Abstract
We have conducted a systematic review and meta-analysis to evaluate the cosmetic applications of Dendropanax morbifera extracts (DMEs). A total of 261 articles were screened; however, after eliminating inappropriate studies, only 16 individual studies were eligible. The comparative standardized mean difference (SMD) between the DME treatment and control groups was used to evaluate the cosmetic properties of DME, including its biocompatibility, whitening effects, and anti-inflammatory and antimicrobial properties. DME treatment exhibited positive results in controlling hyperpigmentation, including effective inhibition of the production of tyrosinase and melanin, with SMDs of 6.85 [4.27, 9.44] and 23.38 [12.94, 33.82], respectively. Moreover, the results confirmed the anti-inflammatory properties in terms of suppressing the expression of interleukin markers (ILs) (SMD = 5.22 [3.12, 7.33]) and reducing NO production (SMD = 6.92 [2.89, 10.96]). DME treatment also effectively inhibited bacteria growth, which causes skin disorders. According to the results, DMEs are shown to be highly biocompatibility, with excellent anti-hyperpigmentation, anti-inflammatory, and antimicrobial properties that contribute significantly to improving skin appearance. The findings provide strong evidence for further research into the in vivo effects of DMEs and their potential cosmetic applications, which could lead to clinical trials in the future.
Keywords: Dendorpanax morbifera, hyperpigmentation, anti-inflammatory, antimicrobial, meta-analysis
1. Introduction
The use of botanical extracts for therapeutic purposes, such as skincare and cosmetics, has gained popularity in recent times. One of the promising candidates is the Dendropanax genus of the Araliaceae family, which has been extensively used in Korea, Japan, South America, and other parts of the world [1,2]. There are approximately 6 to 10 species in this genus, including D. morbifera, D. gonatopodus, D. dentiger, D. capillaris, D. chevalieri, and D. arboreus. In Korea, D. morbifera is native to the tropics and is predominantly found on Jeju Island [2,3,4,5]. The Ministry of Food and Drug Safety, Korea Food and Drug Administration have registered various parts of the D. morbifera plant, including the edible leaf, stems, seeds, bark, and roots, as food additives and alternative folkloric medicine [2].
D. morbifera has various bioactive compounds, both in pure form and crude extracts, demonstrating effectiveness in their biological activities. These compounds are categorized as polyphenols, flavonoids, tannins, pyrimidines, essential oils, terpenoids, phenol carboxylic acids, and alkaloids, which can be applied in medical and cosmetic fields [2,6,7,8,9]. Notably, almost all of these bioactive compounds show antioxidant, anti-inflammatory, anti-cancer, and neuroprotective properties. Some of the most notable compounds are quercetin, rutin, gallic acid, 2,5-dihydroxybenzoic acid, catechin, 4-hydroxybenzoic acid, caffeic acid, syringic acid, p-coumaric acid, trans-ferulic acid, salicylic acid, hesperidin, naringin, resveratrol, myricetin, and trans-cinnamic acid [2].
As the demand for natural and sustainable ingredients in cosmetic products increases, exploring the scientific basis behind the cosmetic and dermatology efficacy of D. morbifera extract (DME) becomes imperative. Recent studies have proven the cosmetic properties of DME, such as antioxidant, anti-inflammatory, antimicrobial, moisturizing, and biocompatibility ones. The DME possesses potent antioxidant properties that are functional for skin-whitening and skin-aging control. The antioxidant properties are demonstrated by high scavenging activities for 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bus(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), reactive oxygen species (ROS) scavenging activities (60%–92%), superoxide dismutase (SOD)-like activities, and low cytotoxicity, owing to their high contents of flavonoid and phenolic compounds [10,11,12,13,14]. Additionally, the extracts show potential anti-inflammatory properties by reducing the production of NO, inducible NO synthase and interleukin markers (ILs), nuclear translocation of nuclear factor-B (NF-B), and tumor necrosis factor- (TNF-) in lipopolysaccharide (LPS)-stimulated RAW264.7 cells [15]. The extract can also mitigate 2,4-dinitrochlorobenzene (DNCB)-induced inflammatory dermatitis in vivo without side effects, suggesting that it can be used for development as a botanical drug to treat atopic dermatitis based on its anti-inflammatory activities [15]. The DME also exhibits antimicrobial activity against Streptococcus mutans, Candida albicans, Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa [4,16,17]. For instance, the hot water and ethanol extracts from the leaves of D. morbifera significantly eliminate Porphyromonas gingivalis after 6 h of incubation, with the minimum inhibitory concentration (MIC) being around 3.13 and 6.25 mg/mL, respectively [18]. The fermented extract of boughs of D. morbifera (200 mg/mL) has strong antimicrobial effects against S. epidermidis and S. aureus with inhibition zone diameters of about 9.3–15.2 mm, suggesting that the extract produced from D. morbifera bough has the potential to produce health-oriented food materials [19]. The 60% ethanol extract of roasted D. morbifera leaves shows the ability to inhibit the growth of Bacillus cereus, S. aureus, and Pichia jadinii with an MIC higher than 250 μg/mL [20]. Moreover, they can inhibit tyrosinase activity and melanin formation in -melanocyte-stimulating hormone (-MSH)-induced melanoma B16/F1 cells, indicating whitening effects [13,14,21]. They also have high promotive effects in growth factor IGF protein expression (156%) and strongly inhibit TGF2 protein expression (78.3%) in human hair cells, making them beneficial for hair growth applications [12]. In addition, 1-tetradecanol isolated from D. morbifera has moisturizing properties that can aid in preventing hair loss and enhancing hair density. The study examined the skin moisturizing effect using HR-1 hairless mice. The transepidermal water loss (TEWL) in the group treated with 1-tetradecanol was significantly lower than that of the control group, showing a reduction of 30% [5].
Although the application of DME to cosmetics has attracted the attention of scientists, no systematic review has addressed this interesting topic. Therefore, this paper presents a comprehensive systematic review and meta-analysis of the existing literature, aiming to elucidate the effectiveness of DME in cosmetic applications. By analyzing and synthesizing findings from the electronic literature, this study provides a comprehensive understanding of the beneficial effects of DME on different skin conditions, such as hyperpigmentation, inflammation, and microbial infections. By integrating diverse articles, it aims to provide valuable insights into the efficacy of DME in cosmetic applications. The findings of this review may guide future research, inform product development in the cosmetics industry, and provide a solid foundation for evidence-based decisions regarding the use of DME in skin care formulations.
2. Materials and Methods
2.1. Literature Search Strategy
This systematic review was designed according to the “Preferred Reporting Items for Systematic Reviews and Meta-Analysis” (PRISMA) 2009 protocol. A literature search for the application of DME in cosmetic products was performed to obtain relevant papers published up to 2024. Data from Korean and English databases were collected from the Cochrane Library, PubMed, Elsevier, EMBASE, and MedRxiv. An electronic search was performed using the following keywords: D. morbifera, DME, antioxidants, anti-inflammatory, antimicrobial, whitening effects, and cosmetic benefits. This systematic review has been registered on PROSPERO under the registration number CRD42024506153.
2.2. Study Selection and Data Extraction
2.2.1. Study Selection
This review included all in vitro studies that investigated the application of DME to improve skin appearance, specifically focusing on anti-aging, whitening effects, and acne treatments. In vitro studies with at least one matched perspective between the control and treatment groups were considered. Individual studies with multiple purposes were selected if they directly compared the treatment and the control groups. The main outcomes were presented as the mean ± standard difference (SD). There were no restrictions on the treatment and control groups, raw materials, extraction methods, or geographical regions. All articles were published in Korean or English and were translated into English for easy data extraction.
Studies on DME applications in other fields, such as medicine and the food industry, were not included. Articles that were partially or entirely duplicated from different studies were also excluded. This study did not include conference abstracts, reviews, primary manuscripts, and seminar presentations.
2.2.2. Data Extraction
The selected studies were screened by three independent reviewers to extract the appropriate data for the systematic review and meta-analysis. The extraction data include the first author’s name, region of study, year of publication, raw materials (stem, leave, branch, and root), extraction method, study design, cosmetic properties (antioxidant, anti-inflammatory, antimicrobial, cell viability, and whitening effect), and primary outcomes (mean ± SD values).
2.3. Meta-Analysis
For all continuous outcomes, the mean and SD of each primary outcome in both the control and DME treatment groups were pooled using the random-effects model and calculated as the SMD (95% confidence interval CI) [22]. The SMD was statistically significant at the 5% level (p < 0.05) if the 0 value was not within the 95% CI. SMDs are categorized into three phases: small (SMD ≤ 0.2), moderate (SMD approximately 0.5), and large (SMD ≥ 0.8) [22]. The heterogeneity of the analysis was quantified using the I2 statistics. Heterogeneity was considered small, moderate, or large when it was less than 25%, approximately 25–50%, or >50%, respectively [22]. The bias was assessed using a funnel plot.
All analyses were performed using Review Manager (version 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) [23].
3. Results
3.1. Characteristics of Included Studies
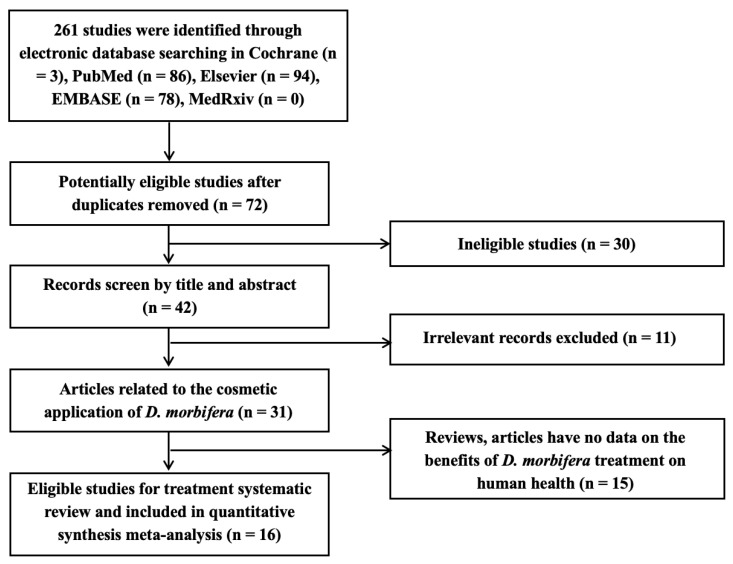
The screening process for the summarized articles is shown in the flowchart (Figure 1). In this study, 261 publications were collected by searching electronic databases using the aforementioned keywords. After the careful screening of these articles by the three reviewers, individual studies were selected to assess the cosmetic properties of DME using a meta-analysis.
Figure 1.
Systematic screening stages of the literature review on D. morbifera extracts (DMEs).
Description of Included Studies
The main characteristics of the 16 included studies are summarized in Table 1. Four studies reported the anti-inflammation effects of DME in skin treatment [12,15,24,25]. Four studies investigated the antimicrobial properties of DME [4,16,19,26]. Seven studies demonstrated the whitening effects of DME for improving skin appearance [3,5,13,14,21,27,28]. The biocompatibility of the extracts with human skin and hair cell lines was investigated in five studies [4,12,13,14,29]. All the included studies were conducted only in Korea between 2010–2024, since D. morbifera is cultivated primarily in Korea. Various parts of D. morbifera, including the leaves (n =15), wood (n = 1), stems (n = 1), and branches (n = 3), were used in the studies. D. morbifera underwent various extraction methods, such as methanol, ethanol, hot water extraction, and microwave-assisted extraction, before being tested in vitro on human cell lines and bacteria. Most studies reported high antioxidant compounds (flavonoids and polyphenolics), suggesting high biocompatibility properties. The studies included three major experimental subject types: normal human cells (HFDPC and HaCaT cells), cancer cells (RAW264.7, L929, B16F10, and EL-4 T), and bacteria (S. aureus, S. mutans, and P. acnes). The extracted data from several main outcomes, such as cell viability (%), inhibition of the expression of pro-inflammatory markers (IL and TNF-) (pg/mL), NO, inhibition of bacterial growth (%), and inhibition of melanin, tyrosinase, and elastase productions (%), were used in the meta-analysis to increase the statistical significance of the results.
Table 1.
Summary of the characteristics of included studies.
| References | Region | Raw D. morbifera | Extraction Methods | Physicochemical Properties | Cosmetic Properties |
Experimental Subjects | Main Outcomes | |
|---|---|---|---|---|---|---|---|---|
| Compounds | Mean ± SD | |||||||
| Akram et al., 2016 [24] | Korea | Leaves | Methanol extraction | — | — | Anti-inflammatory | RAW264.7 cells | Inhibition of the expression of IL and TNF- markers (pg/mL) and NO production (%) |
| Choi et al., 2021 [26] | Korea | Leaves | Ethanol extraction | — | — | Antimicrobial | HFDPC cells S. aureus |
Inhibition of bacterial growth (mm) Cell viability (%) |
| Choo et al., 2019 [15] | Korea | Leaves | Ethanol extraction | — | — | Anti-inflammatory | RAW246.7 cell | Inhibition of the expression of IL and TNF- markers (pg/mL) and NO production (%) |
| Hoang et al., 2022 [13] | Korea | Leaves and wood | Microwave-assisted extraction | Phenolic (mg GAE/ug) Flavonoid (mg QE/g) |
313.03 ± 3.9 32.37 ± 0.9 |
Whitening effect Biocompatibility |
HaCaT cells | Inhibition of tyrosinase production (%) Cell viability (%) |
| Jang et al., 2020 [16] | Korea | Leaves | Ethanol extraction | Phenolic (mg GAE/g) Flavonoid (mg QE/g) |
48.77 ± 1.52 31.86 ± 1.44 |
Antimicrobial | L929 cells S. aureus |
Inhibition of bacterial growth (mm) |
| Kim et al., 2015 [4] | Korea | Branches | Ethanol extraction | — | — | Antimicrobial Biocompatibility |
HaCaT cells S. mutans |
Inhibition of bacterial growth (mm) Cell viability (%) |
| Kim et al., 2021 [3] | Korea | Leaves and stems | Methanol extraction | Phenolic (mg GAE/g) Flavonoid (mg QE/g) |
51.4 ± 1.32 11.3 ± 2.01 |
Whitening effect | — | Inhibition of tyrosinase and melanin production (%) |
| Lee et al., 2015 [5] | Korea | Leaves | n-hexane extraction | — | — | Whitening effect | B16F10 cells | Inhibition of tyrosinase production (%) |
| Lee et al., 2019 [19] | Korea | Leaves and branches | Hot-water extraction | — | — | Antimicrobial | S. aureus | Inhibition of bacterial growth (mm) |
| Mo et al., 2013 [27] | Korea | Leaves | Methanol extract | Flavonoids (mg RE/g) | 98.53 ± 4.09 | Whitening effects | B16F10 cells | Inhibition of tyrosinase and melanin production (%) |
| Park et al., 2013 [14] | Korea | Leaves | Ethanol extraction | — | — | Whitening effect Biocompatibility |
HaCaT cells | Inhibition of melanin production (%) Cell viability (%) |
| Park et al., 2014 [28] | Korea | Leaves | Ethanol extraction | — | — | Whitening effect | B16F10 cells | Inhibition of tyrosinase production (%) |
| Park et al., 2016 [12] | Korea | Leaves | Methanol extraction | Phenolic (mg GAE/g) Flavonoid (mg QE/g) |
74.08 ± 7.18 97.36 ± 2.24 |
Anti-inflammatory Biocompatibility |
HFDPC cells P. acnes |
Inhibition of bacterial growth (mm) Cell viability (%) |
| Park et al., 2017 [25] | Korea | Leaves | Water extraction | — | — | Anti-inflammatory | EL-4 T cells | Inhibition of the expression of IL markers (pg/mL) |
| Park et al., 2019 [29] | Korea | Leaves and branches | Ethanol extraction | — | — | Biocompatibility | HFDPC cells | Cell viability (%) |
| Park et al., 2020 [21] | Korea | Leaves | Water extraction | — | — | Whitening effect | B16F10 cells | Inhibition of tyrosinase and melanin production (%) |
3.2. Quality Assessment of Included Studies
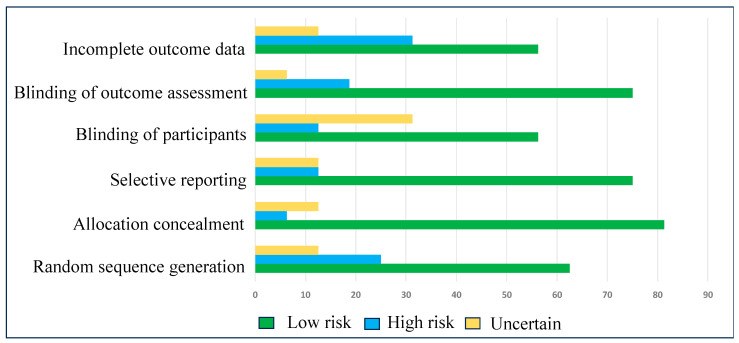
Eligible studies were qualified by considering the bias in the random sequence generation, selective reporting, allocation concealment, blinding of participants, blinding of outcome assessment, and incomplete outcome data, following the Cochrane guidelines [23]. There were three levels of assessment–low risk, high risk, and uncertain–to exhibit the lack of information and uncertainty over the potential for bias. Nearly all the criteria exhibited a low risk of bias, resulting in an evident enhancement of the statistical significance of the meta-analysis (Table 2 and Figure 2).
Table 2.
Risk of bias rating in individual studies.
| Study | Random Sequence Generation | Allocation Concealment | Selective Reporting | Blinding of Participants | Blinding of Outcome Assessment | Incomplete Outcome Data |
|---|---|---|---|---|---|---|
| Akram et al., 2016 [24] | ||||||
| Choi et al., 2021 [26] | ||||||
| Choo et al., 2019 [15] | ||||||
| Hoang et al., 2022 [13] | ||||||
| Jang et al., 2020 [16] | ||||||
| Kim et al., 2015 [4] | ||||||
| Kim et al., 2021 [3] | ||||||
| Lee et al., 2015 [5] | ||||||
| Lee et al., 2019 [19] | ||||||
| Mo et al., 2013 [27] | ||||||
| Park et al., 2013 [14] | ||||||
| Park et al., 2014 [28] | ||||||
| Park et al., 2016 [12] | ||||||
| Park et al., 2017 [25] | ||||||
| Park et al., 2019 [29] | ||||||
| Park et al., 2020 [21] |
Risk of bias rating:  Low risk of bias;
Low risk of bias;  High risk of bias;
High risk of bias;  Uncertain.
Uncertain.
Figure 2.
Risk of bias in individual studies.
3.3. Beneficial Effects of D. morbifera in Cosmetic Applications
3.3.1. Biocompatibility
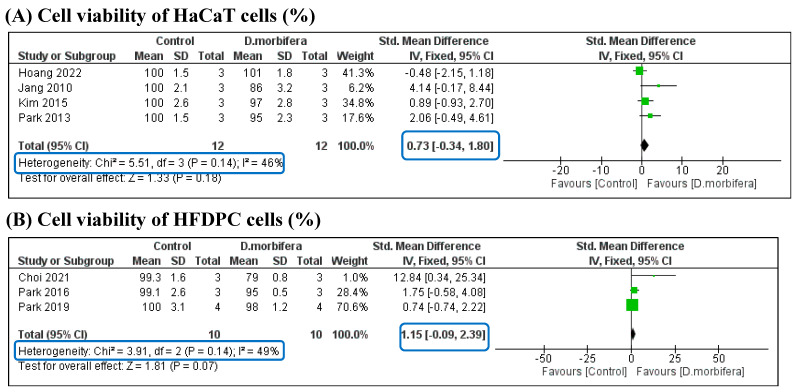
Two types of human cells are commonly used in in vitro experiments for cosmetic applications: normal human skin (HaCaT) cells and normal human follicle dermal papilla (HFDPC) cells. In this study, the biocompatibility of DME was analyzed in two comparisons based on the treatment of the two cell lines (Figure 3 and Table 3). The cytotoxicity of DME on HaCaT and HFDPC cells was compared with that of the control group. The pooled SMD (0.80 [−0.30, 1.90] and 1.15 [−0.09, 2.39], respectively) indicated that there was no difference in the viability of HaCaT and HFDPC cells between the two groups, suggesting that DME is highly biocompatible with human skin fibroblast.
Figure 3.
Comparison of the cell viability (%) of HaCaT (A) and HFDPC (B) cells between the control and the DME treatment groups [4,12,13,14,16,26,29].
Table 3.
Summary of standardized mean differences (SMDs) comparison between the DME treatment and control groups.
| Cosmetic Properties | No. of Studies | Comparison Perspectives | n | SMD (95% CI) | I2
(%) |
|---|---|---|---|---|---|
| Biocompatibility | 5 | Cell viability of HaCaT cells (%) | 12 | 0.73 [−0.34, 1.80] | 46 |
| Cell viability of HFDPC cells (%) | 10 | 1.15 [−0.09, 2.39] | 49 | ||
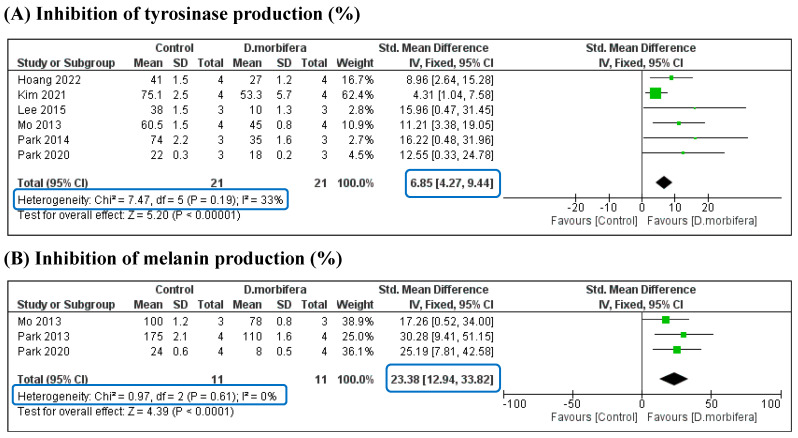
| Whitening effects | 7 | Inhibition of the production of tyrosinase (%) | 21 | 6.85 [4.27, 9.44] | 33 |
| Inhibition of the production of melanin (%) | 11 | 23.38 [12.94, 33.82] | 0 | ||
| Anti-inflammatory properties | 4 | Inhibition of the expression of IL markers (pg/mL) | 18 | 5.22 [3.12, 7.33] | 29 |
| Inhibition of the expression of TNF-α (pg/mL) | 7 | 1.09 [−0.5, 2.67] | 83 | ||
| Inhibition of the production of NO (%) | 7 | 6.92 [2.89, 10.96] | 0 | ||
| Antimicrobial properties | 5 | Inhibition of the growth of bacteria (mm) | 18 | [3.15, 7.42] | 31 |
3.3.2. Whitening Effects
Three comparisons were performed between the mean differences of the control and the DME treatment groups from three perspectives: inhibition of tyrosinase, melanin, and elastase production (%). The results of each study showed that the synthesis of tyrosinase and melanin in the control group was significantly higher than that in the DME treatment group. Meta-analysis confirmed that there were significant differences in the levels of tyrosinase and melanin between the two groups, with SMDs of 6.85 [4.27, 9.44] and 23.38 [12.94, 33.82], respectively (Figure 4 and Table 3). In addition, the analyses were highly significant because of the low heterogeneity of these comparisons (I2 = 0% and 33%). The significant downregulation of tyrosinase and melanin suggests that DMEs are potential ingredients for whitening cosmetic formulations.
Figure 4.
Comparison of downregulating the synthesis of tyrosinase (A) and melanin (B) between the control and the DME treatment groups [3,5,13,14,21,27,28].
3.3.3. Anti-Inflammation Properties
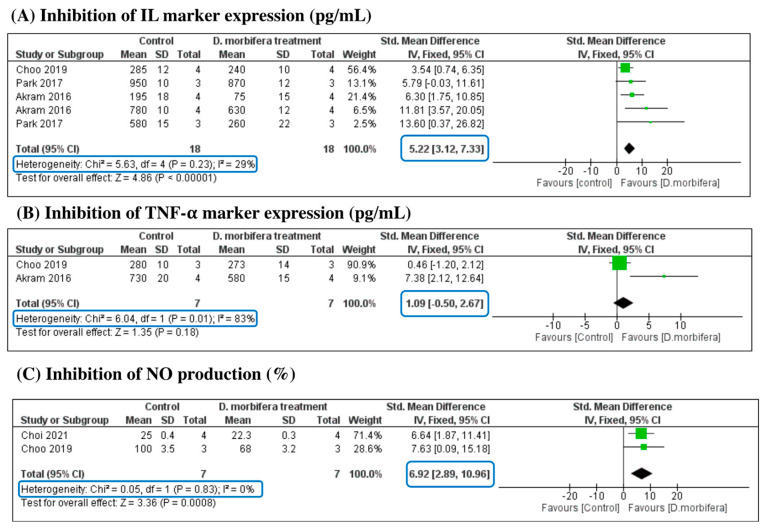
This analysis aimed to evaluate the impact of DME on inflammation signals based on a comparison with three sub-criteria: inhibition of the expression of IL markers (pg/mL), inhibition of the expression of TNF-α (pg/mL), and inhibition of NO production (%). The SMD of each analysis is presented in Figure 5 and Table 3. The meta-analysis indicated that the DME treatment significantly inhibited the expression of pro-inflammatory IL markers, with an SMD of 5.22 [3.12, 7.33] (I2 = 29% and p = 0.23). The production of NO was also reduced in the treatment group compared to the control groups, with significant differences in SMD (6.92 [2.89, 10.96], I2 = 0%, and p = 0.83). However, the expression of TNF- was not significantly different between the two groups, indicating that the DME treatment was ineffective in suppressing the pro-inflammatory TNF-α marker (SMD = 1.09 [−0.5, 2.67]).
Figure 5.
Comparison of inhibition of the expression of pro-inflammatory markers (IL (A) and TNF- (B)) and the production of NO (C) between control and DME treatment groups [15,24,25,26].
3.3.4. Antimicrobial Properties
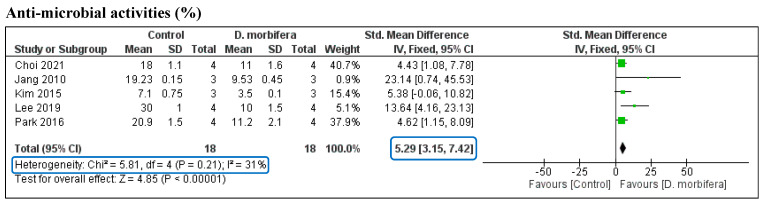
The pooled SMD of the five studies on the antimicrobial activity of DME was assessed by analyzing the statistical differences in bacterial growth between the treatment and the control groups. The pooled SMD was significantly different between the two groups (SMD = 5.29 [3.15, 7.42]) without any heterogeneity (I2 = 31%, p = 0.21) (Figure 6 and Table 3). This indicates that DMEs can effectively suppress bacterial growth and therefore be used in the treatment of skin disorders caused by microbes.
Figure 6.
Comparison of bacterial growth inhibition between the control and the DME treatment groups [4,12,16,17,26].
3.3.5. Subgroup Analysis
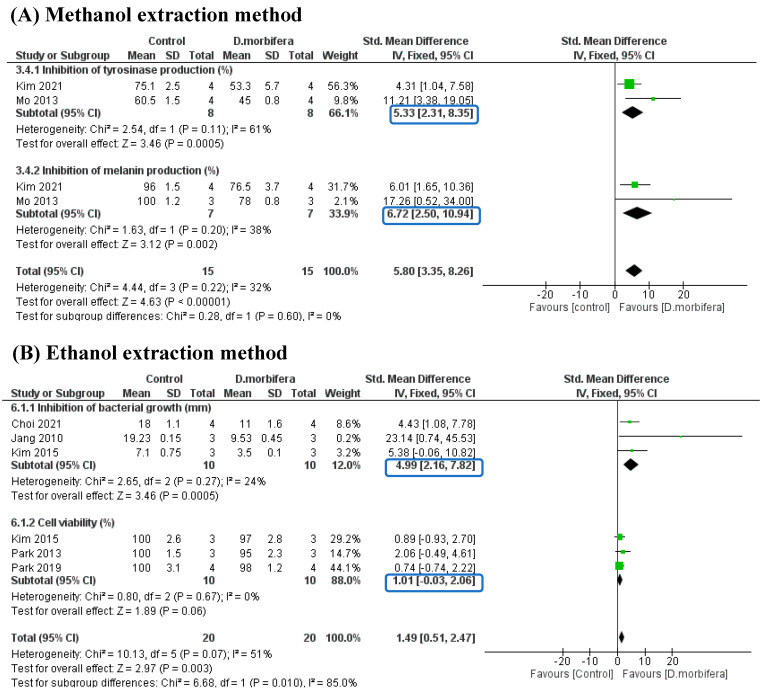
Due to the different extraction methods used in these included studies (e.g., methanol, ethanol, water, n-hexane, and microwave-assisted extractions), subgroup analyses were designed to evaluate the beneficial effects of DME in improving skin appearance. However, due to the limited number of included studies, subgroup analyses were performed only on methanol and ethanol extractions, which could show statistical significance outcomes when comparing the control and treatment groups.
The first subgroup analysis evaluated the efficacy of DME-based methanol extraction in reducing the production of tyrosinase and melanin. Two eligible studies were included in this manner [3,27], which showed significant differences in tyrosinase and melanin production between the two groups. The DME treatment resulted in a decrease in tyrosine and melanin content compared to the control group. This subgroup achieved good statistical significance, with I2 = 32% and p = 0.22 (Figure 7A).
Figure 7.
Subgroup analysis evaluating the efficacy of DME-based methanol extraction [3,27] (A) and DME-based ethanol extraction when compared to the control group [4,14,16,26,29] (B).
In the second subgroup analysis, the ethanol extraction method was used, which was divided into two subcomparisons (Figure 7B). The first subcomparison evaluated the inhibition of bacterial growth (mm), while the second subcomparison addressed cell viability (%). The results showed that bacterial growth was significantly decreased in the DME treatment group compared to the control group (SMD = 4.99 [2.16, 7.82], I2 =24%, and p = 0.27). The cell viability comparison showed no significant difference between the DME and the control group (SMD = 1.01 [−0.03, 2.06] and I2 = 0%), suggesting that DME treatment had a low toxicity and did not affect the cell proliferation of HaCaT and HFDPC cells. This subgroup analysis indicated that the DME treatment helped eliminate harmful factors for the skin, such as bacteria with a high biocompatibility.
4. Analysis Bias
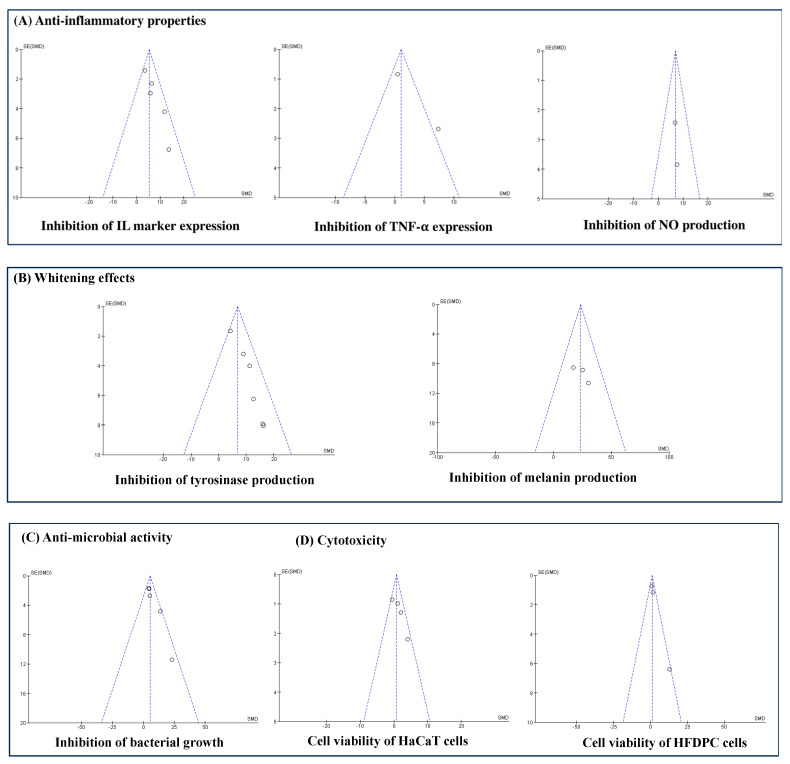
Funnel plots were constructed to detect publication biases. The results indicated that the population of the studies included for each analysis criterion was small, which led to underrated meta-analysis results and publication biases (Figure 8). Therefore, a meta-analysis with numerous prospective studies should be performed to improve the statistical significance and publication bias.
Figure 8.
Publication bias of the included studies on the effectiveness of using DME in cosmetic applications.
5. Discussion
We conducted a systematic review and meta-analysis of 16 in vitro studies to evaluate the cosmetic properties of DMEs. This study found that DME, despite using different extraction methods, contained high flavonoid and phenolic contents, which indicated a high antioxidant activity. The biocompatibility of DMEs was confirmed through a meta-analysis. Treatment with these extracts did not result in significant toxicity to normal human skin or human hair follicle cells compared with the control group. The SMDs between the two groups for HaCaT and HFDPC cells were 0.80 [−0.30, 1.90] (I2 = 47%) and 1.15 [−0.09, 2.39] (I2 = 49%), respectively. These extracts possess potent antioxidant properties that can effectively eliminate the production of ROS, which causes oxidative stress in cell lines, ultimately leading to improved cell proliferation. Considering these properties, this study suggests that these extracts can be safely used in cosmetic applications.
Hyperpigmentation is a skin condition in which specific areas of the skin become darker or have pigmentation [30]. There are different types of this condition, including freckles, sunspots, age spots, post-inflammatory hyperpigmentation (PIH), and melasma. Darkening occurs owing to excess melanin and tyrosinase production [31]. Melanin is synthesized by melanocytes and other specialized cells in the basal layer of the epidermis [30]. For instance, long-term exposure to UV rays leads to more melanin production as a protective response, causing tanning, uneven pigmentation, and dark spot formation. Tyrosinase, an enzyme that catalyzes the conversion of tyrosine to dihydroxyphenylalanine (DOPA), is the initial step of melanin synthesis [31]. DOPA is then converted into dopachrome, which is either pheomelanin or eumelanin. Pheomelanin contributes to red and yellow pigments, while eumelanin produces black and brown pigments. Once synthesized, melanin is transported to melanosomes and transferred to neighboring keratinocytes in the epidermis, thus determining skin color [31]. Therefore, treatment for hyperpigmentation should contain ingredients that inhibit melanin and tyrosinase production to promote skin tone. Hydroquinone, kojic acid, arbutin, and plant extracts inhibit tyrosinase, reduce melanin production, and lighten dark spots [32]. DMEs contain antioxidant compounds which can help reduce skin oxidative stress. This indirectly inhibits melanin and tyrosinase activity, which prevents melanin overproduction and in turn reduces hyperpigmentation [14,31]. Antioxidants also help neutralize free radicals that contribute to skin damage and hyperpigmentation and reduce lipid peroxidation, oxidative stress and various oxidative insults, preventing melanin overproduction [3,5,32]. DME has been shown to have a higher DPPH-radical scavenging activity (82.92 ± 0.49%) than butylated hydroxytoluene (56.71 ± 6.34%) and one that is at the same level as vitamin C (90.11 ± 0.13%), at the highest concentration of 500 μg/mL [4]. Furthermore, the 95% ethanol extractions of D. morbifera (roots, leaves, and stems) in the range of 10–200 μg/mL effectively inhibit intracellular ROS production against hepatocellular injury induced by t-BHP in HepG2 cells [33]. Notably, DMEs have shown the potential to downregulate tyrosinase expression, which results in a decrease in tyrosinase production. These extracts can help prevent the uneven distribution of hyperpigmentation-associated pigmentation by modulating melanin transfer to neighboring skin cells, thereby influencing the transport and distribution of melanin within the skin [3,5,32]. Moreover, DME can promote collagen synthesis, which supports the skin and contributes to overall skin health [3]. Various in vitro studies have investigated the intracellular mechanisms. It has been found that DME inhibits α-melanocyte stimulating hormone (α-MSH)-stimulated intra/extracellular melanogenesis on melanoma B16/F1 cells in a concentration-dependent manner [14]. The ethyl acetate and aglycone fractions of D. morbifera leaf extract are found to be more effective when inhibiting α-MSH (21% and 44% at 25 μg/mL, respectively) than arbutin (15% at 25 μg/mL), a whitening agent [14]. The extraction of D. morbifera leaf has been reported to decrease melanin content by inhibiting the intracellular tyrosinase activity and protein expression of tyrosinase and tyrosinase-related protein-2 (TRP-2) at concentrations of 25 and 50 μg/mL [28]. Another study shows that D. morbifera stem extract has high collagenase and elastase inhibitory activities (79.5% and 23%), with D. morbifera leaf extract exhibiting potential tyrosinase inhibitory activity (53.3%) [3]. In addition, inflammation can contribute to post-inflammatory hyperpigmentation [34], and the compounds found in DME may exhibit anti-inflammatory effects, potentially reducing skin inflammation and subsequent skin darkening. Through the meta-analysis, it is confirmed that DME significantly downregulates tyrosinase and melanin (SMD of 6.85 [4.27, 9.44] and 23.38 [12.94, 33.82], respectively), suggesting that DMEs are potential whitening ingredients.
Inflammation is a natural biological response to injury, irritation, infection, or damage. Inflammation plays a significant role in various skin disorders [35]. While acute inflammation is a protective response to injury or infection, chronic inflammation can develop and worsen certain conditions, such as acne, psoriasis, eczema, and rosacea [35]. Inflammation is critical in developing inflammatory acne lesions (e.g., pimples, blackheads, and whiteheads). The immune system responds to bacteria in the hair follicles, leading to redness and swelling. Psoriasis is a skin condition in which immune cells are activated, causing them to multiply faster than usual [36]. Rosacea is a chronic skin condition that primarily affects the face, causing redness, visible blood vessels, and sometimes red bumps. Inflammation and an overactive immune response are believed to contribute to the development of rosacea [37]. Moreover, inflammation can contribute to changes in skin color through various mechanisms. Post-inflammatory hyperpigmentation occurs when the skin darkens due to the healing process after an inflammatory event [34]. Inflammation increases melanin production and causes hyperpigmentation. Therefore, it is crucial to understand the intracellular mechanisms of inflammation during the treatment of these skin disorders. The inflammatory process is intricate and involves several key steps: recognition of pathogens or damage, activation of signaling pathways (nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1)), release of pro-inflammatory mediators (ILs and TNF-α), vasodilation, increased permeability, migration of immune cells, and phagocytosis [38]. The inflammatory properties of DME have been demonstrated through in vitro and in vivo assays. DME has been shown to significantly and dose-dependently reduce the production of PGE2 and NO and inhibit protein and mRNA expression in COX-2 and iNOS activities [24]. It can also modulate signaling pathways such as NF-κB and MAPK [24,39]. DME effectively inhibits the activity of inflammatory mediators, such as NO, TNF-α, and IL-6, at doses of 200 and 400 μg/mL [15]. In vivo assays have indicated that DME can reduce the levels of pro-inflammatory cytokines (TNF-α, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL12, IL-13, and IFN-γ) in immunized BALB/C mice at high concentrations (125, 250, and 500 mg/kg) [40]. Another group reports that DME markedly inhibits inflammatory cytokines and TGF-β1 expression in diabetic Sprague–Dawley rats when administered at 25 mg/kg [41,42]. In this study, a meta-analysis was designed to compare the expression of these pro-inflammatory mediators (ILs and TNF-α) and NO production between the DME treatment and the control groups. The results showed that DMEs effectively suppressed the expression of IL mediators and NO production compared to the control groups. These findings suggest that DMEs show a potential as anti-inflammation agents.
Microbial infections significantly affect the development and progression of various skin disorders. The skin is a protective barrier against external pathogens; however, when this barrier is weakened, microorganisms (e.g., bacteria, viruses, fungi, and parasites) can penetrate the skin, causing infections [43]. There are several instances of an association between microbial infections and skin disorders. Skin infections caused by S. aureus or Streptococcus pyogenes (S. pyogenes) can result in red sores, blisters, redness, swelling, or pain. Candidiasis, a yeast infection caused by Candida species, generally affects areas with skin folds and can cause diaper rash, thrush, and intertrigo [44]. Propionibacterium acnes contributes to inflammation and colonizes hair follicles, leading to acne [45]. Bacillus oleronius, Bacillus simplex, Bacillus pumilus, and Bacillus cereus, found in Demodex folliculorum, commonly inhabit human hair follicles [46]. They have been identified as inflammation triggers in skin conditions such as rosacea and psoriasis [46]. Bacterial infections can also cause atopic dermatitis by breaking the skin barrier. Therefore, effectively managing skin disorders often involves addressing the underlying microbial infection, which may require topical or systemic antimicrobial agents. DMEs exhibit a wide range of compounds with antimicrobial properties. Phenolic, flavonoid, and terpenoid compounds significantly disrupt microbial cell membranes, inhibit enzymatic activity, and interfere with cellular processes [4,17]. Alkaloids are nitrogen-containing compounds with antimicrobial properties that can disrupt microbial cell membranes, inhibit protein synthesis, and interfere with DNA replication [47]. Saponins, which have detergent-like properties, can disrupt microbial cell membranes, leading to cell lysis [48]. Indeed, DME exhibits high antimicrobial activity against S. mutans and C. albicans at concentrations of 40, 80, and 100 μg/mL [4]. Additionally, DME can inhibit the growth of P. acnes, P. ovale, and Malassezia furfur (M. furtur) with an MIC of 17.3%, 21.6%, and 15.7%, respectively [12]. Another study reports that DME shows antimicrobial effects at a high concentration (>5 mg/disc), resulting in a clear zone of 8.63 ± 0.31 mm for S. aureus and 8.17 ± 0.49 mm for S. epidermidis [16]. A meta-analysis confirmed that DMEs significantly inhibited the growth of bacteria that cause skin disorders with a high statistical significance (I2 = 31%, p = 0.21). DMEs are promising antimicrobial ingredients for cosmetic applications, particularly for treating red sores, blisters, and redness.
A meta-analysis revealed that DMEs show positive effects for nearly all relevant perspectives, including high biocompatibility, suppression of melanin synthesis and tyrosinase production, inhibition of pro-inflammatory markers and NO production, and suppression of bacteria growth.
6. Limitations of Study
This meta-analysis has identified three main limitations. First, this study includes a small number of studies, which may lead to a low reliability of analysis results. Second, because D. morbifera is a medicinal plant cultivated solely in Korea, its statistical significance could not be determined from a geographical perspective, resulting in a low statistical significance worldwide. Finally, all studies were conducted in vitro, which may limit the analytical diversity based on the study design type. Further analyses should be conducted when in vivo studies and clinical trials of DME-based treatments for skin conditions are available.
7. Conclusions
This systematic review and meta-analysis found that DMEs effectively control the symptoms of some skin disorders caused by hyperpigmentation, inflammation, and microbes. The results showed that DMEs showed beneficial effects in inhibiting melanin synthesis, reducing the expression of IL markers and NO production, and suppressing the development of bacteria, compared to the control groups. The meta-analysis achieved a good statistical significance without heterogeneity when comparing the mean differences between the two groups. However, since the meta-analysis was based on in vitro studies, the results may not present a high statistical significance for overall cosmetic applications. Therefore, further meta-analyses should be conducted when in vivo and clinical trial databases become available. Moreover, since the D. morbifera plant is found mainly in East Asia, especially Korea, its worldwide application is still limited. However, the Dendropanax genus has many species worldwide, and this study may provide good evidence for further investigations into the treatment efficiency of other Dendropanax species for skin disorders. In conclusion, with current in vitro evidence, the application of D. morbifera and other Dendropanax species to skin disorder treatment can attract the attention of scientists. Subsequently, literature databases can be enriched and provide a good premise for further applications.
Author Contributions
Y.-C.L. planned the study and contributed the main ideas; J.-Y.M. and L.T.N.N. collected the data, and L.T.N.N. was principally responsible for the writing of the manuscript; Y.-C.L. commented on and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2021R1F1A1047906) and by the Basic Science Research Capacity Enhancement Project through a Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education (2019R1A6C1010016).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Song J.-H., Kang H.-B., Kim J.H., Kwak S., Sung G.-J., Park S.-H., Jeong J.-H., Kim H., Lee J., Jun W. Antiobesity and cholesterol-lowering effects of Dendropanax morbifera water extracts in mouse 3T3-L1 Cells. J. Med. Food. 2018;21:793–800. doi: 10.1089/jmf.2017.4154. [DOI] [PubMed] [Google Scholar]
- 2.Balakrishnan R., Cho D.-Y., Su-Kim I., Choi D.-K. Dendropanax morbiferus and other species from the genus dendropanax: Therapeutic potential of its traditional uses, phytochemistry, and pharmacology. Antioxidants. 2020;9:962. doi: 10.3390/antiox9100962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J., Lee H. Analysis of antioxidant, anti-aging activities and marker components in Dendropanax morbifera Leveille from different areas. J. Investig. Cosmetol. 2021;17:435–445. [Google Scholar]
- 4.Kim R.W., Lee S.Y., Kim S.G., Heo Y.R., Son M.K. Antimicrobial, antioxidant and cytotoxic activities of Dendropanax morbifera léveille extract for mouthwash and denture cleaning solution. J. Adv. Prosthodont. 2016;8:172–180. doi: 10.4047/jap.2016.8.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S.Y., Choi E.-J., Bae D.-H., Lee D.-W., Kim S. Effects of 1-tetradecanol and β-sitosterol isolated from Dendropanax morbifera Lev. on skin whitening, moisturizing and preventing hair loss. J. Soc. Cosmet. Sci. Korea. 2015;41:73–83. doi: 10.15230/SCSK.2015.41.1.73. [DOI] [Google Scholar]
- 6.Park B.-Y., Min B.-S., Oh S.-R., Kim J.-H., Kim T.-J., Kim D.-H., Bae K.-H., Lee H.-K. Isolation and anticomplement activity of compounds from Dendropanax morbifera. J. Ethnopharmacol. 2004;90:403–408. doi: 10.1016/j.jep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Kim J.-Y., Yoon J.-Y., Sugiura Y., Lee S.-K., Park J.-D., Song G.-J., Yang H.-J. Dendropanax morbiferus leaf extract facilitates oligodendrocyte development. R. Soc. Open Sci. 2019;6:190266. doi: 10.1098/rsos.190266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H.Y., Kim K.S., Lee Y.H., Park J.H., Kim J.-H., Lee S.-Y., Kim Y.-M., Kim I.S., Kacew S., Lee B.M. Dendropanax morbifera ameliorates thioacetamide-induced hepatic fibrosis via TGF-β1/Smads pathways. Int. J. Biol. Sci. 2019;15:800. doi: 10.7150/ijbs.30356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H.X., Kang S., Yang S.Y., Kim Y.H., Li W. Chemical constituents from Dendropanax morbiferus H. Lév. Stems and leaves and their chemotaxonomic significance. Biochem. Syst. Ecol. 2019;87:103936. doi: 10.1016/j.bse.2019.103936. [DOI] [Google Scholar]
- 10.Hyun T.K., Kim M.O., Lee H., Kim Y., Kim E., Kim J.S. Evaluation of anti-oxidant and anti-cancer properties of Dendropanax morbifera Léveille. Food Chem. 2013;141:1947–1955. doi: 10.1016/j.foodchem.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Youn J.S., Kim Y.J., Na H.J., Jung H.R., Song C.K., Kang S.Y., Kim J.Y. Antioxidant activity and contents of leaf extracts obtained from Dendropanax morbifera LEV are dependent on the collecting season and extraction conditions. Food Sci. Biotechnol. 2019;28:201–207. doi: 10.1007/s10068-018-0352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park Y.M., Han J.S. A study on the utilization of Dendropanax morbifera Lev. leaf extract for material of functional cosmetics and hair growth products. Asian J. Beauty Cosmetol. 2016;14:277–288. doi: 10.20402/ajbc.2016.0051. [DOI] [Google Scholar]
- 13.Hoang H.T., Park J.S., Kim S.H., Moon J.Y., Lee Y.C. Microwave-assisted Dendropanax morbifera extract for cosmetic applications. Antioxidants. 2022;11:998. doi: 10.3390/antiox11050998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su Ah Park J., Park C.I., Jie Y.J., Hwang Y.C., Kim Y.H., Jeon S.H., Lee H.M., Ha J.H., Kim J., Park S.N. Cellular antioxidant activity and whitening effects of Dendropanax morbifera leaf extracts. Microbiol. Biotechnol. Lett. 2013;41:407–415. doi: 10.4014/kjmb.1311.11001. [DOI] [Google Scholar]
- 15.Choo G.S., Lim D.P., Kim S.M., Yoo E.S., Kim S.H., Kim C.H., Woo J.S., Kim H.J., Jung J.Y. Anti-inflammatory effects of Dendropanax morbifera in lipopolysaccharide-stimulated RAW264.7 macrophages and in an animal model of atopic dermatitis. Mol. Med. Rep. 2019;19:2087–2096. doi: 10.3892/mmr.2019.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang J. Verification of physiological activities of Dendropanax morbiferus leaf ethanol extract as cosmetic ingredient. Korean Soc. Beauty Art. 2020;21:25–37. doi: 10.18693/jksba.2020.21.4.25. [DOI] [Google Scholar]
- 17.Lee J., Park T., Park S., Yang S., Jhee K. The antimicrobial activity of fermented extracts from Korean Dendropanax morbifera. J. Life Sci. 2019;29:29–36. [Google Scholar]
- 18.Cho C.-S., Hwang H.-J., Cho Y.-I., Son S.-E., Kim S., Lee H.-J. Anti-bacterial effect of Dendropanax morbifera L. Leaf extract against Porphyromonas gingivalis. J. Prev. Vet. Med. 2021;45:194–197. doi: 10.13041/jpvm.2021.45.4.194. [DOI] [Google Scholar]
- 19.KiBeom L., Andy K. Antioxidant, cytotoxic, and antidiabetic activities of Dendropanax morbifera extract for production of health-oriented food materials. Afr. J. Biotechnol. 2019;18:124–129. doi: 10.5897/AJB2018.16694. [DOI] [Google Scholar]
- 20.Nakamura M., Jong-Hwan R., Ju-Sung K. Study of antioxidant, antimicrobial, tyrosinase inhibitory, alcohol dehydrogenase, and acetaldehyde dehydrogenase activity of roasted Dendropanax morbifera LEV. leaves with different ethanol concentrations. Thai J. Pharm. Sci. 2018;42:129–137. doi: 10.56808/3027-7922.2377. [DOI] [Google Scholar]
- 21.Park J.U., Yang S.Y., Guo R.H., Li H.X., Kim Y.H., Kim Y.R. Anti-Melanogenic Effect of Dendropanax morbiferus and its active components via protein kinase A/Cyclic adenosine monophosphate-responsive binding protein- and p38 mitogen-activated protein kinase-mediated microphthalmia−associated transcription factor Do. Front. Pharmacol. 2020;11:507. doi: 10.3389/fphar.2020.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; London, UK: 2008. [DOI] [Google Scholar]
- 24.Akram M., Kim K.A., Kim E.S., Syed A.S., Kim C.Y., Lee J.S., Bae O.N. Potent anti-inflammatory and analgesic actions of the chloroform extract of Dendropanax morbifera mediated by the Nrf2/HO-1 pathway. Biol. Pharm. Bull. 2016;39:728–736. doi: 10.1248/bpb.b15-00823. [DOI] [PubMed] [Google Scholar]
- 25.Park J.U., Kang B.Y., Lee H.J., Kim S., Bae D., Park J.H., Kim Y.R. Tetradecanol reduces EL-4 T cell growth by the down regulation of NF-κB mediated IL-2 secretion. Eur. J. Pharmacol. 2017;799:135–142. doi: 10.1016/j.ejphar.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Choi Y.-H., Cho Y.-J., Kim B.-L., Han M.-H., Lee H.-S., Jeong Y.-G. Functional cosmetic effects of Dendropanax, Sea Salt, and other extracts to alleviate hair loss symptoms. Asian J. Beauty Cosmetol. 2021;19:1–11. doi: 10.20402/ajbc.2020.0100. [DOI] [Google Scholar]
- 27.Mo J., Oh S. Tyrosinase inhibitory activity and melanin production inhibitory activity of the methanol extract and fractions from Dendropanax morbifera Lev. Korean J. Aesthet. Cosmetol. 2013;11:275–280. [Google Scholar]
- 28.Park S.A., Lee H.M., Ha J.H., Jeon S.H., Park S.N. Inhibitoiy effects of Dendropanax morbifera leaf extracts on melanogenesis through down-regulation of tyrosinase and TRP-2. Appl. Chem. Eng. 2014;25:468–473. doi: 10.14478/ace.2014.1058. [DOI] [Google Scholar]
- 29.Park T.-H., Park S.-H., Lee J.-Y., Yang S.-A., Jhee K.-H. The Effect of fermented extracts of Korean Dendropanax Morbifera Levéille on hair growth. Korean Soc. Life Sci. 2019;29:455–460. [Google Scholar]
- 30.Schalka S. New data on hyperpigmentation disorders. J. Eur. Acad. Dermatol. Venereol. 2017;31:18–21. doi: 10.1111/jdv.14411. [DOI] [PubMed] [Google Scholar]
- 31.Panzella L., Napolitano A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: Recent advances. Cosmetics. 2019;6:57. doi: 10.3390/cosmetics6040057. [DOI] [Google Scholar]
- 32.Sarkar R., Arora P., Garg K.V. Cosmeceuticals for hyperpigmentation: What is available? J. Cutan. Aesthetic Surg. 2013;6:4–11. doi: 10.4103/0974-2077.110089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee C., Yang M., Moon J.-O. Antioxidant and hepatoprotective effects of the ethanol extract of Dendropanax morbifera Leveille on the T-Butyl Hydroperoxide-Induced HepG2 cell damages. Korean J. Pharmacogn. 2019;50:32–36. [Google Scholar]
- 34.Chaowattanapanit S., Silpa-Archa N., Kohli I., Lim H.W., Hamzavi I. Postinflammatory hyperpigmentation: A comprehensive overview: Treatment options and prevention. J. Am. Acad. Dermatol. 2017;77:607–621. doi: 10.1016/j.jaad.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 35.Bieber T. Disease modification in inflammatory skin disorders: Opportunities and challenges. Nat. Rev. Drug Discov. 2023;22:662–680. doi: 10.1038/s41573-023-00735-0. [DOI] [PubMed] [Google Scholar]
- 36.Singh S., Ramani P., Jayakumar N.D., Pannu S.J., Sharma R.K., Gill S.S. Role of pro-inflammatory and anti-inflammatory cytokines in pathophysiology of psoriasis. Curr. Oral Health Rep. 2022;9:132–145. doi: 10.1007/s40496-022-00320-1. [DOI] [Google Scholar]
- 37.Buddenkotte J., Steinhoff M. Recent advances in understanding and managing rosacea. F1000Research. 2018;7:F1000 Faculty Rev-1885. doi: 10.12688/f1000research.16537.1. [DOI] [Google Scholar]
- 38.Kiwan A.H., Mohamed H.A.K., Hashim O.A.E., Abd-Elraheem S.I., Alkhrsawy A.M. Pro-Inflammatory versus anti-inflammatory cytokines in atopic dermatitis patients: A case control study. J. Cosmet. Dermatol. 2022;21:6163–6168. doi: 10.1111/jocd.15182. [DOI] [PubMed] [Google Scholar]
- 39.Park S.-Y., Karthivashan G., Ko H.M., Cho D.-Y., Kim J., Cho D.J., Ganesan P., Su-Kim I., Choi D.-K. Aqueous extract of Dendropanax morbiferus leaves effectively alleviated neuroinflammation and behavioral impediments in MPTP-induced Parkinson’s mouse model. Oxidative Med. Cell. Longev. 2018;2018:3175214. doi: 10.1155/2018/3175214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birhanu B.T., Kim J.-Y., Hossain M.A., Choi J.-W., Lee S.-P., Park S.-C. An in vivo immunomodulatory and anti-inflammatory study of fermented Dendropanax morbifera Léveille leaf extract. BMC Complement. Altern. Med. 2018;18:222. doi: 10.1186/s12906-018-2282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sachan R., Kundu A., Dey P., Son J.Y., Kim K.S., Lee D.E., Kim H.R., Park J.H., Lee S.H., Kim J.-H. Dendropanax morbifera protects against renal fibrosis in streptozotocin-induced diabetic rats. Antioxidants. 2020;9:84. doi: 10.3390/antiox9010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung M.-A., Oh K., Choi E.J., Kim Y.J., Bae D., Oh D.-R., Kim K., Dong-Wook Kim D.W., Choi C. Effect of aqueous extract of Dendropanax morbifera leaf on sexual behavior in male rats. J. Food Nutr. Res. 2017;5:518–521. doi: 10.12691/jfnr-5-7-10. [DOI] [Google Scholar]
- 43.De Pessemier B., Grine L., Debaere M., Maes A., Paetzold B., Callewaert C. Gut–skin axis: Current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms. 2021;9:353. doi: 10.3390/microorganisms9020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kühbacher A., Burger-Kentischer A., Rupp S. Interaction of Candida species with the skin. Microorganisms. 2017;5:32. doi: 10.3390/microorganisms5020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dréno B., Pécastaings S., Corvec S., Veraldi S., Khammari A., Roques C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018;32:5–14. doi: 10.1111/jdv.15043. [DOI] [PubMed] [Google Scholar]
- 46.Tatu A.L., Clatici V.G., Nwabudike L.C. Rosacea-like demodicosis (but not primary demodicosis) and papulopustular rosacea may be two phenotypes of the same disease—A microbioma, therapeutic and diagnostic tools perspective. J. Eur. Acad. Dermatol. Venereol. 2019;33:e46–e47. doi: 10.1111/jdv.15166. [DOI] [PubMed] [Google Scholar]
- 47.Othman L., Sleiman A., Abdel-Massih R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019;10:911. doi: 10.3389/fmicb.2019.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maatalah M.B., Bouzidi N.K., Bellahouel S., Merah B., Fortas Z., Soulimani R., Saidi S., Derdour A. Antimicrobial activity of the alkaloids and saponin extracts of Anabasis articulate. J. Biotechnol. Pharm. Res. 2012;3:54–57. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.