Abstract
Background
Arm-lifting movements (shoulder flexion) are essential for upper extremity rehabilitation after a stroke. Abnormal flexor synergy (elbow flexion) is frequently observed during shoulder flexion, impeding functional improvement. However, no quantitative method exists for assessing abnormal flexor synergy. This study investigated the validity and responsiveness of a newly developed index to quantitatively evaluate abnormal flexor synergy.
Methods
Participants included 103 patients (mean age: 58.0 ± 10.1 years; 64 men, 39 women) with stroke. Using three-dimensional coordinate data during shoulder flexion obtained from a depth sensor camera, we calculated the abnormal flexor synergy based on our developed index. The abnormal flexor synergy index decreases with increasing flexion of the elbow joint during shoulder flexion (the maximum value is 100% without abnormal flexor synergy). The validity of the abnormal flexor synergy index was assessed by analyzing the correlation between the index and both the Fugl–Meyer Assessment of the Upper Extremity (FMA-UE) four-category scores and the Modified Ashworth Scale (MAS) scores for elbow, wrist, and finger flexors, using Pearson’s and Spearman’s correlation coefficients. Responsiveness was studied in 17 inpatients (mean age: 59.5 ± 8.1 years; 7 men, 10 women) who underwent proximal upper extremity intervention for approximately 3 weeks, evaluating change from admission to discharge using the standardized response mean (SRM).
Results
Significant correlations were observed between the abnormal flexor synergy index and FMA-UE scores: A (r = 0.625, p < 0.001), B (r = 0.433, p < 0.001), C (r = 0.418, p < 0.001), and D (r = 0.411, p < 0.001), as well as MAS scores for elbow flexors (r = -0.283, p = 0.004) and proximal interphalangeal flexors (r = -0.201, p = 0.042). The highest responsiveness was observed in the FMA-UE A score (SRM = 0.81), followed by the abnormal flexor synergy index (SRM = 0.79).
Conclusions
The newly developed index for assessing abnormal flexor synergy demonstrated superior validity and high responsiveness. These results suggest the potential for using this index to evaluate upper extremity function in patients with stroke.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12984-024-01534-3.
Keywords: Stroke, Upper Extremity, Biomechanical Phenomena, Shoulder, Rehabilitation
Background
Stroke is a leading cause of physical disability [1, 2], with upper extremity dysfunction being a primary symptom affecting approximately 70% of patients with stroke [3, 4]. This dysfunction adversely affects daily activities [5] and diminishes health-related quality of life [6]. Stroke survivors often report that the loss of upper extremity function is one of the most distressing long-term outcomes [7]. Consequently, improving upper extremity function is important for stroke survivors and their caregivers.
Rehabilitation of the upper extremity post-stroke requires using the paralyzed limb for training and daily tasks, with functional improvement dependent on the amount of use [8]. Arm-lifting movements (shoulder flexion) are essential for positioning and orienting the hand in the environment [9]. However, after a stroke, pathological co-activation or reciprocal inhibitory changes arise due to central lesions impairing the corticospinal tracts [10]. Specifically, during voluntary single joint movements, excessive and unintended motion occurs in adjacent joints [11, 12]. This stroke-specific abnormal movement is referred to as abnormal synergy. Two main synergies have been identified in the post-stroke upper limb: the flexor synergy, in which shoulder, elbow, and wrist flexion are obligatorily linked, and the opposite extensor synergies [13, 14]. The most common abnormal flexor synergy is elbow flexion during shoulder flexion [15, 16], which is the leading cause of reaching dysfunction [17, 18]. Moreover, this abnormal flexor synergy can lead to long-term issues such as reduced joint mobility and pain, fostering a learned non-use pattern that limits improvement potential in the hemiplegic upper extremity [19]. Therefore, abnormal flexor synergy should be assessed appropriately to safely and effectively rehabilitate the hemiplegic upper extremities.
However, no established method exists for the quantitative assessment of abnormal flexor synergy. The Fugl–Meyer Assessment of the Upper Extremity (FMA-UE), considered the gold standard for evaluating upper extremity motor paralysis, is commonly used to assess abnormal synergistic movements [20], although it is not quantitative. Recently, various quantitative assessment methods for abnormal synergy in the hemiplegic upper limb have emerged. Previous studies have quantified abnormal synergy using different methods. Some used robotic devices to measure elbow torque and stiffness to assess motor impairments, such as spasticity and joint viscoelasticity [21, 22]. Others utilized electromyography to assess abnormal synergy, revealing impaired coordinated movement and muscle activity patterns during upper limb work impairment and dysfunction [23–25]. Further, three-dimensional movement analysis has been used to investigate joint inflexibility and joint connectivity changes [26]. However, these methods typically do not specifically target flexor synergy during shoulder flexion. Furthermore, these methods require extensive preparation, measurement, and analysis time, making them less practical for clinical settings. To eliminate these issues, we developed a specific quantitative assessment method for abnormal flexor synergy during shoulder flexion, which has not been extensively explored in stroke rehabilitation. Moreover, to enhance the clinical feasibility of our study, we used markerless motion capture technology, reducing the complexity and time required for traditional assessments. This offers a more practical and efficient method for routine clinical use. We hypothesized that the developed index would adequately assess abnormal flexor synergy. This study aimed to investigate the validity and responsiveness of a newly developed quantitative assessment method for abnormal flexor synergy in patients with stroke.
Methods
Study design and participants
This retrospective cohort study was conducted according to the STROBE Checklists and included 103 patients with stroke, both inpatients and outpatients, at Keio University Hospital between January 1, 2021, and March 31, 2024. Inclusion criteria were: ≥ 18 years, chronic stroke (> 90 d since onset), and concurrent kinematic and upper extremity functional assessments of shoulder flexion. For the responsiveness study, 17 inpatients who underwent proximal upper-extremity interventions (such as robotics and electrical stimulation) for approximately 3 weeks were selected. Kinematic and upper-extremity function assessments were performed at admission and discharge. This study was conducted in accordance with the Declaration of Helsinki and was reviewed and approved by the Ethics Committee of Keio University (Approval number: 20231079). The opt-out method was applied to obtain informed consent in this study.
Data collection
Medical records were reviewed to collect general characteristics, including age, sex, duration from stroke onset, stroke type, and affected side. Participants in the responsiveness analysis also had an extended hospital stay. Clinical measurements of upper extremity function, FMA-UE [27], and Modified Ashworth Scale (MAS) [28] scores were also obtained from medical records. The FMA-UE [27] score measured upper extremity impairment. The FMA-UE includes 30 motor function items and three reflex function items, scored on a 3-point ordinal scale (0 = cannot perform, 1 = partially performs, and 2 = completely performs), with higher scores indicating better motor function (total score: 0–66 points). The FMA-UE was divided into four categories: A, shoulder/elbow/forearm (0–36); B, wrist (0–10); C, hand (0–14); and D, coordination/speed (0–6). The MAS [28] measured muscle spasticity of the elbow, wrist, and finger flexors (metacarpophalangeal and proximal interphalangeal [PIP] flexors). This is an ordinal scale with a 6-grade criterion (0, 1, 1+, 2, 3, and 4), where higher scores indicated more severe spasticity.
Kinematic analysis
Azure Kinect DK (Microsoft) analyzed hemiplegic shoulder flexion. The test-retest reliability [29] of using Kinect for patients with stroke has been established. Participants sat approximately 2.5 m from the Kinect sensor and performed the maximal shoulder flexion task twice, with their elbows extended as far as possible. The recordings were taken with the Kinect positioned at a height of 1 m. The Kinect data were collected from a dedicated computer.
As preprocessing, three-dimensional coordinate data for the shoulder [Sx, Sy, Sz], elbow [Ex, Ey, Ez], and hand [Hx, Hy, Hz] were acquired. The three-dimensional coordinate data were extracted using dedicated software (ICpro-K2; Hu-tech Co., Ltd., Tokyo, Japan). Spline interpolation was applied to address missing data points. The data were smoothed using a second-order Butterworth filter with a cut-off frequency of 5 Hz and exported as CSV files.
Second, the shoulder flexion angle was calculated from the three-dimensional vectors [Sx, Sy, Sz] and [Ex, Ey, Ez]. The shoulder-floor vertical vector 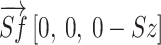 and the shoulder-elbow vector
and the shoulder-elbow vector 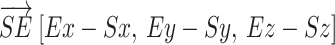 were calculated. The shoulder flexion angle θ between
were calculated. The shoulder flexion angle θ between 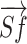 and
and 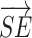 was calculated using formula (I) Furthermore, the flexor synergy parameter was derived from the three-dimensional coordinates of the shoulder (Sx, Sy, Sz), elbow (Ex, Ey, Ez), and hand (Hx, Hy, Hz) using formula (II) In formula II, the maximum value is 100% because the denominator and numerator are equal during elbow extension. In contrast, this value decreases as elbow flexion increases during shoulder flexion, due to the proximity of the shoulder and hand.
was calculated using formula (I) Furthermore, the flexor synergy parameter was derived from the three-dimensional coordinates of the shoulder (Sx, Sy, Sz), elbow (Ex, Ey, Ez), and hand (Hx, Hy, Hz) using formula (II) In formula II, the maximum value is 100% because the denominator and numerator are equal during elbow extension. In contrast, this value decreases as elbow flexion increases during shoulder flexion, due to the proximity of the shoulder and hand.
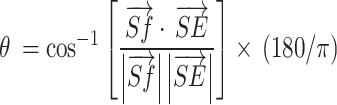 |
I |
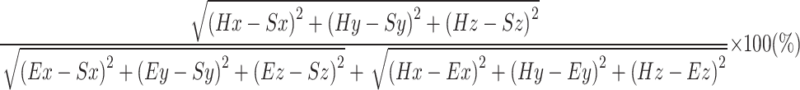 |
II |
Finally, the area under the curve of the flexor synergy parameter from the start of the exercise to maximum shoulder flexion was calculated. To identify the starting point (X0) of shoulder flexion, the shoulder flexion angular velocity was determined, and the first instance at which the shoulder flexion angular velocity was continuously positive was noted. Furthermore, the maximum shoulder flexion point (Xmax) was identified. Next, the time from the starting movement point (X0) to the maximum shoulder flexion point (Xmax) was normalized between 0.0 and 1.0 to calculate “Xi”. Additionally, “Yi”, the flexor synergy parameter, was calculated using formula II, which was derived from the start of the movement (X0) to maximum shoulder flexion (Xmax). The abnormal flexor synergy index was calculated using formula III. This index has a maximum of 100%, with smaller values indicating a higher ratio of abnormal flexor synergy during shoulder flexion.
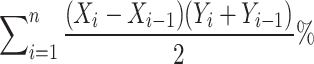 |
III |
Statistics analyses
The validity of the abnormal flexor synergy index was assessed by analyzing the correlation between the index and both the FMA-UE four-category scores and individual MAS scores. Pearson’s correlation coefficient was used to analyze the relationship between the abnormal flexor synergy index and FMA-UE, while Spearman’s correlation coefficient was employed for the correlation between the index and MAS. For the responsiveness analysis, the abnormal flexor synergy index was calculated by matching the maximum shoulder flexion angle to the lower pre- or post-intervention area, and the area under the curve was compared. The responsiveness of each outcome to changes from pre- to post-intervention was determined using the standardized response mean (SRM). The SRM is calculated as the mean difference in scores divided by the standard deviation of paired differences. The magnitude of responsiveness was defined as large for SRM > 0.8, medium for SRM between 0.5 and 0.8, and small for SRM between 0.2 and 0.5 [30]. All statistical analyses were performed using the IBM SPSS Statistics software (version 28.0; IBM, Tokyo, Japan). Statistical significance was set at p ≤ 0.05.
Results
No adverse events were observed in any participants during the study. Preparation for measurement required minimal time, and the measurement was completed in a few seconds for each participant. The general characteristics and upper extremity function of participants in the validity analysis are listed in Table 1. The mean age of the 103 participants was 58.0 years (standard deviation [SD] = 10.1), comprising 64 men and 39 women. The mean FMA-UE total score and abnormal flexor synergy index were 27.1 points (SD = 12.8) and 85.2% (SD = 8.5), respectively.
Table 1.
General characteristics and upper extremity function of participants in the validity analysis
| Characteristics | Values |
|---|---|
| Number | 103 |
| Age (years) a | 58.0 (10.1) |
| Sex (men/women) b | 64/39 |
| Duration from stroke onset (years) c | 5.5 (2.5–9.9) |
| Stroke type (hemorrhage/infarction) b | 68/35 |
| Affected side (right/left) b | 50/53 |
| FMA-UE, total score (0–66) a | 27.1 (12.8) |
| A score (0–36) a | 19.9 (6.3) |
| B score (0–10) a | 2.8 (3.2) |
| C score (0–14) a | 3.9 (3.9) |
| D score (0–6) a | 0.5 (1.3) |
| MAS, elbow flexor (0/1/1+/2/3/4) b | 7/31/55/7/3/0 |
| MAS, wrist flexor (0/1/1+/2/3/4) b | 10/28/48/12/5/0 |
| MAS, MP flexor (0/1/1+/2/3/4) b | 49/26/20/7/1/0 |
| MAS, PIP flexor (0/1/1+/2/3/4) b | 19/15/39/26/4/0 |
| Abnormal flexor synergy index (%) a | 85.2 (8.5) |
a mean (standard deviation), b number, c median (interquartile range)
Abbreviations: FMA-UE, Fugl–Meyer Assessment of the Upper Extremity; MAS, Modified Ashworth Scale; MP, metacarpophalangeal; PIP, proximal interphalangeal
Additional File 1: Group differences in the percentage of abnormal movements during shoulder flexion and upper extremity assessments by the severity of upper extremity paralysis.
Table 2 shows the correlation between the abnormal flexor synergy index and upper extremity functional measure. Significant correlations with the abnormal flexor synergy index were found for FMA-UE A (r = 0.532, p < 0.001), FMA-UE B (r = 0.407, p < 0.001), FMA-UE C (r = 0.355, p < 0.001), and FMA-UE D (r = 0.340, p < 0.001). Table 3 shows the correlation between the abnormal flexor synergy index and spasticity scale. Significant correlations with the abnormal flexor synergy index were found for MAS elbow flexor (r = -0.283, p = 0.004) and MAS PIP flexor scores (r = -0.201, p = 0.042).
Table 2.
Correlation between abnormal flexor synergy index and upper extremity scale
| FMA-UE_A | FMA-UE_B | FMA-UE_C | FMA-UE_D | |
|---|---|---|---|---|
| Abnormal flexor synergy index (%) | 0.532** | 0.407** | 0.355** | 0.340** |
*p value of < 0.05, **p value of < 0.01, correlation using Pearson’s correlation coefficient
Abbreviation: FMA-UE, Fugl–Meyer Assessment of the Upper Extremity
Table 3.
Correlation between abnormal flexor synergy index and spasticity scale
| MAS_ elbow flexor |
MAS_ wrist flexor |
MAS_ finger MP |
MAS_ finger PIP |
|
|---|---|---|---|---|
| Abnormal flexor synergy index (%) | -0.283** | -0.169 | -0.066 | -0.201* |
*p value of < 0.05, **p value of < 0.01, correlation using Spearman’s rank correlation coefficient
Abbreviation: MAS, Modified Ashworth Scale; MP, metacarpophalangeal; PIP, proximal interphalangeal
Table 4 presents the characteristics of the 17 participants in the responsiveness analysis. The mean age was 59.5 years (SD = 8.1), with 7 men and 10 women. The median hospital stay duration was 21 d (interquartile range: 21–24 d). Table 5 shows the responsiveness data for each outcome, with the highest responsiveness in the FMA-UE A score (SRM = 0.81), followed by the abnormal flexor synergy index (SRM = 0.79).
Table 4.
Characteristics of participants in the responsiveness analysis
| Characteristics | |
|---|---|
| Number | 17 |
| Age (years) a | 59.5 (8.1) |
| Sex (men/women) b | 7/10 |
| Duration from stroke onset (years) c | 4.7 (2.0–6.8) |
| Stroke type (hemorrhage/infarction) b | 15/2 |
| Paralysis side (right/left) b | 7/10 |
| Hospital durations (days) c | 21 (21–24) |
a mean (standard deviation), b number, c median (interquartile range)
Table 5.
Responsiveness of each upper extremity outcome (n = 17)
| Indices of responsiveness | FMA-UE_A | FMA-UE_B | FMA-UE_C | FMA-UE_D | MAS_ elbow flexor |
MAS_ wrist flexor |
MAS_ MP flexor |
MAS_ PIP flexor |
Abnormal flexor synergy index |
|---|---|---|---|---|---|---|---|---|---|
| Pre-intervention | 16.5 (6.4) | 2.1 (3.5) | 4.1 (4.9) | 0.5 (1.3) | 1.8 (0.8) | 1.9 (1.1) | 0.9 (1.1) | 2.1 (1.2) | 84.6 (6.9) |
| Post-intervention | 18.0 (7.5) | 3.0 (4.3) | 4.5 (4.8) | 0.5 (1.5) | 1.6 (0.7) | 1.6 (1.1) | 0.6 (1.1) | 1.7 (1.3) | 88.4 (6.0) |
| Mean difference | 1.47 | 0.88 | 0.47 | 0.06 | -0.12 | -0.35 | -0.24 | -0.41 | 3.82 |
| SD of paired differences | 1.81 | 1.36 | 0.87 | 0.24 | 0.60 | 0.49 | 0.83 | 0.94 | 4.83 |
| Standardized response mean (SRM) | 0.81 | 0.65 | 0.54 | 0.24 | -0.20 | -0.72 | -0.28 | -0.44 | 0.79 |
Abbreviations: FMA-UE, Fugl–Meyer Assessment of the Upper Extremity; MAS, Modified Ashworth Scale; MP, metacarpophalangeal; PIP, proximal interphalangeal; SD, standard deviation
Discussion
Abnormal flexor synergy, which is frequently observed in patients with stroke, has no quantitative and convenient assessment method. In the present study, abnormal flexor synergy was quantitatively calculated using a newly developed index. The validity and responsiveness of this index were investigated, revealing mild to moderate correlations with upper extremity functional outcomes, indicating better validity and high responsiveness.
Validity
The abnormal flexor synergy index significantly correlated with all categories of the FMA-UE and MAS elbow and finger flexor scores. The significant correlation with all FMA-UE scores may be attributed to the association between the abnormal flexor synergy index and the severity of upper-extremity dysfunction. Upper extremity performance, including segmentation, accuracy, and coordination, was associated with the severity of impairment in patients with stroke [31]. As the FMA-UE is an indicator of upper extremity dysfunction severity, our findings align with this notion. The significant correlation with MAS elbow and finger flexor scores may be attributed to spasticity being a contributing factor to abnormal flexor synergy. Abnormal flexor synergy arises from various factors, including motor paralysis, muscle weakness, contracture, and spasticity [32]. However, spasticity is a velocity-dependent muscle tone disturbance, while abnormal synergy involves coordinated motor disturbance, making these two phenomena distinct. Therefore, the observed correlation between the abnormal flexor synergy index and MAS was likely modest. The significant correlation with the upper limb distal scores (FMA-UE B and C, and MAS PIP flexor) may be related to abnormal upper limb proximal-distal interaction. The proximal kinematics of stroke survivors are influenced by finger function [23]. Importantly, the abnormal flexor synergy index showed a moderate positive correlation with the FMA-UE A score (a measure of proximal motor function including synergy), suggesting that the developed measure captures abnormal flexor synergy in the proximal upper limb. Hence, our findings confirm the concurrent validity of these scales.
Responsiveness
The FMA-UE A score and abnormal flexor synergy index showed good responsiveness. The FMA-UE has demonstrated high responsiveness in patients with chronic stroke [33], which aligns with our findings. In contrast, a systematic review and meta-analysis of kinematic assessments in patients with stroke reported low responsiveness [34]. Nonetheless, our developed kinematic indicator was highly responsive. This difference may be because our index captured abnormal flexor synergy, including various aspects such as motor paralysis, spasticity, and abnormal synergies of the hemiplegic upper limb.
Clinical implication
Abnormal flexor synergy index provides a quantitative and simplified assessment of upper extremity motor function. The European evidence-based recommendations for clinical assessment of the upper limb in neurorehabilitation (CAULIN) recommend including kinematic assessments alongside general upper extremity functional assessments [35]. Kinematic assessments can detect subtle changes and provide valuable information for individualized treatment planning and evaluation [36], aligning with this index. An increase in the abnormal flexor synergy index, even with the same shoulder flexion angle, implies an expanded reaching range and a deviation from the flexion synergy pattern. In the future, the optimal reaching range can be calculated using this index. Notably, our method can easily assess abnormal flexor synergy. Although kinematic assessments are increasingly used in research, no quantitative method for assessing abnormal flexor synergy has been established. Recent research using depth sensor cameras has mainly focused on interventions combined with VR technology [37], home-based applications [38], and alternatives to existing assessments [39]. While motion analysis has been conducted, it remains limited to calculating movement time, transition, and range of motion [40]. Furthermore, they are not yet widely applied in clinical practice [35] owing to their complexity and lack of user-friendliness [41]. By contrast, our measurement of the abnormal flexor synergy index took only a few seconds, making it suitable for clinical settings. Future studies should investigate its efficacy in larger cohorts and clinical trials.
Limitations
The present study has several limitations. First, the population is unbalanced due to selection bias attributable to its retrospective design. The severity of upper limb paralysis among participants was unevenly distributed. According to the FMA-UE total score, ≤ 19 points indicated severe, 20–47 points indicated moderate, and ≥ 48 points indicated mild impairment [42]. The classifications of participants in this study were as follows: 32 (31.1%) severe, 61 (59.2%) moderate, and 10 (9.7%) mild in the validity analysis, and 10 (58.8%) severe, 5 (29.4%) moderate, and 2 (11.8%) mild in the responsiveness analysis. Therefore, the developed indicator may not be suitable for patients with mild upper extremity paralysis. However, these results are clinically relevant because abnormal synergy is more common in patients with severe to moderate upper extremity paralysis. Thus, this indicator could be a new measure for assessing patients with severe to moderate upper extremity paralysis. Second, the clinical data collected only included FMA-UE and MAS, leaving it unclear whether participants had cognitive or higher brain function. However, all participants were chronic stroke survivors living independently at home, a population generally at low risk for significant cognitive impairment or higher brain dysfunction. Third, MAS reliability and validity reporting was inconsistent. While some studies indicate insufficient reliability and validity of the MAS [43], others have shown its reliability [44, 45] and validity [46, 47], showing an inconsistent trend. Nevertheless, the MAS remains the most commonly used spasticity assessment tool in clinical settings, and many studies have investigated the correlation between the developed indicators and the MAS, making its use in this analysis reasonable. Furthermore, we did not investigate reliability in this study, as it depends on the device used to capture the three-dimensional coordinate data. However, the reliability of using Kinect for patients with stroke has already been demonstrated [29], ensuring the reliability of the data in this study. Therefore, the developed measure is expected to be highly reliable.
Conclusion
The assessment of abnormal flexor synergy using the newly developed index demonstrated better validity and responsiveness. The results of the present study support the use of this index to quantitatively measure upper extremity function in patients with stroke.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- FMA-UE
Fugl–Meyer Assessment of the Upper Extremity
- MAS
Modified Ashworth Scale
- MP
Metacarpophalangeal
- PIP
Proximal interphalangeal
- SD
Standard deviation
- SRM
Standardized response mean
Author contributions
DI developed the kinematic analysis methods, conducted the kinematic analysis, and wrote the main manuscript. MK assisted in developing the kinematic analysis methods, supervised the entire study, and revised the manuscript. YH contributed to the development of the kinematic analysis and participated in data analysis. TK and YY assisted with data collection. RT provided consultations on the statistical analysis. TT supervised the entire study and revised the manuscript. All authors read and approved the final manuscript.
Funding
AMED under grant number JP19he2302006 and the Japan Society for the Promotion of Science (JSPS) KAKENHI grant number 23H00458 supported part of this research.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was reviewed and approved by the Ethics Committee of Keio University (Approval number: 20231079). The opt-out method was applied to obtain informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feigin VL. Stroke in developing countries: can the epidemic be stopped and outcomes improved? Lancet Neurol. 2007;6:94–7. 10.1016/S1474-4422(07)70007-8. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9:105–18. 10.1016/S1474-4422(09)70266-2. [DOI] [PubMed] [Google Scholar]
- 3.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS, Stroke. Neurologic and functional recovery the Copenhagen Stroke Study. Phys Med Rehabil Clin N Am. 1999;10:887–906. 10.1016/S1047-9651(18)30169-4. [PubMed] [Google Scholar]
- 4.Lawrence ES, Coshall C, Dundas R, Stewart J, Rudd AG, Howard R, et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnicpopulation. Stroke. 2001;32:1279–84. 10.1161/01.str.32.6.1279. [DOI] [PubMed] [Google Scholar]
- 5.Veerbeek JM, Kwakkel G, van Wegen EEH, Ket JCF, Heymans MW. Early prediction of outcome of activities of daily living after stroke: a systematic review. Stroke. 2011;42:1482–8. 10.1161/STROKEAHA.110.604090. [DOI] [PubMed] [Google Scholar]
- 6.Franceschini M, La Porta F, Agosti M, Massucci M, ICR2 group. Is health-related-quality of life of stroke patients influenced by neurological impairments at one year after stroke? Eur J Phys Rehabil Med. 2010;46:389–99. [PubMed] [Google Scholar]
- 7.Pollock A, St George B, Fenton M, Firkins L. Top 10 research priorities relating to life after stroke–consensus from stroke survivors, caregivers, and health professionals. Int J Stroke. 2014;9:313–20. 10.1111/j.1747-4949.2012.00942.x. [DOI] [PubMed] [Google Scholar]
- 8.Dobkin BH. Strategies for stroke rehabilitation. Lancet Neurol. 2004;3:528–36. 10.1016/S1474-4422(04)00851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang CE, Beebe JA. Relating movement control at 9 upper extremity segments to loss of hand function in people with chronic hemiparesis. Neurorehabil Neural Repair. 2007;21:279–91. 10.1177/1545968306296964. [DOI] [PubMed] [Google Scholar]
- 10.McMorland AJ, Runnalls KD, Byblow WD. A neuroanatomical framework for upper limb synergies after stroke. Front Hum Neurosci. 2015;9:82. 10.3389/fnhum.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zackowski KM, Dromerick AW, Sahrmann SA, Thach WT, Bastian AJ. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain. 2004;127:1035–46. 10.1093/brain/awh116. [DOI] [PubMed] [Google Scholar]
- 12.Schieber MH, Poliakov AV. Partial inactivation of the primary motor cortex hand area: effects on individuated finger movements. J Neurosci. 1998;18:9038–54. 10.1523/JNEUROSCI.18-21-09038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–80. 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- 14.Brunnstrom S. Motor testing procedures. Am Phys Ther Assoc. 1966;46:357–75. 10.1093/ptj/46.4.357. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Fu Y, Ye B, Babineau J, Ding Y, Mihailidis A. Technology-based Compensation Assessment and Detection of Upper extremity activities of stroke survivors: systematic review. J Med Internet Res. 2022;24:e34307. 10.2196/34307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118:495–510. 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 17.Ellis MD, Schut I, Dewald JPA. Flexion synergy overshadows flexor spasticity during reaching in chronic moderate to severe hemiparetic stroke. Clin Neurophysiol. 2017;128:1308–14. 10.1016/j.clinph.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sukal TM, Ellis MD, Dewald JPA. Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications. Exp Brain Res. 2007;183:215–23. 10.1007/s00221-007-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin MF, Kleim JA, Wolf SL. What do motor recovery and compensation mean in patients following stroke? Neurorehabil Neural Repair. 2009;23:313–9. 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- 20.Hadjiosif AM, Branscheidt M, Anaya MA, Runnalls KD, Keller J, Bastian AJ, et al. Dissociation between abnormal motor synergies and impaired reaching dexterity after stroke. J Neurophysiol. 2022;127:856–68. 10.1152/jn.00447.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Ruit M, van der Velden LL, Onneweer B, Benner JL, Haarman CJW, Ribbers GM, et al. System identification: a feasible, reliable and valid way to quantify upper limb motor impairments. J Neuroeng Rehabil. 2023;20:67. 10.1186/s12984-023-01192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopke JV, Hargrove LJ, Ellis MD. Coupling of shoulder joint torques in individuals with chronic stroke mirrors controls, with additional non-load-dependent negative effects in a combined-torque task. J Neuroeng Rehabil. 2021;18:134. 10.1186/s12984-021-00924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phan T, Nguyen H, Vermillion BC, Kamper DG, Lee SW. Abnormal proximal-distal interactions in upper-limb of stroke survivors during object manipulation: a pilot study. Front Hum Neurosci. 2022;16:1022516. 10.3389/fnhum.2022.1022516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McPherson LM, Dewald JPA. Abnormal synergies and associated reactions post-hemiparetic stroke reflect muscle activation patterns of brainstem motor pathways. Front Neurol. 2022;13:934670. 10.3389/fneur.2022.934670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Israely S, Leisman G, Carmeli E. Impaired coordination and recruitment of muscle agonists, but not abnormal synergies or co-contraction, have a significant effect on motor impairments after stroke. Adv Exp Med Biol. 2020;1279:37–51. 10.1007/5584_2020_528. [DOI] [PubMed] [Google Scholar]
- 26.Reisman DS, Scholz JP. Aspects of joint coordination are preserved during pointing in persons with post-stroke hemiparesis. Brain. 2003;126:2510–27. 10.1093/brain/awg246. [DOI] [PubMed] [Google Scholar]
- 27.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. 10.2340/1650197771331. [PubMed] [Google Scholar]
- 28.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–7. 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 29.Mobini A, Behzadipour S, Saadat M. Test-retest reliability of Kinect’s measurements for the evaluation of upper body recovery of stroke patients. Biomed Eng OnLine. 2015;14:75. 10.1186/s12938-015-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen J. Statistical power analysis for the behavior sciences. Volume II. Hillsdale: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 31.Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123:940–53. 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen JB, Christensen MS, Farmer SF, Lorentzen J. Spastic movement disorder: should we forget hyperexcitable stretch reflexes and start talking about inappropriate prediction of sensory consequences of movement? Exp Brain Res. 2020;238:1627–36. 10.1007/s00221-020-05792-0. [DOI] [PubMed] [Google Scholar]
- 33.Wei XJ, Tong KY, Hu XL. The responsiveness and correlation between Fugl-Meyer Assessment, Motor Status Scale, and the Action Research Arm Test in chronic stroke with upper-extremity rehabilitation robotic training. Int J Rehabil Res. 2011;34:349–56. 10.1097/MRR.0b013e32834d330a. [DOI] [PubMed] [Google Scholar]
- 34.Villepinte C, Verma A, Dimeglio C, De Boissezon X, Gasq D. Responsiveness of kinematic and clinical measures of upper-limb motor function after stroke: a systematic review and meta-analysis. Ann Phys Rehabil Med. 2021;64:101366. 10.1016/j.rehab.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Prange-Lasonder GB, Alt Murphy M, Lamers I, Hughes AM, Buurke JH, Feys P, et al. European evidence-based recommendations for clinical assessment of upper limb in neurorehabilitation (CAULIN): data synthesis from systematic reviews, clinical practice guidelines and expert consensus. J Neuroeng Rehabil. 2021;18:162. 10.1186/s12984-021-00951-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thrane G, Sunnerhagen KS, Persson HC, Opheim A, Alt Murphy MM. Kinematic upper extremity performance in people with near or fully recovered sensorimotor function after stroke. Physiother Theory Pract. 2019;35:822–32. 10.1080/09593985.2018.1458929. [DOI] [PubMed] [Google Scholar]
- 37.Luo Z, Lim AE, Durairaj P, Tan KK, Verawaty V. Development of a compensation-aware virtual rehabilitation system for upper extremity rehabilitation in community-dwelling older adults with stroke. J Neuroeng Rehabil. 2023;20:56. 10.1186/s12984-023-01183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo L, Wang J, Wu Q, Li X, Zhang B, Zhou L, et al. Clinical study of a wearable remote rehabilitation training system for patients with stroke: Randomized controlled pilot trial. JMIR Mhealth Uhealth. 2023;11:e40416. 10.2196/40416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheng B, Chen X, Cheng J, Zhang Y, Xie SSQ, Tao J, et al. A novel scoring approach for the Wolf motor function test in stroke survivors using motion-sensing technology and machine learning: a preliminary study. Comput Methods Programs Biomed. 2024;243:107887. 10.1016/j.cmpb.2023.107887. [DOI] [PubMed] [Google Scholar]
- 40.Lee YM, Lee S, Uhm KE, Kurillo G, Han JJ, Lee J. Upper limb three-dimensional reachable workspace analysis using the Kinect sensor in hemiplegic stroke patients: a cross-sectional observational study. Am J Phys Med Rehabil. 2020;99:397–403. 10.1097/PHM.0000000000001350. [DOI] [PubMed] [Google Scholar]
- 41.Kwakkel G, van Wegen EEH, Burridge JH, Winstein CJ, van Dokkum LEH, Alt Murphy M, et al. Standardized measurement of quality of upper limb movement after stroke: consensus-based core recommendations from the second stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair. 2019;33:951–8. 10.1177/1545968319886477. [DOI] [PubMed] [Google Scholar]
- 42.Woodbury ML, Velozo CA, Richards LG, Duncan PW. Rasch analysis staging methodology to classify upper extremity movement impairment after stroke. Arch Phys Med Rehabil. 2013;94:1527–33. 10.1016/j.apmr.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Fleuren JF, Voerman GE, Erren-Wolters CV, Snoek GJ, Rietman JS, Hermens HJ, et al. Stop using the Ashworth Scale for the assessment of spasticity. J Neurol Neurosurg Psychiatry. 2010;81:46–52. 10.1136/jnnp.2009.177071. [DOI] [PubMed] [Google Scholar]
- 44.Gregson JM, Leathley MJ, Moore AP, Smith TL, Sharma AK, Watkins CL. Reliability of measurements of muscle tone and muscle power in stroke patients. Age Ageing. 2000;29:223–8. 10.1093/ageing/29.3.223. [DOI] [PubMed] [Google Scholar]
- 45.Li F, Wu Y, Li X. Test-retest reliability and inter-rater reliability of the modified Tardieu Scale and the Modified Ashworth Scale in hemiplegic patients with stroke. Eur J Phys Rehabil Med. 2014;50:9–15. [PubMed] [Google Scholar]
- 46.Min JH, Shin YI, Joa KL, Ko SH, Shin MJ, Chang JH, et al. The correlation between Modified Ashworth Scale and biceps T-reflex and inter-rater and intra-rater reliability of biceps T-reflex. Ann Rehabil Med. 2012;36:538–43. 10.5535/arm.2012.36.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin FM, Sabbahi M. Correlation of spasticity with hyperactive stretch reflexes and motor dysfunction in hemiplegia. Arch Phys Med Rehabil. 1999;80:526–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.


