Abstract
Background
Systemic amyloidosis is a kind of clinical syndrome in which amyloid is deposited between the cells of various organs in the body, resulting in gradual failure of the function of the affected organs. Depending on the site of amyloid deposition, it may show various clinical symptoms of multiple system involvement.
Patient concerns
A 44-years-old female with spontaneous giant retroperitoneal hematoma was admitted to the emergency department of Peking Union Medical College Hospital in Mar 2023.
Diagnoses
She was found with a extremely X-factor deficiency and diagnosed with AL amyloidosis according to pathological findings finally.
Interventions and outcomes
She received a variety of treatments to improve her coagulation function and underwent chemotherapy for AL in the hematology department which improved her coagulation function and was discharged to her local hospital for follow-up treatment.
Conclusion
This case provides a new reference for emergency doctors in the diagnosis and treatment of acute severe hemorrhagic diseases.
Keywords: Immunoglobulin light chain amyloidosis (AL), Factor X deficiency, Case report
Introduction
Systemic amyloidosis is a disease caused by the immersion of highly ordered fibrils consisting of low molecular weight sub-units of multiple proteins in extracellular tissues and organs. There are 18 known types of systemic amyloidosis, the main types of which include: immunoglobulin light chain amyloidosis (AL), transthyretin amyloidosis (ATTR), reactive amyloidosis (AA), other types such as dialysis-related amyloidosis, hereditary amyloidosis, and organ-specific amyloidosis [1]. The clinical features can present different manifestations of multi-system organs involvement, common including waxy skin, muscle enlargement, cardiac conduction abnormalities and heart failure, liver enlargement, kidney disease or other else depending on the type of precursor protein, tissue distribution, and amount of amyloid deposition.
A patient diagnosed of AL amyloidosis with spontaneous giant retroperitoneal hematoma and severe X-factor deficiency was admitted to the emergency department of Peking Union Medical College Hospital in Mar 2023, which provides a new clinical idea for patients with acute and severe coagulation abnormalities. The process of diagnosis and treatment will be reported as follows.
Case presentation
Condition of pre-hospitalization
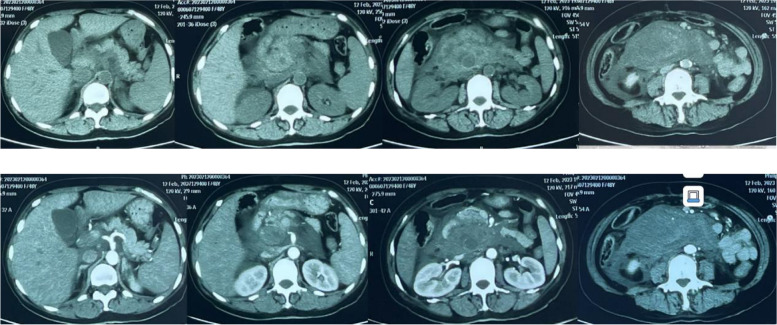
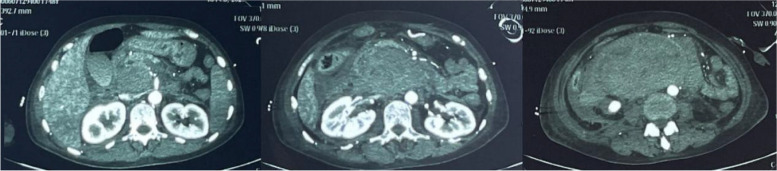
A 44-years-old female went to hospital mainly because of "upper abdominal pain for 17 days". In the early morning of Feb. 12th 2023, she had a sudden severe and persistent epigastric pain without obvious inducement, accompanied by vomiting. She went to local hospital and took the blood routine with a result of leukocyte is 7.77 × 109/L, in which neutrophil% (Neut) is 74%, hemoglobin (Hb) is 71g/L, platelet (Plt) is 393 × 109/L. Liver and kidney function, myocardial enzyme, amylase were normal. Within 4 h, the Hb decreased to 45g/L rapidly, with abnormal coagulation function including prothrombin time (PT) 45s, partial thromboplastin time (APTT) 38s, fibrinogen (Fbg) 1.09 → 0.73g/L. Arterial blood gas (ABG) showed a PH of 7.25 with a lactate level of 5.2mmol/L. Enhanced abdominal CT showed a huge retroperitoneal hematoma, abdominal effusion (Fig. 1). On the same day, a right femoral arteriography was performed, no clear contrast medium leakage was found during the operation, so no interventional embolization was performed. She was transferred to the ICU for endotracheal intubation assisted mechanical ventilation, blood transfusion, pain relief on Feb. 13th. With a monitoring level of Hb 80-90g/L. Fever occurred during hospitalization with a Tmax 38.5℃, accompanied by jaundice in skin and sclera. Further more examinations were done, including leukocyte 16–20 × 109/L, Neut% 80%. Liver function included aspartate aminotransferase (AST) 13 → 90u/L, alanine aminotransferase (ALT) 11 → 81u/L, total bilirubin/direct bilirubin (Tbil/Dbil) normal → 156/149μmol/L. Blood culture of aerobic and anaerobic with two sets were both negative. Qualified sputum culture showed Stenotrophomonas maltophilia positive. Enhanced CT was re-examined on Feb. 18th, roughly the same as before (Fig. 2). When the infection state was almost controlled with cephalosporins, she was transferred to a general ward after deventilation and extubation on Feb. 25th. The gastric tube drainage was dark green at the beginning, but with a intermittent bright red—reddish-brown fluid on Feb. 27th. She arrived at the emergency department of our hospital on Feb. 28th. CT scan showed double lower lung cord shadow, bilateral pleural effusion, and huge retroperitoneal hematoma (about 15cm in length), which was roughly the same as before. She was treated with antibiotics, acid inhibition and fluid rehydration, then she was admitted to the emergency generalized ward on Mar. 1st.
Fig. 1.
Abdominal enhanced CT scan on Feb. 12.th 2023
Fig. 2.
Abdominal enhanced CT scan on Feb. 18.th 2023
Past history: Adenomyosis with anemia was diagnosed in 2021 with a Hb of 70g/L. Total laparoscopic hysterectomy was performed in Sep. 2022, and Hb recovered to 80g/L after surgery. Novel corona virus infection history in Dec. 2022. Hypertension for six years, SBPmax 160mmHg, irregular medication.
Condition of hospitalization
Physical examination showed that irritability, anemic in appearance, part cooperation, inquiries roughly to the point. Yellow in sclera and skin of the whole body. The upper abdomen was full and tender with a suspicious rebound pain. Improved examination included blood routine with leukocyte 14 × 109/L, Neut% 77.8%, Hb 82g/L, Plt 803 × 109/L. Reticulocyte% 7.39%, with 219 × 109/L. Biochemical function included albumin (Alb) 36g/L, Alt 60U/L, Ast 58U/L, Tbil/Dbil 93.5/75.2μmol/L. Coagulation showed PT 29.8s, APTT 39.1s, Fbg 0.90g/L, D-dimer 1.65mg/L, international normalized ratio 2.67, fibrinogen degradation products 5.5μg/ml. Inflammatory markers, tumor index and immune index had no significant abnormality. Blood smear showed neutrophilrophil 78%, lymphocytes 13%, increased number of platelets, roughly normal shape. A 1:1 mixing test showed both APTT and PT can be completely corrected immediately and t hours later. Blood coagulation factor activities included FVIII 153.4%↑, FXII 49.4%↓, FIX 58.4%↓, FX 6.0%↓. Factor II,V,VII,XI factors were normal. were almost normal.
Process of diagnosis and treatment
The patient had a fever with Tmax of 37.9℃ and intermittent complaints of abdominal pain after admission. She was given plasma infusion to correct coagulation function, piperacillin / tazobactam for anti-infection, cooling, pain relief, and rehydration. Dark green fluid was drained at the beginning of the gastric tube, which turned to reddish brown in the morning of Mar. 2nd. She was treated with strictly fasting for solids and liquids, rehydration, continuously pumping nexium and citrine. At noon, dyspnea and hypoxia occurred with oxyhemoglobin saturation 87–90%@room air → 100%@nasal catheter 5L/min, checking ABG with PH 7.46, pCO2 34, pO2 50, total Hb 72g/L. She was transferred to EICU on Mar. 2nd. Then enhanced CT was taken which showed retroperitoneal hematoma (about 16.9*8.3mm), compression of the superior arteries of duodenum, inferior vena cava, pancreas and mesentery, multiple peripheral exudation and effusion, and no obvious contrast agent extravasation (Fig. 3). Assisted of endotracheal intubation with ventilator, vasoactive drugs and active fluid rehydration were given. Red blood cells, plasma, human fibrinogen, prothrombin complex concentrates (PCCs), vitamin K1 were infused. The etiological examination was gradually improved including urine immunofixation electrophoresis (IFE) showed free λ M protein positive. Blood IFE showed λ positive, the serum free light chain (sFLC) -κ 20.4 mg/L, sFLC—λ 1252.5 mg/L, sFLC κ/λ 0.016. No M protein was found in serum protein electrophoresis (SPE). Cardiac ultrasound showed possible myocardial amyloidosis, bilateral atrium enlargement, moderate tricuspid valve insufficiency. Bone marrow smear showed active hyperplasia, normal morphology, and increased thrombocytosis. The proportion of hematopoietic and adipose tissue is roughly normal in bone marrow biopsy. Special dyeing results showed congo red and potassium permanganate congo red both negative. Under effective treatment the tracheal intubation was removed, and she was transferred back to the general ward. Gradually discontinued nesin and stanine pumps, and continued infusion of vitamin K1, PCCs, plasma infusion and corresponding anti-infection therapy. Subsequently, the patient's symptoms were improved, the gastric drainage was dark green fluid, about 200ml per day, still intermittent night upper abdominal pain, accompanied by acid reflux and heartburn, body temperature was normal since Mar. 4th. The level of Hb was stable (61 → 80 → 99 → 102g/L), and coagulation was slightly improved including PT 25s, APTT 40s, Fbg 1.3g/L. Myocardial biopsy was scheduled. Upper gastrointestinal contrast showed gastric wall peristalsis decreased, contrast agent passed slowly through pylorus, duodenal curvature expanded, and the possibility of duodenal compression stenosis (Fig. 4). The patient was allowed to transit to liquid diet without discomfort gradually. Pathology of myocardium showed some cardiomyocytes were deformed, individual blood vessel walls in the interstroma were thickened, and a little powdery stain deposition was seen around the blood vessel walls. Combined with special staining, it was considered amyloidosis. Immunohistochemical results were κ partial positive, λ positive. Special staining results were congo red focal, potassium permanganate congo red focal, alcoholized congo red all positive, but masson staining negative. Delivered myocardium pathology showed κ negative, λ positive under gold immunoelectron microscopy, consistent with amyloidosis (AL-λ type). Report of abdominal wall biopsy included congo red staining with polarizing lens showed focal apple green double refraction, indicating amyloid deposition. Special staining were fused congo red, congo red focal, and potassium permanganate fused congo red focal all positive. PET-CT showed bilateral ventricles and atria positive, SUVmax 8.2. The metabolism of tongue, spleen, abdominal and pelvic subcutaneous fat and peripheral bone marrow were increased. For further diagnosis and treatment, she was transferred to the hematology department on Mar. 24th. At the same time, the patient developed fever again with a Tmax 38℃considered of infection. After comprehensive discussion by the professional team, it was concluded that the diagnosis of systemic amyloidosis was clear and the primary disease treatment should be started as soon as possible to reduce amyloid deposition and improve the function of affected organs. DBD programs were started on Mar. 29th, specifically including daretumab 800mg every week*8 times (then every 2 weeks *8 times, once every 4 weeks thereafter); bortezomib 2mg, d1, d8, d15, d22; dexamethasone 40mg, d1,d8,d15,d22. The infusion process was successful and her coagulation function were improved obviously. The patient discharged to the local hospital for follow-up treatment. All the disease changes and treatment procedures were showed in Table 1.
Fig. 3.
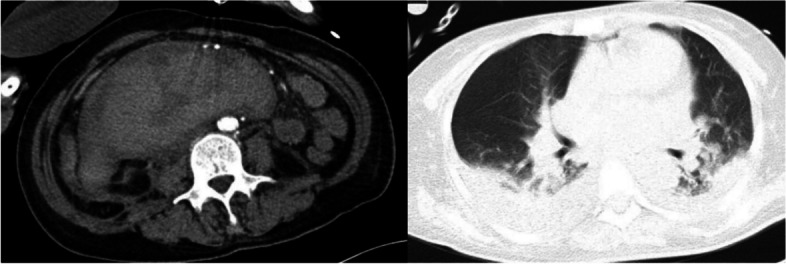
Enhanced CT + CTA of the thoracic and abdominal pelvis on Mar 2.nd 2023
Fig. 4.
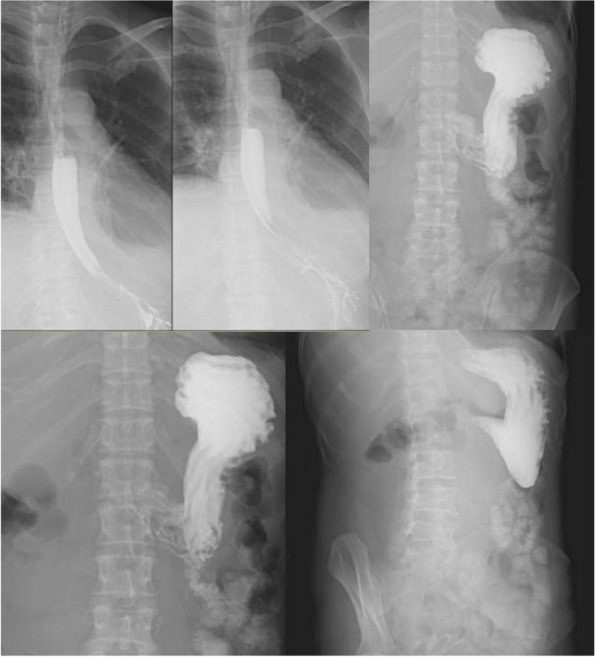
Upper gastrointestinal contrast using meglumine diatrizoate on Mar 8.th 2023
Table 1.
Disease changes and treatment procedures
| Time node | Clinical manifestation | Examination and treatment |
|---|---|---|
| Feb. 12th 2023 | Upper abdominal pain for 17 days | Hb 71 → 45g/L, CT showed retroperitoneal hematoma with no contrast medium leakage |
| Feb. 13th 2023 | Fever and jaundice | Blood transfusion, anti-infection |
| Feb. 18th 2023 | None | CT roughly the same as before |
| Feb. 25th 2023 | Infection state almost controlled | Deventilation and extubation |
| Feb. 27th 2023 | Gastric tube drainage with a intermittent bright red - reddish-brown fluid | Fasting for solids and liquids |
| Feb. 28th 2023 | Same as above | Arrived at the emergency department of PUMCH |
| Mar. 1st 2023 | Low fever, abdominal pain | Admitted to the emergency generalized ward, plasma infusion, anti-infection, cooling, pain relief, rehydration, |
| Mar. 2nd 2023 | Reddish brown of gastric tube drainage | Transferred to EICU and red blood cells, plasma, human fibrinogen, PCCs, vitamin K1 were infused |
| Mar. 4th 2023 | Symptoms were improved | Back to the general ward |
| Mar. 8th to 24th 2023 | Transit to liquid diet without discomfort gradually |
Myocardium and abdominal wall biopsy pathology λ + , congo red + , consistent with amyloidosis (AL-λ type) |
| Mar. 24th 2023 | Same as above | Transferred to the hematology department, developed fever again |
| Mar. 28th 2023 | Same as above | Comprehensive discussion by the professional team |
| Mar. 29th 2023 | Same as above | Daretumab + bortezomib + dexamethasone |
Discussion
Spontaneous hemorrhagic diseases are common in the emergency department, and the common bleeding sites include skin and mucosa, joints, digestive tract, respiratory tract, vagina, and solid organs such as intracranial, liver, kidney, etc. Amyloidosis may be associated with bleeding of various organs. Perivascular amyloidosis deposits can lead to amyloid angiopathy, which increases capillary vulnerability and impairs capillary vasomotor. So vascular amyloidosis without coagulation deficiency can lead to bleeding from the vessel wall. The bleeding event reported in this case, which started with spontaneous abdominal giant hematoma and was later diagnosed as amyloidosis disease, was rarely reported. Through screening the coagulation function and various coagulation factors of the patient, we found that the X factor activity in the patient was extremely low. Since the onset of the patient was not early and there was no related family history, it was considered that the patient was highly likely to have secondary X factor deficiency. Combined with the elevated free light chain, the result of myocardial and abdominal wall biopsy, the final diagnosis of AL was confirmed.
Factor X is a vitamin K-dependent factor which is essential for the clotting cascade of common pathways and plays a key role in fibrin formation. Factor X deficiency is one of the rarest coagulation disorders, accounting for 10% of all rare bleeding diseases, among which the incidence of hereditary factor X deficiency is 1:100,000, and some patients are even found by auxiliary laboratory tests after completing other routine disease treatment without any bleeding tendency [2, 3]. Secondary factor X deficiency is associated with liver disease, vitamin K1 deficiency, use of the anticoagulant warfarin, acute myelogenous leukemia, infectious diseases, primary amyloidosis, malignancy, and connective tissue diseases, and there have been cases of post-COVID-19 factor X deficiency [4–7].
Secondary factor X deficiency is a specific manifestation of AL, with an incidence of 9–14 cases per million every year in the United States [1], but given the potential for missed diagnosis of amyloidosis, it is reasonable to speculate that the prevalence may be higher. Laboratory tests often indicate prolonged PT/APTT and reduced X factor. Therefore, patients with AL amyloidosis sometimes experience abnormal bleeding, such as bleeding in the skin and soft tissues, gastrointestinal tract, kidney, spleen, liver, or other sites [8, 9]. The possible mechanism of bleeding caused by X factor deficiency in AL patients is that FXa in these patients can be temporarily cut by plasminase to FXa-β and then cut to Xa33/13, both of which enhance tissue plasminogen activator mediated plasminogen activation under certain conditions [10]. Positive congo red staining is an essential diagnostic basis for AL amyloidosis [11]. However, biopsy is not necessary to diagnose cardiac amyloidosis [12]. X-factor levels may rise in weeks to months with effective treatment [9]. The morbidity and mortality of AL amyloidosis are not caused by the proliferation of monoclonal plasma cells, but by the action of toxic monoclonal proteins. In addition to fatal bleeding in important organs, the effect of toxic monoclonal proteins on cardiomyocytes leading to cardiac function impairment is the most important determinant of survival for patients [13, 14].
Patients with spontaneous hemorrhagic disorders are usually not first seen by a hematologist, but by a community physician, emergency physician, surgeon, or any other specialty related to the site of the first bleeding. It’s common that the primary diseases behind spontaneous hemorrhage can’t be diagnosed in time. But literature reports show that the diagnostic efficiency of amyloidosis complicating diseases can be improved after efforts to popularize relevant knowledge [15, 16]. One study showed that there was a significant delay in the diagnosis of amyloidosis, with a median diagnosis of seven months. Thirty-seven percent of patients reported a delay of more than a year from the onset of symptoms, and they had multiple visits to medical institutions before being diagnosed. More than 10% of these patients were diagnosed more than three years after the onset of symptoms [17]. Among them, amyloidosis with bleeding as the first symptom is more rare. As emergency physicians, it is difficult for us to identify the main cause of hemorrhagic diseases at the first time sometimes. We often conduct a preliminary examination through blood routine and coagulation function test before conducting subsequent etiological examinations, including plasma correction test, coagulation factor function, hematuria light chain determination, bone marrow puncture, etc. It was full of challenge for us during the process of etiological screening. In-depth learning of amyloidosis can greatly accelerate the rate of which emergency physicians can identify related diseases with bleeding as the main manifestation in their work. Transfusions of PCCs, fresh frozen plasma (FFP), tranexamic acid are commonly used to treat bleeding events induced by factor X deficiency. In the process of large-scale use of the above protocols, it is necessary to be vigilant about the risk of thrombotic complications, as well as the potential risks of infectious diseases transmitted by FFP, allergic reactions, transfusion-related lung injury, and circulating overload. Therefore, novel drug substitution therapy containing X factor has became an optimal choice for immediate or preventive treatment in patients with X factor deficiency during bleeding [18–21].
Acknowledgments
Patient consent
The patient give informed consent before we write the case report.
Abbreviations
- AL
Immunoglobulin light chain amyloidosis
- ATTR
Transthyretin amyloidosis
- AA
Reactive amyloidosis
- Hb
Hemoglobin
- Neut
Neutrophil
- Plt
Platelet
- Alb
Albumin
- PT
Prothrombin time
- APTT
Partial thromboplastin time
- Fbg
Fibrinogen
- ABG
Arterial blood gas
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- Tbil
Total bilirubin
- Dbil
Direct bilirubin
- PCCs
Prothrombin complex concentrates
- IFE
Urine immunofixation electrophoresis
- SPE
Serum protein electrophoresis
- Sflc
Serum free light chain
- FFP
Fresh frozen plasma
- PUMCH
Peking Union Medical College Hospital
Authors’ contributions
YqS composed this manuscript. HdZ provided the report idea and guidance. All authors contributed to the refinement of this report and approved the final draft.
Funding
There is no fund for this case report.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
None.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vaxman I, Gertz M. When to Suspect a Diagnosis of Amyloidosis. Acta Haematol. 2020;143:304–11. [DOI] [PubMed] [Google Scholar]
- 2.Rehab Y AL-Ansari , Ghufran Alofi, Nasser Aljarah, et al. A case report of congenital factor X deficiency in an adult patient. SAGE Open Medical Case Reports 2022;10: 1–3. [DOI] [PMC free article] [PubMed]
- 3.Masayoshi Souri, TsukasaOsaki, Yuji Shimura, et al. Identification of non-neutrophilralizing anti-factor X autoantibodies in three Japanese cases of autoimmune acquired factor X Deficiency. Haemophilia 2023;29:555–563. [DOI] [PubMed]
- 4.Ichinose A, Osaki T, Souri M. Autoimmune coagulation factor X deficiency as a rare acquired hemorrhagic disorder: a literature review. Thromb Haemost. 2022;122:320–8. [DOI] [PubMed] [Google Scholar]
- 5.Patel G, Hari P, Szabo A, et al. Acquired factor X deficiency in light-chain (AL) amyloidosis is rare and associated with advanced disease. Hematol Oncol Stem Cell Ther. 2019;12(1):10–4. [DOI] [PubMed] [Google Scholar]
- 6.Bangolo A, Waykole T, Niazi B, et al. A rare cause of acquired factor X deficiency in an 87-year-old female. Case Rep Hematol. 2021;2021:1138329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humayun O, Durrani T, Ullah R, et al. Post-COVID Factor X Deficiency: A Case Report From Pakistan. Cureus. 2022;14(11):e31473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuda M, Katoh N, Ikeda S. Clinical manifestations at diagnosis in Japanese patients with systemic AL amyloidosis: a retrospective study of 202 cases with a special attention to uncommon symptoms. Intern Med. 2014;53(5):403–12. [DOI] [PubMed] [Google Scholar]
- 9.Arahata M, Takamatsu H, Morishita E, et al. Coagulation and fibrinolytic features in AL amyloidosis with abnormal bleeding and usefulness of tranexamic acid. Int J Hematol. 2020;111(4):550–8. [DOI] [PubMed] [Google Scholar]
- 10.Talbot K, Meixner SC, Pryzdial EL. Proteolytic modulation of factor Xa-antithrombin complex enhances fibrinolysis in plasma. Biochim Biophys Acta. 2013;1834(6):989–95. [DOI] [PubMed] [Google Scholar]
- 11.Fernández de Larrea C, Verga L, Morbini P, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood 2015; 125(14): 2239–44. [DOI] [PubMed]
- 12.Gillmore JD, Maurer MS, Falk RH, Merlini G, et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation. 2016;133(24):2404–12. [DOI] [PubMed] [Google Scholar]
- 13.Comenzo RL, Reece D, Palladini G, et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia. 2012;26(11):2317–25. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Dispenzieri A, Lacy MQ, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30(9):989–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCausland KL, White MK, Guthrie SD, et al. Light Chain (AL) Amyloidosis: The Journey to Diagnosis. Patient. 2018;11(2):207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muchtar E, Dispenzieri A, Lacy MQ, et al. Overuse of organ biopsies in immunoglobulin light chain amyloidosis (AL): the consequence of failure of early recognition. Ann Med. 2017;49(7):545–51. [DOI] [PubMed] [Google Scholar]
- 17.Lousada I, Comenzo RL, Landau H, et al. Light chain amyloidosis: patient experience survey from the amyloidosis research consortium. Adv Ther. 2015;32(10):920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grottke O, Moser O, Farrag A, et al. Plasma-derived factor X therapy for treatment of intracranial bleeding in a patient with Factor X deficiency: a case report. Transfusion. 2019;59:2228–33. [DOI] [PubMed] [Google Scholar]
- 19.Zimowski KL, McGuinn CE, Abajas YL, et al. Use of plasma-derived factor X concentrate in neonates and infants with congenital factor X deficiency. J Thromb Haemost. 2020;18:2551–6. [DOI] [PubMed] [Google Scholar]
- 20.Huang JN, Liesner R, Austin SK, et al. Plasma-derived factor X concentrate compassionate use for hereditary factor X deficiency: long-term safety and efficacy in a retrospective data-collection study. Res Pract Thromb Haemost. 2021;5: e12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeanette Payne, Glaivy Batsuli, Andrew D, et al. A review of the pharmacokineics, efficacy and safety of high-purity factor X for the prophylactic treatment of hereditary factor X deficiency. Haemophilia 2022;28:523–531. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.