ratios (OR) or mean differences (MD) with 95% confidence intervals (CI).
Results
Our meta-analysis, involving data from 14 studies, revealed a significant increase in both 30-day (OR 2.52; 95% CI: 2.07 to 3.07) and 1-year mortality (OR 2.58; 95% CI: 1.49 to 4.45) among frail patients. The risk of acute cardiac and cerebrovascular complications was comparable in all patients (OR 1.03; 95% CI: 0.89 to 1.19). However, frailty was associated with a significant increase in the risk of acute kidney injury (OR 2.31; 95% CI: 1.26 to 4.23). Frail patients were more likely to have longer hospital stays and higher readmission rates compared to their non-frail counterparts.
Conclusion
Our study confirms the critical impact of frailty on mortality and morbidity in CABG patients and advocates for the integration of frailty assessments into the preoperative evaluation process. Addressing frailty can lead to more individualized patient care and better outcomes, urging a paradigm shift towards comprehensive, patient-centric management in cardiac surgery.
Prospero register
CRD42024521327.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12893-024-02728-1.
Keywords: Frailty, CABG, Mortality, Cardiac surgery, Elderly, Systematic review, Meta-analysis
Introduction
Coronary artery bypass grafting (CABG) has long been established as the superior intervention for patients with complex multi-vessel coronary artery disease or distal/bifurcation left main stenosis, offering more durable long-term results compared to percutaneous coronary intervention (PCI) [1, 2]. CABG is associated with low mortality rates of 1–2% even among high-risk patients [3, 4]. However, the mid-term survival and quality of life following CABG increasingly hinge on non-cardiac comorbidities rather than the complexity of coronary lesions [5]. This shift comes at a time of global increase in life expectancy, as older CABG patients have a higher prevalence of risk factors such as diabetes mellitus, chronic kidney disease, chronic obstructive pulmonary disease, and notably, geriatric syndromes like frailty [6, 7].
The recognition of these changes has influenced recent coronary revascularization guidelines, which now recommend a heart-team approach when selecting treatments for older patients with stable coronary artery disease [8]. Of all non-cardiac comorbidities, frailty presents a unique set of challenges. It is a complex syndrome characterized by diminished strength, endurance, and physiological function that increases patient morbidity and mortality [9]. However, current practice recommendations are mostly based on recent revascularization trials which often exclude frail patients. This exclusion raises significant concerns regarding the applicability of trial results to a substantial subset of the population undergoing CABG. Prior evidence indicates that frail patients face increased perioperative mortality following cardiac surgery. Nevertheless, no meta-analysis to date has comprehensively examined the effect of frailty on CABG outcomes [10]. This gap in knowledge impacts the ability of clinicians to accurately assess risk and tailor treatment strategies for this vulnerable patient group.
Our systematic review and meta-analysis aim to consolidate existing data to assess the impact of frailty on outcomes following CABG.
Methods
Eligibility criteria
This review included studies involving adult patients (aged 18 years and older) undergoing CABG. Studies were selected if they provided explicit information on the assessment of frailty among participants before undergoing CABG. There were no restrictions regarding the patient’s gender, ethnicity, or geographic location to ensure the broad applicability of the findings.
The exposure group included patients classified as frail based on predefined criteria using validated frailty scales such as Fried Frailty Index, Tilburg Frailty Indicator (TFI), or other similar assessments documented in the studies. The comparison group included patients undergoing CABG who were determined to be non-frail, using the same frailty assessments as the exposure group to maintain consistency across comparisons.
Outcomes
The primary outcomes of interest were all-cause mortality within 30 days and one-year post-surgery, and major adverse cardiac events (MACE), which included myocardial infarction, stroke, and re-hospitalization due to cardiac complications within one year of surgery. Secondary outcomes assessed in the study were hospital readmission rates within 30 days and one year, the total number of days patients spent in the hospital post-CABG, and postoperative complications such as surgical site infections, renal failure, or prolonged ventilation.
Study design
The review considered randomized controlled trials (RCTs), prospective and retrospective cohort studies, and other observational studies. Exclusion criteria encompassed case reports, case series, editorials, commentaries, and studies not published in peer-reviewed journals. Studies published only as abstracts or presentations at conferences were also excluded due to the potential lack of detailed data and peer review. All studies must have been published in English, with the literature search covering publications from the inception of the databases until March 2024.
Information sources
A comprehensive and systematic search strategy was employed to identify relevant studies from PubMed, Cochrane Library, Embase, and Scopus databases. Reference lists of included studies and relevant systematic reviews were manually searched to identify additional studies not captured in the electronic searches. Contact was made with authors for additional data or clarification of study methodologies when necessary.
The last search of each database was conducted on March 31, 2024. The search strategy was designed as follows:
((“Coronary Artery Bypass Grafting” [Title/Abstract] OR CABG [Title/Abstract] OR “heart bypass surgery” [Title/Abstract]) AND (Frailty [MeSH Terms] OR frail [Title/Abstract] OR “frail patients” [Title/Abstract] OR vulnerability [Title/Abstract]) AND (Mortality [MeSH Terms] OR “major adverse cardiac events” [Title/Abstract] OR MACE [Title/Abstract] OR “hospital readmission” [Title/Abstract] OR “postoperative complications” [Title/Abstract] OR “length of hospital stay” [Title/Abstract])) Filters applied for language: English, publication date: since database inception to March 31, 2024, and study types: RCTs, cohort studies, observational studies.
Study selection process
Two reviewers (SChen & SZ) independently conducted an initial assessment of titles and abstracts of the identified studies. A thorough review of full texts of relevant studies was then performed by the same reviewers (SChen & SZ). Any inconsistencies in reviewer opinions regarding study inclusion were resolved by consulting a third, experienced reviewer (SCai). To enhance efficiency and organization during the screening phase, online tool Rayyan was used. The duplicates were also identified by the Rayyan, but the final decision to delete duplicate reports was done by the authors.
Data extraction
Two independent reviewers (SZ & SCai) carried out data extraction using a specially designed form, which was tested on a subset of studies to ensure its effectiveness in capturing all pertinent data points clearly and concisely. A third reviewers (HW) then cross-checked the data entries to correct discrepancies and confirm data integrity. For each study, the form recorded author details, publication year, location, and study design, the total number of subjects, their age and gender distributions, and frailty assessment methods.
The primary outcomes included all-cause mortality and MACE within 30 days and one year after the surgery. Secondary outcomes included hospital readmission rates, duration of hospital stays, and a detailed account of postoperative complications. For each outcome, data were collected across all compatible measures, time points, and analyses provided in the studies. In cases where multiple time points or analyses were reported, priority was given to the most clinically relevant and commonly reported time points, such as immediate postoperative outcomes and follow-ups at one year, and 5 years. For data that were unclear or missing from reports, the study authors were contacted to provide further details or clarification to ensure the completeness and accuracy of the data set. To address potential duplication, included studies were carefully reviewed to identify overlapping datasets. Any studies with overlapping data were excluded from pooled analyses to ensure accurate effect size estimation. Such studies were retained only for narrative synthesis if exclusivity of the data could not be verified.
Study risk of bias assessment
The Newcastle Ottawa Scale (NOS) for cohort studies was used to evaluate the risk of bias in each included study [11]. This scale assesses the quality of prospective cohorts through a detailed examination of three critical domains: the method of selection of study groups, the degree of comparability between these groups, and the accuracy of outcome or exposure ascertainment. Studies are awarded up to nine stars across these domains, with up to four stars for selection, two for comparability, and three for outcome assessment. A study scoring 7 to 9 stars is typically considered to have a low risk of bias, indicating high methodological quality. Studies with the score of 4 to 6 are considered as having a moderate risk of bias, suggesting some methodological concerns that could affect the results, while a score below 4 stars denotes a high risk of bias, pointing to significant issues that could substantially skew the outcomes. Two independent reviewers conducted the bias assessment for each study and any disagreements were resolved through discussion or consultation with a senior reviewer to reach a consensus.
Statistical analysis
Meta-analyses were conducted using STATA version 14.2 software [12]. A random-effects model was used due to the anticipated methodological and clinical heterogeneity. The results were presented as pooled odds ratios (OR) with 95% confidence intervals (CI) for dichotomous outcomes such as mortality, readmissions, adverse cardiac and cerebrovascular events, and mean differences (MD) with 95% CI for continuous outcomes like the length of hospital stay.
To determine which studies were eligible for each synthesis, we tabulated the characteristics of the interventions from each study and compared them against the planned groups for each synthesis as outlined in our protocol. We employed forest plots to visually display the meta-analysis results, where individual study effects were represented by squares sized according to the study’s weight, and their 95% CIs were depicted by horizontal lines. The overall effect size and its CI were indicated by a diamond shape at the bottom of each plot. The I² statistic, along with the chi-squared test, was used to identify the presence and extent of statistical heterogeneity among the studies.
Given that the number of studies included in each analysis was less than ten, subgroup analyses were not performed, and traditional methods of assessing publication bias like funnel plots were not applicable. Instead, we used the Doi plot and Luis Furuya-Kanamori (LFK) index as alternative methods to evaluate the potential presence of publication bias [13]. These tools provide both a visual and statistical means to detect asymmetry in the meta-analysis results, offering valuable insights even with a smaller sample of studies.
Results
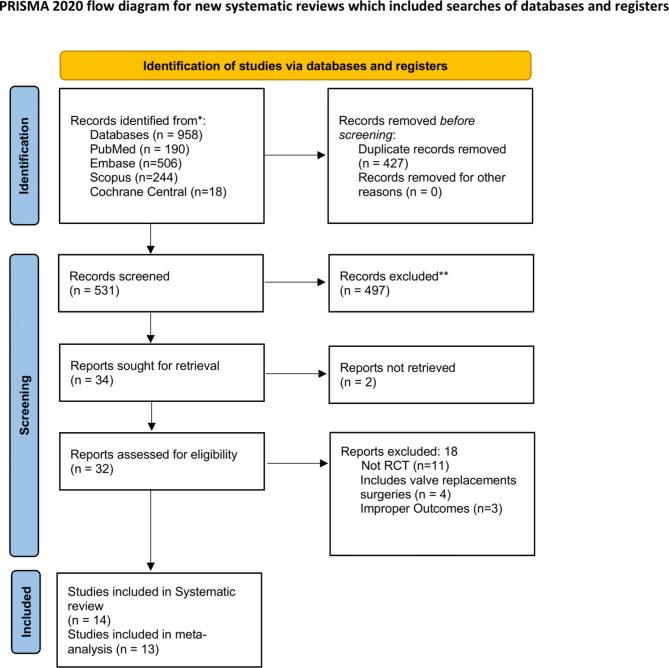
The initial database searches yielded a total of 958 records across various databases. Specifically, we retrieved 190 citations from PubMed, 506 from Embase, 244 from Scopus, and 18 from Cochrane Central. Of them, 427 duplicate records were removed, and 531 citations underwent primary screening, and 497 records were further excluded due to irrelevance or failure to meet preliminary criteria. Full text of 32 articles were retrieved for secondary screening. Of these, 18 reports were excluded for reasons such as not being randomized controlled trials (n = 11), intervention included valve replacements surgeries (n = 4), or reporting irrelevant outcomes (n = 3). Ultimately, 14 studies met al.l the inclusion criteria for the systematic review, and 13 were deemed suitable for the inclusion in meta-analysis (Fig. 1) [14–23].
Fig. 1.
PRISMA flowchart
Characteristics of included studies
The characteristics of the included studies are shown in Table 1. The included studies spanned a diverse range of cohorts, methodologies, and geographical locations. Studies varied from retrospective to prospective cohort designs and included a considerable number of participants ranging from a few hundred to over two million. Most studies focused on elderly populations typical of CABG patients, with mean ages generally in the mid-sixties to early seventies. Gender distribution across the studies predominantly skewed towards males, which is reflective of the higher incidence of coronary artery disease in this demographic. Body mass index (BMI) was reported in several studies, with averages suggesting that most patients were in the overweight category. Comorbid conditions such as diabetes mellitus, hypertension, and dyslipidaemia were commonly reported, underscoring the complex medical profiles often seen in CABG patients. Other significant comorbidities included heart failure, cerebrovascular disease, and atrial fibrillation, which are critical considerations for surgical outcomes and postoperative recovery.
Table 1.
Characteristics of the included studies (N = 14)
| Author and year | Study design | Country | Study participants | Sample size | Mean age in years | Gender distribution | BMI | Comorbidity - DM | Comorbidity - HTN | Comorbidity - Dyslipidemia | Heart failure / LVEF@ | Cerebrovascular disease / stroke | Atrial Fibrillation | Charlson index score | Elixhauser index score | STS / EURO score | Frailty Assessment Tool | Outcomes Assessed | NOS score (Risk of bias) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chan Hyeong Kim 2022 [15] | Retrospective cohort | South Korea | Elderly patients who underwent primary isolated CABG procedure | 508 | 67.3 ± 9.7 |
M = 78% F = 22% |
24.7 ± 3.6 | 297 (58.5%) | 337 (66.3%) | 257 (50.6%) | 45 (8.9%) | 59 (11.6%) | 23 (4.5%) | NR | NR | Median 1.3 (0.9–2.3) | Patients’ laboratory data and vital signs | Early mortality rate, Post operative complications, 1- and 3-year survival rates, 1- and 3-year cumulative incidence rates of MACCE | 6 (moderate risk) |
| Diem T. T. Tran 2018 [16] | Retrospective Cohort Study | Canada | ≥ 40 years undergoing CABG | 40 083 | 65.84 ± 9.85 |
M = 79.6% F = 20.4% |
Frail – 3179 (36.1%) Not frail – 8966 (28.7%) |
Frail – 4909 (55.8% ) Not frail – 13,999 (44.8%) |
Frail − 8045 (91.4%) Not frail – 26,984 (86.3%) |
NR |
Frail ≥ 50–19,746 (63.1) 35–49–7896 (25.2) 20–34–3084 (9.9) < 20–554 (1.8) Not frail ≥ 50–5237 (59.5) 35–49–2348 (26.7) 20–34–1010 (11.5) < 20–208(2.4) |
NR |
Frail – 856 (9.7%) Not frail − 1899 (6.1%) |
NR | NR | NR | Johns Hopkins Adjusted Clinical Groups (ACG) indicator | Mortality rates | 4 (moderate risk) |
| Daniel Reichart 2018 [17] | Prospective Cohort study | 16 European centres of cardiac surgery - Finland, France, Italy, Germany, Sweden and the UK. | Elderly patients aged 65 years and above undergoing isolated CABG | 6156 | 66.4 ± 9.6 |
M = 83% F = 17% |
Class 1–2–27.6 ± 4.1 Class 3–4–27.5 ± 4.2 Class 5–7–27.7 ± 4.2 |
Class 1–2–740 (30.7%) Class 3–4–1170 (33.0%) Class 5–7–77 (38.5%) |
NR | NR |
Class 1–2–619 (25.7%) Class 3–4–1146 (32.4%) Class 5–7–86 (43.0%) |
Class 1–2–94 (3.9%) Class 3–4–242 (6.8%) Class 5–7–19 (9.5%) |
Class 1–2–146 (6.1%) Class 3–4–337 (9.5%) Class 5–7 − 33 (16.5%) |
NR | NR |
Class 1–2–2.3 ± 3.1 Class 3–4–3.2 ± 4.5 Class 5–7–7.4 ± 9.4 |
Clinical Frailty Scale (CFS) | hospital/30-day death, Length of stay in ICU, postoperative atrial fibrillation, Stroke, Prolonged inotropic support, Deep sternal wound infection, KDIGO acute kidney injury, Renal replacement therapy, E-CABG bleeding grades 2–3 | 7 (low risk) |
| Joshua Solomon 2021 [18] | Prospective Cohort study | CaNRda | Elderly patients (60 years and above) undergoing urgent or elective isolated CABG | 500 | 71.4 ± 6.4 years |
M = 79% F = 21% |
Frail – 27.6 ± 5.6 Not frail − 28.1 ± 4.3 |
Frail − 39 (56%) Not frail − 43 (33%) |
Frail – 58 (83%) Not frail − 91 (69%) |
Frail − 56 (80%) Not frail - 95 (72%) |
Frail − 17 (24%) Not frail – 21 (16%) |
Frail − 7 (10%) Not frail – 8 (6%) |
Frail − 10 (14%) Not frail – 14 (11%) |
NR | NR |
Frail – 4.5 ± 3.5 Not frail – 2.4 ± 2.4 |
Essential Frailty Toolset (EFT) | all-cause mortality, postoperative hospital length of stay ≥ 14 days, discharge to a healthcare facility, all-cause readmission at 30 days | 5 (moderate risk) |
| Martyna Kluszczyńska 2021 [19] | Prospective Cohort study | Poland | Elderly patients (60 years and above) qualified for CABG | 180 | 69.3 ± 6.2 |
M = 69% F = 31% |
NR | 30.6% | 60.0% | NR | NR | NR | NR | NR | NR | NR | Tilburg Frailty Indicator (TFI) | impact of frailty syndrome on the prognosis of patients after CABG | 7 (low risk) |
| Martyna Kluszczynska 2021 [20] | Prospective observational study | Poland | Elderly patients (60 years and above) qualified for CABG | 108 | 70.1 ± 6.4 |
M = 69.4% F = 31.5% |
78.3 ± 13.3 | NR | NR | NR | NR | NR | NR | NR | NR | NR | Tilburg Frailty Indicator (TFI) | Influence of frailty syndrome on kinesiophobia after CABG according to the gender of patients. | |
| Ajar Kochar 2023[21] | retrospective cohort study | USA | Elderly patients underwent CABG | 13,554 | median age was 69 years (IQR-63,72) |
M = 98.6% F = 1.4% |
NR |
Frail – 4263 (67.0) Non frail – 870 (29.3)) |
Frail − 6251 (98.2) Non frail – 1313 (44.2) |
NR |
Frail – 2042 (32.1) Not frail – 322 (10.8) |
NR |
Frail – 3375 (53.0 Not frail (10 (0.3) |
NR | NR | NR | Veterans Administration Frailty Index (VA-FI) | 5-year all-cause mortality, days alive and out of the hospital within the first postoperative year, 30-day, 90-day and 1-year mortality | 6 (moderate risk) |
| Arum Lim 2022[22] | retrospective cohort study | South Korea | Adults > 19 years who underwent CABG surgery | 896 | 66.0 ± 9.0 |
M = 78.0% F = 22.0% |
24.7 ± 3.2 | 498 (55.6%) | 617 (68.9%) | 295 (32.9%) | 99 (11.0%) | NR | 18 (2.1%) | 2.3 ± 1.9 | NR | NR | Frailty Index-Laboratory (FI-LAB) | association between laboratory based-frailty and patient health outcomes after CABG surgery | 5 (moderate risk) |
| Vishal Dobaria 2020[23] | retrospective cohort study | California, USA | Patients ≥ 18 years undergoing isolated coronary artery bypass grafting (CABG) | 2,137,618 | 67.4 ± 10.7 |
M = 72.4% F = 27.6% |
NR |
Frail – 30,533 (35.6) Not frail – 846,458 (39.6) |
Frail − 51,887 (60.4) Not frail − 1,646,618 (77.0) |
NR |
Frail – 34,913 (40.7) Not frail – 474,571 (22.2) |
NR |
Frail – 26,768 (31.2) Not frail – 572,458 (26.8) |
NR |
Frail – 3.8 ± 2.1 Not frail – 2.9 ± 1.8 |
NR | Johns Hopkins Adjusted Clinical Groups (ACG) indicator | impact of frailty on in-hospital mortality, complications, resource use, length of stay, hospitalization costs and trends in mortality. | 3 (high risk) |
| Nicholas J. Goel 2020[24] | Prospective Cohort study | USA | Adult (age ≥ 18 years) patients undergoing valve replacement surgery, coronary artery bypass grafting (CABG), or both | 1,507,899 |
Median 70 (65–75) |
M = 67.9% F = 32.1% |
NR |
Frail – 19,333 (26.5%) Non frail – 443,153 (30.9%) |
NR | NR |
Frail – 12,164 (16.7%) Non frail – 35,447 (2.4%) |
NR | NR |
Frail < 2–33,253 (45.7%) 2–3–25,498 (35.0%) > 3–14,067 (19.3%) Non frail < 2–920,461 (64.1%) 2–3–244,394 (17.0%) > 3–270,226 (18.8) |
NR | NR | Johns Hopkins Adjusted Clinical Groups (ACG) indicator | in-hospital mortality, in-hospital complications, FTR – Failure to Rescue, nonhome discharge, 30-day readmission, length of hospitalization, and hospital costs | 5 (moderate risk) |
| Mehrnoosh Bakhtiari 2024 | Prospective Cohort study | Iran | patients aged over 60 years undergoing elective CABG | 170 |
Median 66 (62–70) |
M = 75.3% F = 24.7% |
Frail 26.1 ± 3.3 Not frail 26.1 ± 3.2 |
Frail − 29 (25.9%) Not frail – 20 (34.5%) |
NR | NR | NR | NR |
Frail – 30 (51.7%) Not frail – 52 (46.4%) |
NR | NR |
Frail 1.9 (1.5–2.4) Not frail – 1.5 (1.3–1.9) |
Frail Scale and Clinical Frail scale | Length of ward hospitalization, Length of ICU Hospitalization, Pneumonia, Atrial fibrillation, Mortality, Sepsis, Euro score, Readmission | 5 (moderate risk) |
| ANR Johnson 2024 | retrospective cohort study | CaNRda | patients 18 years of age or older who underwent isolated CABG surgery | 50,682 | NR |
M = 76.8% F = 23.2% |
Non frail – 28.6 (5.4) Frail – 29.1 (6.0) |
NR | NR | NR | NR | NR | NR |
Non frail – 1.5 (1.3) Frail – 3.2 (1.8) |
Non frail – 2.7 (4.4) Frail – 9.4 (6.8) |
NR | Clinical frailty scale | Mortality, average hospital length of stay between initial surgery and discharge, readmission within one year of discharge, Charlson comorbidity score, Elixhauser score, ADG. | 3 (high risk) |
| Aslihan Aykut MD 2022 | Retrospective Cohort Study | Turkey | adult patients who underwent coronary artery bypass grafting (CABG) with a cardiopulmonary bypass (CPB) and aortic cross-clamp (CC) | 455 | Median 61 (53–68) |
M = 82.2% F = 17.8% |
27.97 (25.3–30.8) | 158 (34.7%) | 201 (44.2%) | NR | NR | 15 (3.3%) | 15 (3.3%) | NR | NR | NR | preoperative frailty index | Acute Kidney Injury | 4 (moderate risk) |
| Nozomu Sugimoto 2023 | retrospective cohort study | Japan | adults aged ≥ 65 years and diagnosed with angina pectoris and acute myocardial infarction who had undergone CABG | 35,015 |
Median 74 (69–78) |
M = 74.4% F = 25.6% |
Not frail < 18.5–1685 (4.9%) 18.5 to 25- 22,302(64.9%) > 25–9921 (28.9) Frail < 18.5–52 (8.2%) 18.5 to 25–416(65.7%) > 25–158 (25.0) |
Not frail 14,755 (42.9%) Frail − 265 (46.6%) |
Not frail - 20,301 (59.0%) Frail − 370 (58.5%) |
Not frail 11,291 (32.8%) Frail − 170 (26.9%) |
Not frail 11,575 (33.7%) Frail − 176 (27.8%) |
Not frail 977 (2.8%) Frail − 19 (3.0%) |
NR |
Not frail 0–5534 (16.1%) 1–11,004 (32.0%) 2–9588 (27.9%) ≥ 3–8256 (24.0%) Frail 0–11 (1.7%) 1–79 (12.5%) 2–113 (17.9%) ≥ 3–430 (67.9%) |
NR | NR | The Hospital Frailty Risk score (HFRS) | Discharge to home, Aspiration pneumonia, Delirium, Disuse syndrome, Heart failure, Sepsis, Stroke. | 4 (moderate risk) |
#Risk of bias score: 0–3 = high risk; 4–6 = moderate risk and 7–9 = low risk
@ - proportion with LV failure / LVEF less than 40%
NR - Not Reported
Outcomes assessed ranged from early and long-term mortality rates to more specific complications like major adverse cardiac and cerebrovascular events (MACCE), Acute Kidney Injury (AKI), readmissions and lengths of hospital and ICU stays. The Newcastle-Ottawa Scale (NOS) was utilized to determine the risk of bias within each study, with scores varying from moderate to low risk, indicating a generally reliable and valid collection of data for drawing conclusions on the researched topics. This variability in study design, participant demographics, and assessed outcomes enriches the analysis but also introduces heterogeneity that must be carefully interpreted within the context of broader clinical implications. Only one studies was not included in the meta-analysis as it did not specifically report the desired outcomes. The study findings showed that frailty syndrome did not affect kinesiophobia among patients after CABG. The level of kinesiophobia was significantly higher among women compared to men.
Frailty assessment tools
Frailty assessment tools were designed to measure vulnerability in patients and predict adverse outcomes, particularly in surgical contexts such as CABG. Tools like the Frail Scale and Clinical Frailty Scale (CFS) focused primarily on physical and functional domains, while others, including the Essential Frailty Toolset (EFT) and Frailty Index-Laboratory (FI-LAB), integrated physiological and biochemical parameters for a broader assessment. Tools such as the Tilburg Frailty Indicator (TFI) and Johns Hopkins ACG Indicator adopted a multidimensional approach, incorporating physical, psychological, and social dimensions. These tools shared a common objective: to stratify risk and guide clinical decision-making. However, they differed in their depth of assessment—ranging from quick screening instruments like the Frail Scale to comprehensive evaluations like the TFI—and in their reliance on subjective assessments, objective lab values, or electronic health record data.
Clinicians chose tools based on the clinical setting and patient needs. For emergency or preoperative contexts, rapid tools like the Frail Scale, EFT, or CFS were practical. For comprehensive evaluations, the TFI and CFS were more suitable for long-term planning. Data-driven systems with access to electronic health records efficiently utilized tools like the VA-FI or HFRS. Outcome-specific considerations were also important: the EFT, CFS, and FI-LAB were particularly effective for predicting mortality, while the TFI and HFRS were better suited for evaluating long-term care needs. This diversity of tools provided clinicians with the flexibility to select the most appropriate method, balancing practicality, precision, and the specific clinical context. Though detailed reporting on each tool is beyond the scope of this review, more insights can be found in the article by Sutton et al., which critically evaluates the reliability and validity of these instruments [24].
Mortality
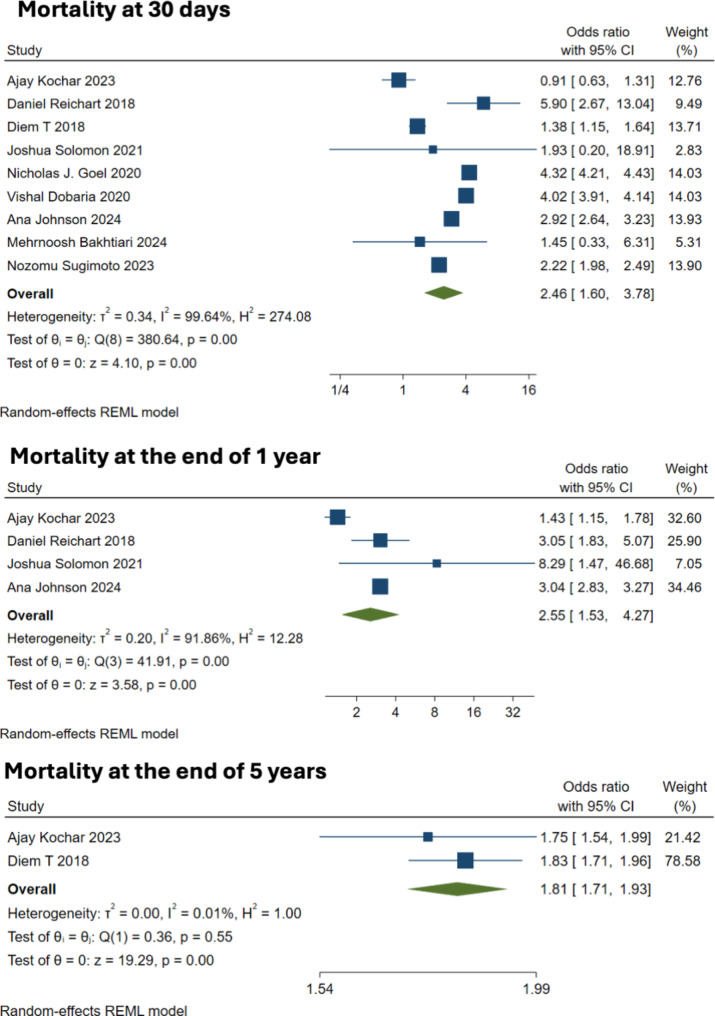
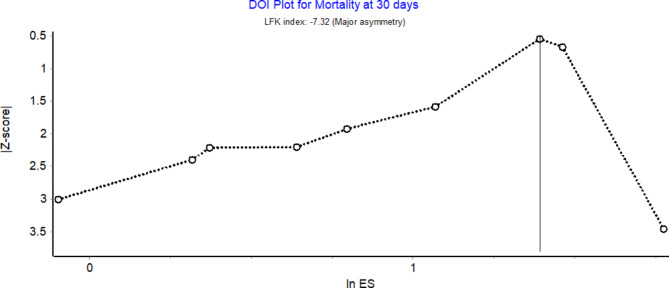
Nine studies with 350 patients reported data on the 30-day mortality outcome. Frailty was associated with a statistically significant increase in short-term mortality risk, with a pooled OR of 2.52, with a 95% CI from 2.07 to 3.07, and a 95% prediction interval (PI) from 1.36 to 4.68, (Fig. 2A). The analysis showed considerable heterogeneity (I² = 98%, tau² = 0.058), suggesting notable variability across studies (Q = 380.64, p < 0.001). However, the DOI plot showed major asymmetry with LFK index of -7.32 (Fig. 3). Similarly, frailty correlated with increased 1-year mortality that was reported in four studies. A random-effects model resulted in the OR of 2.58 (95% CI: 1.49 to 4.45) with a 95% PI ranging from 0.24 to 27.79 (Fig. 2B), with significant heterogeneity (I² = 93%, tau² = 0.228) among the studies (Q = 41.91, p < 0.001). Long-term outcomes, assessed at 5 years post-CABG, were pooled from two studies, with OR of 1.81 (95% CI: 1.71 to 1.93), without heterogeneity (I² = 0%, tau² = 0), further confirming a consistent effect across studies (Q = 0.36, p = 0.548). Frail patients had increased risk of death across all time points in the pooled analysis. Sensitivity analysis by leaving out 1 study at a time, did not significantly alter the pooled effect size obtained for mortality at 30 days and 1 year.
Fig. 2.
Forest plot showing the association between frailty and mortality (30 days, 1 year and 5 year)
Fig. 3.
Doi plot for mortality outcomes
Adverse cardiac and cerebrovascular events
In the meta-analysis evaluating acute cardiac and cerebrovascular events following CABG, four studies were included. The pooled odds ratio (OR) was 1.03 (95% CI: 0.89–1.19), indicating no significant increase in risk for these events among CABG patients. The analysis demonstrated no heterogeneity across studies, with a prediction interval from 0.75 to 1.42, supporting consistency of findings across different studies. Statistical tests for overall effect (z = 0.39, p = 0.695) and homogeneity (Q = 2.19, p = 0.533) further confirmed the stability and uniformity of the effect size. However, it is notable that nearly 97% of the weight in this analysis was attributed to a single study (Kim et al., 2020), with the remaining three studies contributing minimal weight.
Acute kidney injury (AKI)
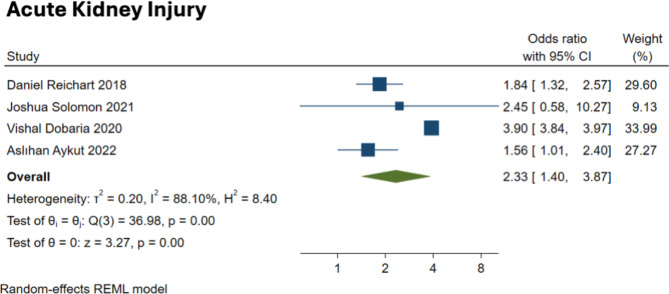
The analysis concerning acute kidney injury (AKI) included data from four studies, revealing a significantly elevated risk associated with frailty, with the pooled OR of 2.31, with a 95% CI from 1.26 to 4.23 (Fig. 4). However, this outcome displayed substantial heterogeneity (I² = 92%), with a prediction interval ranging widely from 0.15 to 34.65, suggesting that the effect size might vary significantly across different settings or study conditions. The test for overall effect showed a significant increase in risk (z = 2.70, p = 0.007), while the test of homogeneity indicated significant variability among the studies (Q = 36.98, p < 0.001). The Pooled effect size for AKI did not differ significantly in the sensitivity analysis by leaving out one study at a time.
Fig. 4.
Forest plot showing the association between frailty and complications (AKI)
Hospital readmission
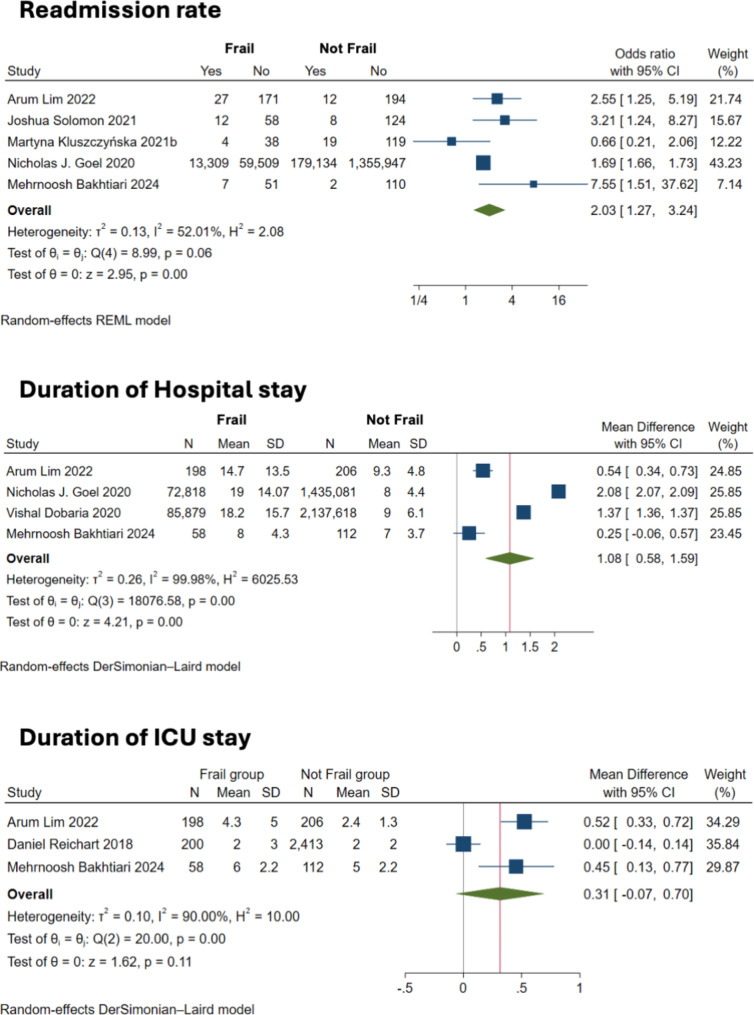
The meta-analysis assessing the risk of hospital readmission in frail patients after CABG incorporated data from five studies. The analysis reported a pooled OR of 2.04 with a 95% CI from 1.25 to 3.34, indicating a statistically significant increased risk of readmission. Moderate heterogeneity was observed (I² = 55.5%) and the test for overall effect was significant (z = 2.85, p = 0.005), affirming the higher likelihood of readmission among frail patients compared to their non-frail counterparts (Fig. 5A).
Fig. 5.
Forest plot showing the association between frailty and readmission rates and length of hospital stay
Duration of stay in hospital
Four studies reported data on the mean duration of hospital stays. As shown in Fig. 5B, frailty was associated with longer hospital stays post-CABG, with a mean difference (MD) of 1.084 days (95% CI: 0.580 to 1.588). This result showed extremely high heterogeneity (I² = 99.98%), with the prediction interval ranging from − 1.358 to 3.525, suggesting substantial variability across studies. The finding suggests that frailty contributes to prolonged hospitalization.
Mean ICU stay
Intensive Care Unit (ICU) duration of stay was reported un three studies, with the pooled MD of 0.315 days (95% CI: -0.067 to 0.696), although this increase was not statistically significant (z = 1.62, p = 0.1059). Heterogeneity was substantial (I² = 90%), indicating considerable variation in the ICU stays reported by the studies (Fig. 5C). The wide prediction interval from − 4.409 to 5.038 also reflects this variability, suggesting that while some frail patients may require longer ICU stays, the effect is not consistent across all settings or patient groups.
Discussion
The findings from our meta-analysis clearly highlight the pronounced impact of frailty on various outcomes in patients after CABG. Specifically, our study reveals that frailty is associated with a significantly increased risk of death across all time points, higher risk of AKI, longer hospital stays, and increased rate of hospital readmission.
Our results reported an increased risk of 30-day mortality among frail patients, with a pooled odds ratio of 2.86. This finding is consistent with previous research that has identified frailty as a critical predictor of poor postoperative outcomes in cardiac surgery patients. A study by Afilalo et al. also noted heightened mortality risks associated with frailty, reinforcing the crucial nature of this condition as a determinant of immediate postoperative survival [25]. Furthermore, our analysis extended these observations to long-term outcomes, demonstrating that frailty continues to influence survival rates up to five years after the surgery, with a pooled odds ratio of 1.81. This finding provides an important update to the existing body of research, since previous studies tended to focus more on short-term outcomes. Our results suggest that the effects of frailty persist well beyond the initial recovery period, emphasizing the need for ongoing management strategies tailored to frail individuals.
While previous studies have explored the relationship between frailty and postoperative complications, our study specifically investigated adverse cardiac and cerebrovascular complications. We found a non-significant increase in the risk of MACCE, with a pooled odds ratio of 1.37. Although not statistically significant, the direction of the effect is consistent with previous studies suggesting that frail patients undergoing various surgical procedures, including cardiac surgery, may experience increased incidence of multi-system complications [26–28]. In the context of AKI, our findings are particularly notable, with a significant pooled odds ratio of 2.86, pointing to almost three-time higher odds of AKI in frail patients. This aligns with other studies that have similarly reported an increased incidence of AKI in frail patients following cardiac procedures [29, 30]. Our results reinforce the vulnerability of this population to severe complications such as AKI, which can drastically affect outcomes and healthcare costs.
Moreover, our analysis indicates that frail patients are likely to experience longer hospital stays post-CABG, with an average increase of 1.3 days compared to their non-frail counterparts. This finding is in agreement with the broader literature that consistently shows prolonged recovery times among frail individuals due to their reduced physiological reserves and increased care needs [31–34]. Lastly, the increased likelihood of hospital readmission among frail patients, as demonstrated by an odds ratio of 1.77 for readmission, suggest that frailty not only affects immediate and long-term mortality but also impacts the broader trajectory of recovery and rehospitalization. This cycle of readmission is a critical issue, as it indicates potential gaps in the continuum of care for these high-risk patients.
The increased risks associated with frailty in CABG patients can be attributed to a combination of physiological, psychological, and systemic factors [35]. Frailty is marked by diminished physiological reserves and impaired stress responses, leading to exacerbated post-surgical complications such as prolonged hospital stays, proinflammatory state, and AKI. Frail patients often have coexisting cognitive impairments which complicate postoperative recovery and adherence to medical advice, potentially leading to increased incidences of delirium and extended hospitalization. Furthermore, frail individuals may lack adequate social support, which can hinder their ability to manage postoperative care and result in higher readmission rates [36, 37]. Additionally, standard surgical recovery protocols may not be adequately suited for frail patients, leading to suboptimal management and higher risks of drug interactions due to polypharmacy, further contributing to the observed outcomes. These factors collectively underscore the need for tailored healthcare strategies to mitigate these risks and improve recovery outcomes for frail patients undergoing CABG.
This meta-analysis demonstrates several strengths. Utilizing a random-effects model, the study effectively manages the inherent heterogeneity among the included studies, which differ in design, demographics, and frailty assessments. Furthermore, the systematic review process was rigorously conducted according to established protocols, ensuring a thorough and reproducible selection and analysis of relevant studies.
However, the study has some limitations. Notably, there was a significant heterogeneity observed in several outcomes like 30-day mortality and complications. This indicates variability in study designs or frailty definitions, which could skew the interpretation of the results. Inclusion of predominantly high-income country studies may also limit the applicability of the findings to lower-income settings where different healthcare systems and patient demographics prevail. Additionally, relatively small number of studies in some analyses could impact the robustness of the findings. Finally, as the meta-analysis is based on published studies, it may be susceptible to publication bias, potentially omitting studies with non-significant findings.
The implications for clinical practice are significant, particularly concerning the management of frail patients undergoing CABG. The increased risk of mortality, complications, and longer hospital stays, highlighted in this review, further emphasize the crucial need for preoperative frailty assessments to identify high-risk patients who might benefit from customized perioperative care strategies. Implementing specialized care pathways that involve multidisciplinary teams could address the unique needs of these patients, potentially improving outcomes and reducing complication rates and readmissions. Additionally, these findings emphasize the need for enhanced post-discharge support and monitoring to prevent high readmission rates. This highlights the importance of continuous care coordination between hospital teams and primary care providers.
Future research should focus on further exploring the mechanisms by which frailty influences CABG outcomes to inform the development of targeted interventions. It is essential to standardize the definition and measurement of frailty to decrease heterogeneity and enhance result comparability across studies. Further research should focus on assessing the effectiveness of specific interventions, designed to reduce frailty-associated risks in CABG patients, such as preoperative optimization programs, personalized rehabilitation plans, and enhanced postoperative surveillance. Additional research is needed in various geographic and economic settings to verify the generalizability of the findings and to examine how differences in healthcare systems might affect outcomes for frail patients.
Conclusion
This meta-analysis confirms that frailty significantly elevates the risk of adverse outcomes in patients undergoing CABG. Our findings highlight the urgent need to recognize frailty as a crucial prognostic factor in CABG and to adjust clinical practices accordingly. Incorporating comprehensive frailty assessments into the preoperative evaluation and developing customized management strategies may potentially improve postoperative outcomes for this vulnerable population.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
SChen: conceived and designed the study. SChen, SZ, SCai and HW collected the data and performed the analysis. SChen was involved in the writing of the manuscript and is responsible for the integrity of the study. All authors have read and approved the final manuscript.
Funding
None.
Data availability
All data generated or analysed during this study are included in this published article or its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dąbrowski EJ, Kożuch M, Dobrzycki S. Left Main Coronary Artery Disease—Current Management and Future Perspectives. J Clin Med. 2022;11:5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spadaccio C, Benedetto U. Coronary artery bypass grafting (CABG) vs. percutaneous coronary intervention (PCI) in the treatment of multivessel coronary disease: quo vadis? —a review of the evidences on coronary artery disease. Ann Cardiothorac Surg. 2018;7:506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rai P, Taylor R, Bittar MN. Long-term survival in patients who had CABG with or without prior coronary artery stenting. Open Heart. 2020;7:e001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adelborg K, Horváth-Puhó E, Schmidt M, Munch T, Pedersen L, Nielsen PH et al. Thirty-Year Mortality After Coronary Artery Bypass Graft Surgery: A Danish Nationwide Population-Based Cohort Study. Circ: Cardiovascular Quality and Outcomes. 2017;10. [DOI] [PubMed]
- 5.Shahian DM, O’Brien SM, Sheng S, Grover FL, Mayer JE, Jacobs JP, et al. Predictors of Long-Term Survival After Coronary Artery Bypass Grafting Surgery: Results From the Society of Thoracic Surgeons Adult Cardiac Surgery Database (The ASCERT Study). Circulation. 2012;125:1491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dani SS, Minhas AMK, Arshad A, Krupica T, Goel SS, Virani SS, et al. Trends in Characteristics and Outcomes of Hospitalized Young Patients Undergoing Coronary Artery Bypass Grafting in the United States, 2004 to 2018. J Am Heart Association. 2021;10:e021361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alkhouli M, Alqahtani F, Kalra A, Gafoor S, Alhajji M, Alreshidan M, et al. Trends in Characteristics and Outcomes of Hospital Inpatients Undergoing Coronary Revascularization in the United States, 2003–2016. JAMA Netw Open. 2020;3:e1921326. [DOI] [PubMed] [Google Scholar]
- 8.Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145. [DOI] [PubMed]
- 9.Doody P, Lord JM, Greig CA, Whittaker AC. Frailty: Pathophysiology, Theoretical and Operational Definition(s), Impact, Prevalence, Management and Prevention, in an Increasingly Economically Developed and Ageing World. Gerontology. 2023;69:927–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JA, Yanagawa B, An KR, Arora RC, Verma S, Friedrich JO, et al. Frailty and pre-frailty in cardiac surgery: a systematic review and meta-analysis of 66,448 patients. J Cardiothorac Surg. 2021;16:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- 12.StataCorp. Stata Statistical Software: Release 18. College Station. TX: StataCorp LLC; 2023. [Google Scholar]
- 13.Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc. 2018;16:195–203. [DOI] [PubMed] [Google Scholar]
- 14.Kim CH, Kang Y, Kim JS, Sohn SH, Hwang HY. Association Between the Frailty Index and Clinical Outcomes after Coronary Artery Bypass Grafting. J Chest Surg. 2022;55:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran DTT, Tu JV, Dupuis J, Bader Eddeen A, Sun LY. Association of Frailty and Long-Term Survival in Patients Undergoing Coronary Artery Bypass Grafting. JAHA. 2018;7:e009882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichart D, Rosato S, Nammas W, Onorati F, Dalén M, Castro L, et al. Clinical frailty scale and outcome after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2018;54:1102–9. [DOI] [PubMed] [Google Scholar]
- 17.Solomon J, Moss E, Morin J, Langlois Y, Cecere R, De Varennes B, et al. The Essential Frailty Toolset in Older Adults Undergoing Coronary Artery Bypass Surgery. JAHA. 2021;10:e020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kluszczyńska M, Młynarska A, Mikulakova W. Influence of Frailty Syndrome on Kinesiophobia According to the Gender of Patients after Coronary Artery Bypass Surgery. Healthcare. 2021;9:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kluszczyńska M, Młynarska A. Influence of frailty syndrome on patient prognosis after coronary artery bypass grafting. Adv Clin Exp Med. 2021;30:923–31. [DOI] [PubMed] [Google Scholar]
- 20.Kochar A, Deo SV, Charest B, Peterman-Rocha F, Elgudin Y, Chu D, et al. Preoperative frailty and adverse outcomes following coronary artery bypass grafting surgery in US veterans. J Am Geriatr Soc. 2023;71:2736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim A, Choi M, Jang Y, Lee H. Preoperative frailty based on laboratory data and postoperative health outcomes in patients undergoing coronary artery bypass graft surgery. Heart Lung. 2022;56:1–7. [DOI] [PubMed] [Google Scholar]
- 22.Dobaria V, Hadaya J, Sanaiha Y, Aguayo E, Sareh S, Benharash P. The Pragmatic Impact of Frailty on Outcomes of Coronary Artery Bypass Grafting. Ann Thorac Surg. 2021;112:108–15. [DOI] [PubMed] [Google Scholar]
- 23.Goel NJ, Iyengar A, Kelly JJ, Han JJ, Brown CR, Desai ND. Volume of frail patients predicts outcome in frail patients after cardiac surgery. J Thorac Cardiovasc Surg. 2022;163:151–e1606. [DOI] [PubMed] [Google Scholar]
- 24.Sutton JL, Gould RL, Daley S, Coulson MC, Ward EV, Butler AM, Nunn SP, Howard RJ. Psychometric properties of multicomponent tools designed to assess frailty in older adults: A systematic review. BMC Geriatr. 2016;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, et al. Frailty Assessment in the Cardiovascular Care of Older Adults. J Am Coll Cardiol. 2014;63:747–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakano M, Nomura Y, Suffredini G, Bush B, Tian J, Yamaguchi A, et al. Functional Outcomes of Frail Patients after Cardiac Surgery: An Observational Study. Anesth Analg. 2020;130:1534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pozzi M, Mariani S, Scanziani M, Passolunghi D, Bruni A, Finazzi A, et al. The frail patient undergoing cardiac surgery: lessons learned and future perspectives. Front Cardiovasc Med. 2023;10:1295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishijima TF, Esaki T, Morita M, Toh Y. Preoperative frailty assessment with the Robinson Frailty Score, Edmonton Frail Scale, and G8 and adverse postoperative outcomes in older surgical patients with cancer. Eur J Surg Oncol. 2021;47:896–901. [DOI] [PubMed] [Google Scholar]
- 29.Brown JR, Rezaee ME, Nichols EL, Marshall EJ, Siew ED, Matheny ME. Incidence and In-Hospital Mortality of Acute Kidney Injury (AKI) and Dialysis‐Requiring AKI (AKI‐D) After Cardiac Catheterization in the National Inpatient Sample. J Am Heart Association. 5:e002739. [DOI] [PMC free article] [PubMed]
- 30.Vives M, Hernandez A, Parramon F, Estanyol N, Pardina B, Muñoz A, et al. Acute kidney injury after cardiac surgery: prevalence, impact and management challenges. Int J Nephrol Renovasc Dis. 2019;12:153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunha AIL, Veronese N, de Melo Borges S, Ricci NA. Frailty as a predictor of adverse outcomes in hospitalized older adults: A systematic review and meta-analysis. Ageing Res Rev. 2019;56:100960. [DOI] [PubMed] [Google Scholar]
- 32.Khandelwal D, Goel A, Kumar U, Gulati V, Narang R, Dey AB. Frailty is associated with longer hospital stay and increased mortality in hospitalized older patients. J Nutr Health Aging. 2012;16:732–5. [DOI] [PubMed] [Google Scholar]
- 33.Keeble E, Roberts HC, Williams CD, Van Oppen J, Conroy SP. Outcomes of hospital admissions among frail older people: a 2-year cohort study. Br J Gen Pract. 2019;69:e555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wozniak H, Beckmann TS, Dos Santos Rocha A, Pugin J, Heidegger C-P, Cereghetti S. Long-stay ICU patients with frailty: mortality and recovery outcomes at 6 months. Ann Intensiv Care. 2024;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakhtiari M, Shaker F, Shirmard FO, Jalali A, Vakili-Basir A, Balabandian M, et al. Frailty efficacy as a predictor of clinical and cognitive complications in patients undergoing coronary artery bypass grafting: a prospective cohort study. BMC Cardiovasc Disord. 2024;24:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kojima G, Liljas AEM, Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. 2019;12:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394:1376–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article or its supplementary information files.