Abstract
The three members of the genus capripoxvirus (CaPV), lumpy skin disease virus (LSDV), sheeppox virus (SPPV), and goatpox virus (GTPV) have common hosts and areas of overlapping geographical distribution with Rift Valley fever virus (RVFV). Hence, to ensure more cost-effective disease surveillance we developed and evaluated a Luminex assay for the simultaneous detection of antibodies against CaPV and RVFV in domestic ruminants. In cattle, the assay had a sensitivity (Se) of 98.7% and a specificity (Sp) of 98.3% in detecting anti-LSDV antibodies; both diagnostic parameters were 100% for the detection of anti-RVFV antibodies in this species. In sheep and goats, Se and Sp were 100% for the detection of anti-SPPV and anti-GTPV antibodies while they were 100% and 98.9%, respectively for the detection of anti-RVFV antibody. The assay did not cross react with anti-parapoxvirus antibodies of cattle, sheep, and goats. This multiplex serological assay offers a practical tool for accurate detection and monitoring of the immunological status of domestic ruminant populations against veterinary and socio-economically important capripox- and phleboviral infections, thus has the potential to aid in the strategic application of vaccination programmes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12985-024-02602-9.
Keywords: Capripoxvirus, Luminex, LSDV, SPPV, GTPV, Serology, A34, RVFV, Rift valley fever
Background
Capripox diseases, namely sheeppox (SPP), goatpox (GTP), and lumpy skin disease (LSD) affect millions of ruminants of many low-income households in endemic countries. Additionally, these diseases have a significant impact on those countries’ ruminant industries which are often major sources of country revenue. These diseases are caused by the sheeppox virus (SPPV), goatpox virus (GTPV), and lumpy skin disease virus (LSDV), respectively. The viruses are members of the Capripoxviridae genus within the Poxviridae family. Geographically, these viruses are found in Africa, the Middle East, Europe, and Asia and due to their mortality, morbidity, and effects on animal productivity, they have been a cause for concern in their affected regions [1]. Control measures have been implemented in many countries, where testing for capripox diseases is required for trade purposes; vaccination rounds are well established; and sero-surveillance and monitoring are performed periodically [2–4].
Rift Valley fever (RVF) is a zoonotic disease, caused by the Rift Valley fever virus (RVFV). This disease has several common hosts to capripoxvirus, including sheep, goats, and cattle. Although, its geographic extension is more contained than that of capripox, both are present simultaneously in several parts of Africa and the Middle East. RVF control programmes are not routinely implemented [5, 6].
RVFV is a zoonotic arbovirus in the genus Phlebovirus of the Phenuiviridae family, in the Bunyavirales order, transmitted by mosquito bites as well as by contact with infected animals [7, 8]. Sheep, goats, cattle, and buffaloes are among the most affected [6, 9, 10], including high rate of neonatal deaths, and abortions [5]. In humans, encephalitis, haemorrhagic fever and death are some of the possible severe outcomes of RVFV infections [11–16]. The disease has seasonality which is related to the levels of rainfall [12, 17].
Since RVF in humans displays symptoms that are often associated with influenza or malaria, it is often misdiagnosed in endemic regions [18]. In animals, it is also often misdiagnosed as brucellosis, bluetongue, enterotoxemia, bovine ephemeral fever, vibriosis, trichomonosis, Nairobi sheep disease, heartwater, ovine enzootic abortion, peste des petits ruminants, anthrax or Schmallenberg disease, among others [19, 20].
RVF is endemic in sub-Saharan Africa, with occasional outbreaks in the Middle East [21, 22].
Trade and livestock mobility are the main causes of RVF spread [5]. Additionally, due to seasonality of rain and the presence of insect vectors in neighbouring regions, as well as rainfall level variations due to climate change, the expansion of RVF is expected in the future [13, 14, 23].
Even though there are vaccines available against RVF, in most RVF endemic countries, control programmes are not routinely implemented [5, 6].
Since these two diseases have common hosts and overlap geographically, we developed and evaluated a duplex serological Luminex assay for simultaneous and differential detection of anti-capripoxvirus and anti-RVFV antibodies in cattle, sheep, and goats.
For RVF, the serological diagnostic gold standard is the virus neutralization test (VNT) [5]. Additionally, enzyme-linked immunosorbent assays (ELISAs) are available [5, 15]. Both techniques are sensitive and specific, but the VNT is labour intensive, requires the use of live virus and takes several days to produce results; thus, it is not routinely applied and only in high-biocontainment laboratories. In addition, both assays target only one disease at a time [16, 24].
Multiplex serological assays rely on coupled beads to detect multiple analytes in one sample [6, 24]. The technological platform used in this study is Luminex, where magnetic polystyrene beads were coated with different antigens to detect antibodies in serum samples [25]. This type of assay has been successfully used in the detection of antibodies against West Nile, Dengue and Ebola, among others [6, 26, 27].
Capripox diseases are routinely assayed in many countries where RVF is also present [6]. This assay offers the possibility to test for the presence of antibodies against both targets simultaneously, thus allowing the determination of the immunological status of the two diseases in a host’s population within endemic regions.
Methods
Target determination
Expression and purification of capripoxvirus target protein
As an antigenic target for capripoxvirus, we used the homolog of the vaccinia virus C-type lectin-like protein A34 (LSDV ORF 123). The expression and purification of the antigen were performed as previously described [28]. This recombinant antigen was successfully used in ELISA to detect antibodies against capripoxvirus in sheep, goats and cattle [28]. After the initial proof of concept, the protein, based on the corresponding sequence of LSDV NI-2490 (NC_003027), was outsourced for production to GenScript, Inc. (Piscataway, NJ, USA).
Expression and purification of RVFV nucleoprotein
The plasmid pET 32a-RVFV NP for expression of the RVFV nucleocapsid (NP) protein was used to transform BL21a cells (Thermo Fisher Scientific, Waltham, MA, USA). The transformation was confirmed by DNA amplification using the T7 promoter and T7 terminator primers. The thermocycler conditions used were: (1X) 95 °C, 10 min; (30X) 95 °C, 30 s; 55 °C, 30 s; 72 °C, 1 min 30 s; (1X) 72 °C, 2 min; hold at 4 °C. Cells were grown in Luria–Bertani (LB) broth medium (Thermo Fisher Scientific, Waltham, MA, USA) containing carbenicillin (Merck KGaA, Darmstadt, Germany), and protein expression was induced with 0.3% L-arabinose and 1 mM IPTG (Merck KGaA, Darmstadt, Germany) for five hours. The initial expression test was performed at time points T0h, T1h, T2h, T3h, T4h, and T5h. Upon mechanical lysis of the cells, the recombinant NP was purified by an Invitrogen Probond kit (Thermo Fisher Scientific, Waltham, MA, USA) which is based on affinity purification using a nickel column to bind His-tagged proteins. Since we wanted a native conformation of the protein, we followed the commercial protocol described by the manufacturer under native purification conditions (10 mM imidazole for binding, 20 mM for washing, and 250 mM imidazole in the elution). For buffer exchange, an Amicon concentrator (Merck KGaA, Darmstadt, Germany) with a MWCO of 3 kDa was used to exchange the original buffer with storage buffer (50 mM Tris HCl, 150 mM NaCl, 25 mM Sucrose, and protease inhibitor pills, pH 8.0).
RVFV nucleoprotein quantification
Pierce BCA for protein quantification was used following the manufacturer's instructions (Thermo Fisher Scientific, Waltham, MA, USA).
Western blot
The proteins were analysed by SDS-PAGE. Antibody binding in both goat and cattle positive sera, was confirmed by Western blot.
The preparation of proteins for the SDS-PAGE gels and Western blots followed standard procedures. The protein preparations (20 μL) were heated at 80 °C for 10 min in 4X LDS sample buffer (Thermo Fisher Scientific, Waltham, MA, USA). The samples were loaded onto an SDS-PAGE (NUPAGE 10% (v/v)) gel (Thermo Fisher Scientific, Waltham, MA, USA) and transferred to a 0.2 μm PVDF membrane (Thermo Fisher Scientific, Waltham, MA, USA). The membrane was probed for 1 h at room temperature with anti-penta His antibody (1:1000) (Merck KGaA, Darmstadt, Germany), washed three times with PBS containing 0.5% (v/v) Tween 20 (PBS-T) and probed for 1 h with diluted (1:5000) goat anti-mouse antibodies conjugated to horseradish peroxidase (Merck KGaA, Darmstadt, Germany). For detection, ECL substrate (GE Healthcare, Chicago, IL, USA) was used according to the manufacturer’s instructions.
RVFV NP ELISA
100 ng per well of RVFV NP purified antigen in carbonate/bicarbonate buffer pH 9.6 vf = 50 μL was shaken overnight at 4 °C in a 96 well plate (Polysorb, Nalgene). The plate was washed 3X with 1XPBS-T and blocked with 50 μL of protein-free blocking buffer (Thermo Fisher Scientific, Waltham, MA, USA), hereon known as BB1, for 30 min at 37 °C. The diluted sera were added and incubated for 90 min at 37 °C. The plate was washed 3X with 1XPBS-T, and an HRP-conjugated secondary antibody (Merck KgaA, Darmstadt, Germany), diluted 1:10 k, was added and incubated for 45 min at RT. The plate was washed 3X with 1XPBS-T, and 100 μL of TMB per well was added and incubated in the dark for 10 min. 100 μL of stop solution was added per well and the plate was read at 450 nm.
Virus neutralization test
The virus neutralization tests were carried out as previously described [28].
Capripox serum samples
Experimental and field sera
Four types of reference population sera were used in this study: (a) bovine LSD-positive sera (n = 77) obtained from naturally LSDV-infected animals from Bulgaria (NDRVMI, Sofia, Bulgaria) and the Republic of North Macedonia (School of Veterinary Medicine, University “Ss Cyril and Methodius”, Skopje, Republic of North Macedonia). These samples were confirmed LSD-positive by VNT; (b) bovine LSD-negative sera (n = 59) from countries where the disease is historically not present, namely France (provided by IDVet, Montpellier, France), Austria (provided by AGES, Austria), and the Republic of North Macedonia (sera collected before 2010) (School of Veterinary Medicine, University “Ss Cyril and Methodius”, Skopje, Republic of North Macedonia); (c) Capripox-positive sheep and goat sera (n = 27) from experimentally infected animals from Pirbright (UK), LCV (Mali) [29] and AHI (Ethiopia) [30], which were confirmed positive by VNT; and (d) Capripox-negative sheep and goat sera (n = 181) from Austria (provided by AGES, Austria), where the disease is historically not present. See Supplementary Table 1.
Specificity control sera
Parapoxvirus-positive sera were used to determine cross-reactivity. They consisted of 12 orf naturally infected goat samples (provided by Stéphane Bertagnoli, Ecole Vétérinaire de Toulouse, Toulouse, France), 4 pseudocowpox (PCP)-positive cattle sera from Zambia (provided by Maureen Ziba from CVRI, Zambia), and one bovine papular stomatitis (BPS) (provided by AGES, Austria). See Supplementary Table 1.
Sera from longitudinal studies
We used three sets of samples from longitudinal studies. The first set comprised samples collected at 0, 6, 12, 18, 20, 23, 26, and 30-days post-infection (DPI) from two cattle that were experimentally infected with a virulent South African LSDV Neethling strain [31]. The second set consisted of samples collected at 0, 7, 14, 21, 28, 35, 42, 49, and 56 DPI from four goats experimentally infected with GTPV Oman 84. This was part of a larger GTPV study with experimentally infected Ethiopian goats [30]. The third set consisted of samples from a single sheep produced by LCV (Mali) [29]. The sheep was experimentally infected with SPPV Algeria/93 Djelfa and samples were collected at 0, 7, 14, 21, and 28 DPI.
RVF serum samples
Sera
Four types of reference populations of RVF sera were used in this study: (a) bovine RVF-positive sera (n = 6) obtained from Zambia (provided by Maureen Ziba from CVRI, Zambia), field-infected cattle from Côte d’Ivoire (provided by Dr. Emmanuel Couacy-Hymann, Laboratoire Central de Pathologie Animale, Bingerville), and commercially available bovine RVF-positive serum samples (IDVet, Montpellier, France). These samples were confirmed to be positive by cELISA (IDvet, Montpellier, France); (b) bovine negative sera (n = 56) from countries where the disease is historically not present, namely France (provided by IDVet, Montpellier, France), Austria (provided by AGES, Austria) and, the United Kingdom (the Pirbright Institute); (c) RVF-positive sheep and goat sera (n = 13) from a goat experimental infection in Teramo, Italy (provided by Chiara Pinoni from Diagnostica e Sorveglianza Malattie Esotiche, Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise "G.Caporale"), and sheep and goat field infections collected during routine screening at the Laboratoire Vétérinaire de Kinshasa, DRC. These samples were confirmed to be positive by cELISA (IDvet, Montpellier, France); and (d) negative sheep and goat sera (n = 180) from Austria (provided by AGES, Austria), where the disease is historically not present. All the samples were confirmed to be negative by cELISA (IDvet, Montpellier, France). See Supplementary Table 1.
Luminex multiplex serological assay
Coupling of target antigens to beads
The viral target antigens were covalently coupled to polystyrene carboxyl magnetic beads, in regions 34 and 63 (Bio-Rad, Hercules, California, USA) following a modified protocol based on Anderson et al. [32]. Briefly, 1.2 ×106 beads placed into a 1.5 ml Eppendorf tube were washed, vortexed and sonicated once with 100 μL of sterile water and 3 times with 100 μL of activation buffer (0.1 M sodium phosphate (monobasic), pH 6.2). Separation was performed via a magnetic separator stand (Thermo Fisher Scientific, Waltham, MA, USA). After a final round of vortexing and sonication, the beads were resuspended in 80 μL of activation buffer. 10 μL of freshly made 50 mg/mL Sulfo-NHS (Thermo Fisher Scientific, Waltham, MA, USA) and 10 μL of 50 mg/mL EDC (Merck KGaA, Darmstadt, Germany) diluted in activation buffer were added to the microspheres. After a 20-min incubation in the dark on a plate shaker, the beads were magnetically separated and resuspended in 500 μL of coupling buffer (0.1 M PBS pH 7.4) followed by vortexing and sonication. After two rounds of washing with coupling buffer, vortexing and sonication the beads were resuspended in 100 μL of coupling buffer. 10 μg of purified RVFV NP, or A34-CaPV antigens in 300 μL of coupling buffer were added to the corresponding resuspended carboxyl magnetic beads (regions 63 for RVFV or 34 for A34-CaPV). Per bead type, the final volume was 400ul in coupling buffer. The beads were incubated for 2 h on a plate shaker at 200 rpm at room temperature in the dark, vortexing at 10-min intervals. They were then magnetically separated, washed twice with 1000 μL of wash buffer (0.1 M PBS, 0.05% Tween 20 pH 7.4), followed by vortexing and sonication. After the magnetic separation of the beads, the microspheres were resuspended once in 250 μL of BB1, then vortexed and sonicated as described above. Then the beads were separated via the magnetic separator, resuspended in 100 μL of BB1 and stored at 4 °C in the dark. The number of microspheres recovered after the coupling reaction was counted with a haemocytometer.
Luminex multiplex assay
The filtered microplate (Merck KGaA, Darmstadt, Germany) was pre-wetted by filling each well twice with 50 μL of wash buffer (10X PBS, 0.05%Tween 20) and once with 50 μl of BB1. The liquid was removed through the filter at the bottom of the plate via a vacuum manifold (Merck KGaA, Darmstadt, Germany). A 1000 coupled beads per antigen per sample or control in a final volume of 5 μL of BB1 each were added to 100 μL of serum diluted 1:500 in BB1 in the case of cattle samples and 1:2000 in BB1 in the case of sheep or goat samples. The plate covered with its lid and wrapped in aluminium foil was incubated overnight at 4 °C on a horizontal orbital microplate shaker set at 300 rpm. The liquid was then removed with the vacuum manifold. 150 μL of wash buffer were added to each well and the liquid was removed with the vacuum manifold. This was repeated 4 times. 50 µl of diluted species-specific (anti-ruminant) biotin-conjugated secondary antibody (Merck KGaA, Darmstadt, Germany), hereon known as SGB, diluted at 1:10 k in BB1 for bovine samples and 1:40 k in BB1 for sheep or goat samples, was added to each well. The plate was incubated for 30 min in the dark, at room temperature on a horizontal orbital microplate shaker set at 300 rpm. The liquid was then removed with the vacuum manifold and washed 5 times with 150 μL of wash buffer. 50 µl of diluted streptavidin–PE diluted 1:250 in BB1 was added to each well. The plate was incubated for 10 min in the dark, at room temperature on a horizontal orbital microplate shaker set at 300 rpm. The liquid was removed with a vacuum manifold and the wells were washed 5 times with 150μL of reading buffer (0.01 M PBS pH 7.4). 50 µl of reading buffer was added to the wells. The plate was shaken at 300 rpm for 1 min. Further, 75 μL of reading buffer was added to each well (final volume 125 μL). The plate was read in the BioPlex 200 Luminex machine (Bio-Rad, Hercules, CA, USA) (50 beads/region and the median value obtained for each reaction event per bead set using a volume of 50 μL/sample).
Unless otherwise stated, all samples were analysed in duplicate and average readings calculated. For all samples, the multiplex assay mean fluorescence intensity (MFI) data were corrected for background levels by subtracting the no-serum-added control signal from the serum-added signals.
Optimization of RVF and capripox sera for the duplex serological luminex assay
For cattle, a serial dilution of IgG and IgM positive RVF and capripox positive and negative sera from 1:500 to 1:4 k and secondary antibody dilutions of 1:5 k and 1:10 k were prepared to establish the best dilution to use for both serum types. Supplementary Figs. 1 and 2 show the results of RVFV NP and A34-CaPV antigen coated beads. The chosen dilution, based on signal to noise ratio, for cattle was set at 1:500 for the serum and 1:10 k for the secondary antibody.
For sheep and goats, a serial dilution of goat positive RVF and capripox sheep and goat positive and negative sera from 1:500 to 1:4 k and secondary antibody dilutions at 1:40 k and 1:60 k were produced to determine the best dilution to use for both serum types. Supplementary Figs. 3 and 4 show the results of RVFV NP and A34-CaPV antigen coated beads. The chosen dilution, based on signal to noise ratio, for sheep and goat was set at 1:2 k for the serum and 1:40 k for the secondary antibody. As shown in Supplementary Fig. 5, the coated beads were specific for their targeted controls.
Statistical analysis
The Luminex fluorescence values, relevant information about the samples, and the OD results were compiled in Microsoft Excel (Microsoft Corporation, Redmond, DC, USA). The background-subtracted MFI values were calculated in Microsoft Excel, and these data were imported into the R software for further analysis. In addition to R base functions, the dplyr package was used for data frame manipulation. The Shapiro–Wilk test was used to calculate the sample distribution. The pROC package was used for the receiver operating characteristic (ROC) analysis, and the ggplot2 package was used for the graphical representation of the data. The Youden index = sensitivity + specificity-1, was used to determine the cut-off from the ROC analysis. The maximum value for the Youden index was used as a criterion for selecting the optimal cut-off point.
Results
Expression, purification, and characterization of RVFV nucleoprotein
Plasmid pET 32a-RVFV NP was used to transform BL21a bacterial cells for expression of RVFV nucleoprotein (RVFV NP). Upon amplification of the target DNA, we observed a band of the expected molecular weight (1490 bp) (Supplementary Fig. 6).
One of the colonies was grown and induced with arabinose and IPTG to express RVFV NP.
As shown in Supplementary Fig. 7, the induced fractions accumulated over 5 h, producing the thickest protein band at t = 5 h.
To further confirm protein expression, we tested the induced and uninduced bacterial lysates by Western blot, using a His-tag antibody (Supplementary Fig. 8). We observed a protein band at the expected molecular weight of 43 kDa. We detected the presence of a band in the uninduced lane, suggesting low levels of constitutive protein expression (“leaky vector”).
An Invitrogen Probond kit was used to purify the expressed RVFV NP which, once purified, had a molecular weight of 43 kDa on an SDS PAGE (Supplementary Fig. 9).
After the purified protein was quantified, ELISA plates were coated and the protein tested using positive and negative sera, as an additional method for characterization. The serially diluted sera (from 1:2 to 1:32) showed the expected decreasing absorbance pattern ranging from 2.8 to 1.8 for IgG positive samples, 0.95 to 0.3 for IgM positive samples and 0.4 to 0.1 for the negative control samples (Supplementary Fig. 10).
Evaluation of the duplex serological luminex assay
Cattle sera
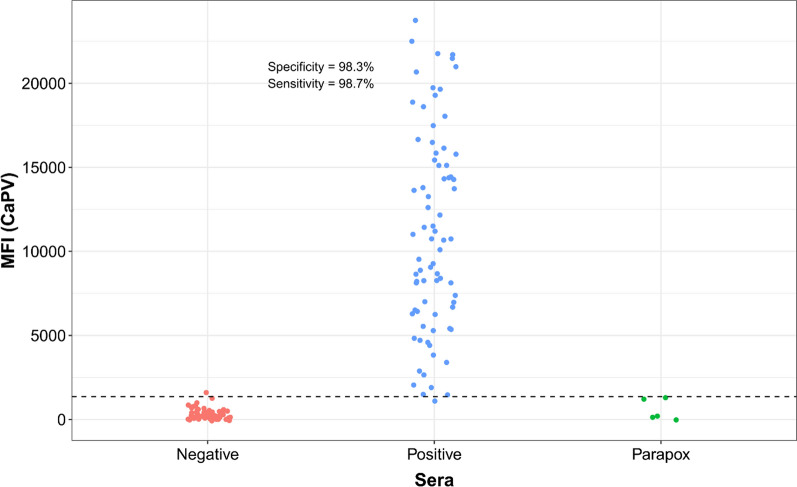
Cattle serum samples were tested using the above-determined conditions for the cattle serological duplex Luminex assay (Fig. 1). Of 77 LSD reference positive samples 76 (98%) were detected by the A34-CaPV Luminex, while none of them were detected by the RVF Luminex. Of 59 negative cattle samples, 58 were below the established threshold.
Fig. 1.
Distribution of the A34-CaPV Luminex values for LSD positive and negative reference cattle sera and parapox positive sera
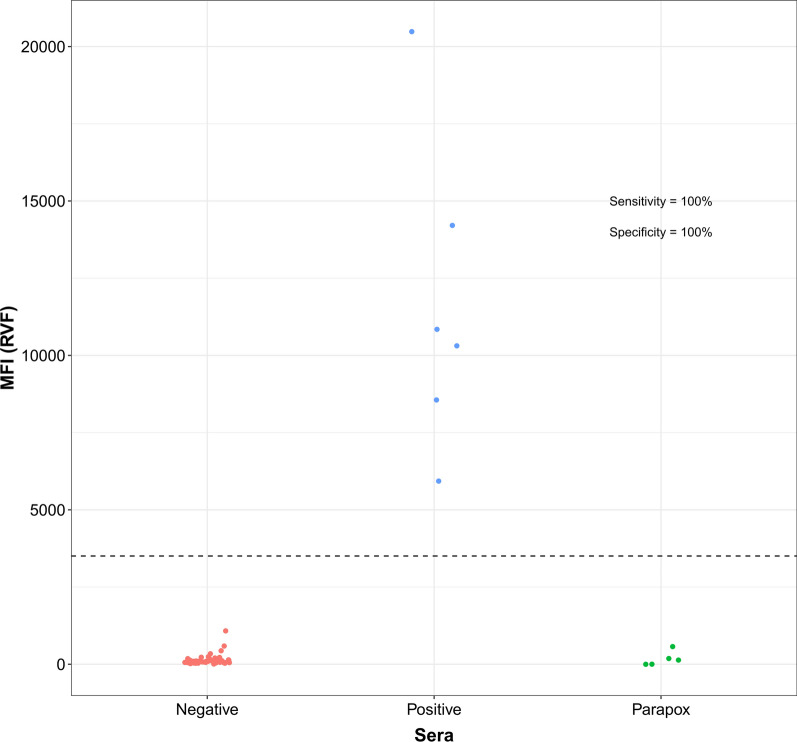
Using the threshold determined by ROC analysis (Supplementary Fig. 11) for the A34-CaPV Luminex, sensitivity (Se) of 98.7% and specificity (Sp) of 98.3% were established for cattle (Fig. 1). For RVF Luminex, the Se and Sp were 100% on the bovine samples tested (Fig. 2). This was based on six positive and 56 negative RVF samples. Five parapox positive sera tested for specificity control were negative by the assay.
Fig. 2.
Distribution of the RVF Luminex values in RVF positive and negative reference cattle sera and parapox positive sera
Capripox cattle longitudinal study
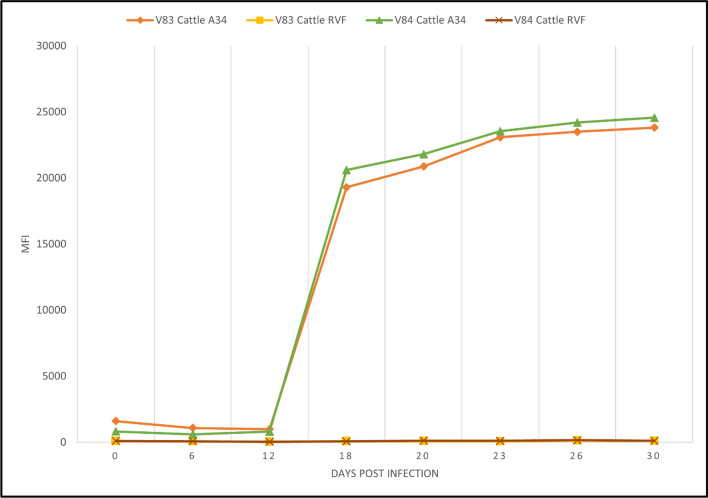
For cattle, capripox antibodies from two animals experimentally infected with Neethling strain were detected after 12 DPI. The positivity was maintained with an upward trend until the end collection at 30 DPI. RVF controls (negative) were included (Fig. 3).
Fig. 3.
Kinetics of serological responses in two cattle experimentally infected with LSDV measured by Luminex. Animals showed specific LSDV seropositivity after day 12 post infection, while for RVFV, the samples were seronegative at every time point tested
Sheep and goat sera
Sheep and goat serum samples were tested using the above-determined conditions for the sheep and goat duplex serological Luminex assay.
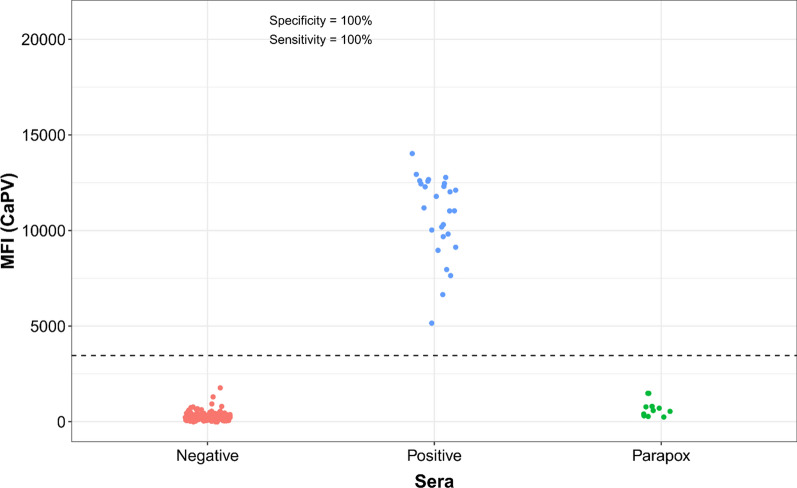
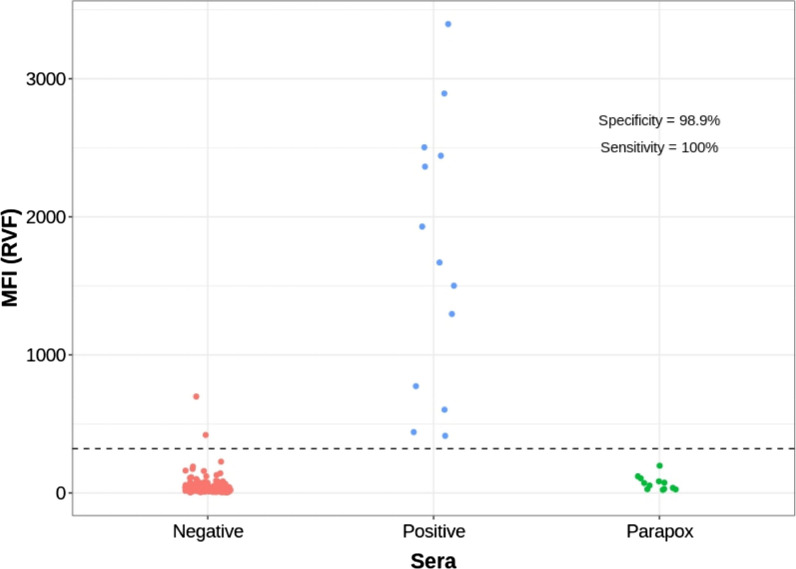
Using the threshold determined by ROC analysis (Supplementary Fig. 11) for the A34-CaPV Luminex, the sensitivity and specificity were 100% on 181 negative and 27 positive sheep and goat samples tested (Fig. 4). For RVF Luminex, the sensitivity was of 100% and the specificity was 98.9%, on the 180 negative and 13 positive sheep and goat samples tested. (Fig. 5). 12 parapox positive sera tested for specificity control were negative by the assay.
Fig. 4.
Distribution of the A34-CaPV Luminex values in capripox reference positive and negative sheep and goat sera, and parapox positive sera
Fig. 5.
Distribution of the RVF Luminex values in reference RVF sheep and goat positive and negative and parapox positive sera
Capripox sheep and goat longitudinal study
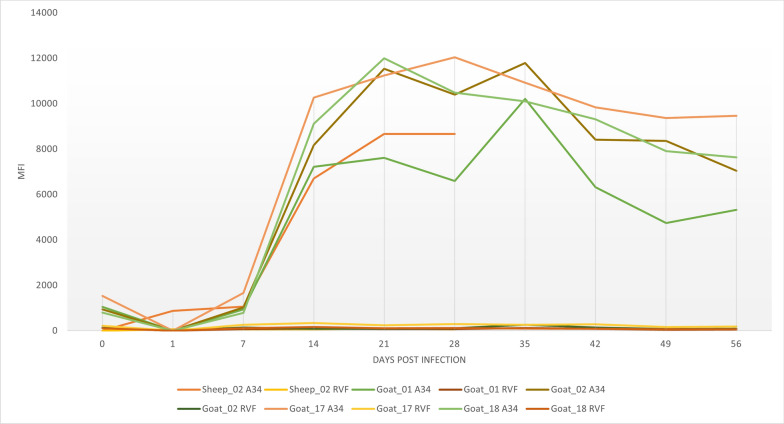
Additionally, capripox sheep and goat serum samples from longitudinal studies previously collected were tested. One sheep and four goat sera panels were collected. Capripox antibodies for the sheep and goat panels were detected after 07 DPI. The positivity was maintained until the end collection at 56 DPI for goat and 28 DPI for sheep. RVF controls (negative) were included (Fig. 6).
Fig. 6.
Kinetics of serological responses in sera from one sheep and four goats experimentally infected with Algeria/93 Djelfa and Oman 84 respectively. Sera collected at the indicated time points were tested using the duplex serological Luminex. Animals showed A34-CaPV seropositivity after day 7 post infection, while RVF remained seronegative at every time point tested
Capripox longitudinal study
The results of the longitudinal studies show that anti-capripoxvirus antibodies can be detected in all three species tested starting at 14 DPI and to at least 56 DPI in goats. For cattle and sheep, we detected antibodies to at least 30 DPI and 28 DPI, respectively.
Unfortunately, for cattle and sheep, we could only detect antibodies up to 30 DPI and 28 DPI, respectively, since we did not have samples collected after those days.
Discussion
We developed a duplex serological assay based on Luminex technology capable of detecting antibodies against capripoxvirus and RVFV in cattle, sheep, and goats. The two diseases were selected based upon their similar vector-borne transmissibility, common host species affected and their overlapping geographical distribution.
Serological assays for capripox, including ELISAs, have been developed with greater or lesser success [33–38]. Additionally, a commercial ELISA has been recently made available [39].
Some of the existing RVF ELISAs are based on IgM capture while others on indirect IgG binding [40].
There are several Luminex-based assays available to detect antibodies against RVFV alone or in a panel with other diseases [24, 41, 42]. However, none of the existing assays is capable of detecting anti-capripox and anti-RVFV antibodies in one assay.
Since the secondary antibody used for the Luminex assay here developed has a higher binding affinity for goat and sheep than it does for cattle, we selected two serum and secondary dilution conditions; one for sheep and goat and one for cattle. The antigenic targets were tested using positive and negative sera for both diseases in all three species, as well as positive parapox sera for specificity control.
The nucleoprotein is the most abundant protein in phlebovirus-infected cells and strongly immunogenic, thus a reliable antigen for a serological assay. Moreover, in a recent study by Paweska et al., a large-scale validation of an indirect ELISA based on RVFV NP antigen was performed using serum panels from animals classified as infected and uninfected by the VNT. Results of this study demonstrated high diagnostic accuracy (> 90% sensitivity and specificity) of the RVFV NP iELISA compared to VNT [16].
Although we successfully reproduced the validated RVFV iELISA based on recombinant NP to establish antigen reactivity [16], we confirmed the sera status, as positive or negative using the commercially available IDVet cELISA.
One of the weaknesses of our assay is that the antibody detection is limited by the selected antigenic target. We selected A34 of CaPV and the nucleoprotein of RVFV. These targets have intrinsic detection limitations, which are incorporated into the assay. For example, we have previously shown that A34-CaPV ELISA requires higher serum concentration for detection in vaccinated animals [28]. Furthermore, since LSD, SPP and GTP are serologically indistinguishable, the selected target A34 detects any or all of the three capripoxvirus members. Distinction can be made based on the animal species infected [28]. Additionally, we observed high background in some of the European negative sera tested. This likely could be accounted for some level of cross-reactivity background resulting from infections with other members of the Bunyaviridae genus endemic in many European countries including Schmallenberg [43] and Toscana virus [44].
Conclusions
The assay developed and preliminarily evaluated in this study is a single surveillance tool for rapid, accurate and simultaneous detection of antibodies against RVFV and LSDV. It is intended for use in endemic regions where both viruses overlap affecting the same ruminant species. In addition, it would allow for early detection and response to zoonotic spread of RVFV and, more cost-efficient monitoring of herd immunity. This information could be used for the development of disease hot spot maps and the identification of hyper-epidemic areas to generate informed strategies for vaccination programmes.
Supplementary Information
Acknowledgements
The authors thank the Pirbright Institute for the capripox sera provided for this study. We thank AGES (Austria), Emiliya Ivanova and Gabriela Goujgoulova (National Diagnostic and Research Veterinary Institute, Bulgaria), Kiril Krstevski and Igor Djadjovski (School of Veterinary Medicine, University “Ss Cyril and Methodius”, Skopje, Republic of North Macedonia), Maureen Ziba (CVRI, Zambia), Chiara Pinoni (Diagnostica e Sorveglianza Malattie Esotiche, Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise"G.Caporale") and Stéphane Bertagnoli (Ecole Nationale Vétérinaire de Toulouse, France) for kindly contributing materials used for experiments. We thank William G. Dundon (IAEA/FAO Animal Production and Health Laboratory, Seibersdorf) for his critical reading and editing of the manuscript.
Abbreviations
- DPI
Days post infection
- ELISA
Enzyme-linked immunosorbent assay
- GTP
Goatpox
- GTPV
Goatpox virus
- LSDV
Lumpy skin disease virus
- LSD
Lumpy skin disease
- RVFV
Rift valley fever virus
- RVF
Rift valley fever
- SPP
Sheeppox
- SPPV
Sheeppox virus
- VNT
Virus neutralization test
Author contributions
Conceptualization, C.E.L. and F.J.B.; methodology, F.J.B., and T.B.K.S.; formal analysis, C.E.L., F.J.B., M.T.B.; resources, C.G.T.M., P.J.v.V. and J.T.P.; writing, original draft preparation, F.J.B. and C.E.L.; writing—review and editing, T.B.K.S., C.G.T.M., J.T.P., P.J.v.V., R.G., and G.C.; visualization, M.T.B.; supervision, C.E.L., R.G., and G.C. All authors read and approved the final manuscript.
Funding
This study was supported by funds received from the government of Japan for the IAEA Peaceful Uses Initiative (Project “Detection of emerging and re-emerging animal and zoonotic pathogens at the animal-human interface”). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Availability of data and materials
The data presented in this study are available upon request from the corresponding author.
Declarations
Ethics approval and consent to participate
No animal experiments were carried out in the framework of this study. Serum samples were previously described or collected and submitted to the laboratories as part of routine diagnostic service and official surveillance programs, where according to national and legislation, ethical approval was not required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tuppurainen ESM, Venter EH, Shisler JL, Gari G, Mekonnen GA, Juleff N, et al. Review: Capripoxvirus diseases: current status and opportunities for control. Transbound Emerg Dis. 2017;64(3):729–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haegeman A, De Vleeschauwer A, De Leeuw I, Vidanović D, Šekler M, Petrović T, et al. Overview of diagnostic tools for Capripox virus infections. Prev Vet Med. 2020;181: 104704. [DOI] [PubMed] [Google Scholar]
- 3.Mirzaie K, Barani S, Bokaie S. A review of sheep pox and goat pox: perspective of their control and eradication in Iran. J Adv Vet Anim Res. 2015;2(4):373–373. [Google Scholar]
- 4.Bhanuprakash V, Hosamani M, Singh RK. Prospects of control and eradication of capripox from the Indian subcontinent: a perspective. Antiviral Res. 2011;91(3):225–32. [DOI] [PubMed] [Google Scholar]
- 5.Gerken KN, LaBeaud AD, Mandi H, L’Azou Jackson M, Breugelmans JG, King CH. Paving the way for human vaccination against Rift Valley fever virus: a systematic literature review of RVFV epidemiology from 1999 to 2021. PLoS Negl Trop Dis. 2022;16(1): e0009852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindahl JF, Ragan IK, Rowland RR, Wainaina M, Mbotha D, Wilson W. A multiplex fluorescence microsphere immunoassay for increased understanding of Rift Valley fever immune responses in ruminants in Kenya. J Virol Methods. 2019;1(269):70–6. [DOI] [PubMed] [Google Scholar]
- 7.Msimang V, Thompson PN, Jansen P, van Vuren S, Tempia CC, Kgaladi J, Khosa J, Burt FJ, Liang J, Rostal MK, Karesh WB, Paweska JT. Rift valley fever virus exposure amongst farmers, farm workers, and veterinary professionals in central South Africa. Viruses. 2019;11(2):140. 10.3390/v11020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumley S, Horton DL, Hernandez-Triana LLM, Johnson N, Fooks AR, Hewson R. Rift Valley fever virus: strategies for maintenance, survival and vertical transmission in mosquitoes. J Gen Virol. 2017;98(5):875–87. [DOI] [PubMed] [Google Scholar]
- 9.Jori F, Alexander KA, Mokopasetso M, Munstermann S, Moagabo K, Paweska JT. Serological evidence of Rift Valley fever virus circulation in domestic cattle and African buffalo in Northern Botswana (2010–2011). Front Vet Sci. 2015;2:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaBeaud AD, Cross PC, Getz WM, Glinka A, King CH. Rift Valley fever virus infection in African buffalo (Synceruscaffer) herds in rural South Africa: evidence of interepidemic transmission. Am J Trop Med Hyg. 2011;84(4):641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholas DE, Jacobsen KH, Waters NM. Risk factors associated with human rift valley fever infection: systematic review and meta-analysis. Trop Med Int Health. 2014;19(12):1420–9. [DOI] [PubMed] [Google Scholar]
- 12.Paweska JT, van Jansen VP. Chapter 8—Rift valley fever virus: a virus with potential for global emergence. In: Johnson N, editor. The role of animals in emerging viral diseases [internet]. Boston: Academic Press; 2014. p. 169–200. [Google Scholar]
- 13.Rolin AI, Berrang-Ford L, Kulkarni MA. The risk of Rift Valley fever virus introduction and establishment in the United States and European Union. Emerg Microbes Infect. 2013;2(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omosa E, Bett B, Kiage B. Climate change and Rift Valley fever disease outbreak: implications for the food environment of pastoralists. Lancet Planet Health. 2022;6:S17. [Google Scholar]
- 15.Kim HJ, Nah JJ, Moon JS, Ko YJ, Yoo HS, Kweon CH. Competitive ELISA for the detection of antibodies to Rift Valley fever virus in goats and cattle. J Vet Med Sci. 2012;74(3):321–7. [DOI] [PubMed] [Google Scholar]
- 16.Pawęska JT, van Jansen VP, Msimang V, Lô MM, Thiongane Y, Mulumba-Mfumu LK, et al. Large-scale international validation of an indirect ELISA Based on recombinant nucleocapsid protein of rift valley fever virus for the detection of IgG antibody in domestic ruminants. Viruses. 2021;13(8):1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies F, Linthicum K, James A. Rainfall and epizootic Rift Valley fever. Bull World Health Organ. 1985;63(5):941. [PMC free article] [PubMed] [Google Scholar]
- 18.Oragwa AO, Oragwa FC, Oluwayelu DO. Serologic evidence of silent Rift Valley fever virus infection among occupationally exposed persons in northern Nigeria. J Infect Dev Ctries. 2022;16(05):881–7. [DOI] [PubMed] [Google Scholar]
- 19.Adamu AM, Enem SI, Ngbede EO, Owolodun OA, Dzikwi AA, Ajagbe OA, et al. Serosurvey on sheep unravel circulation of rift valley fever virus in Nigeria. EcoHealth. 2020;17(3):393–7. [DOI] [PubMed] [Google Scholar]
- 20.Mansfield KL, Banyard AC, McElhinney L, Johnson N, Horton DL, Hernández-Triana LM, et al. Rift Valley fever virus: a review of diagnosis and vaccination, and implications for emergence in Europe. Vaccine. 2015;33(42):5520–31. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Yuan Y, Liu Y, Zhang L. Arm race between Rift Valley fever virus and host. Front Immunol. 2022;13:7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madani TA, Al-Mazrou YY, Al-Jeffri MH, Mishkhas AA, Al-Rabeah AM, Turkistani AM, et al. Rift valley fever epidemic in saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin Infect Dis. 2003;37(8):1084–92. [DOI] [PubMed] [Google Scholar]
- 23.Bett B, Kiunga P, Gachohi J, Sindato C, Mbotha D, Robinson T, et al. Effects of climate change on the occurrence and distribution of livestock diseases. Prev Vet Med. 2017;1(137):119–29. [DOI] [PubMed] [Google Scholar]
- 24.Ragan IK, Davis AS, McVey DS, Richt JA, Rowland RR, Wilson WC. Evaluation of fluorescence microsphere immunoassay for detection of antibodies to rift valley fever virus nucleocapsid protein and glycoproteins. J Clin Microbiol. 2018;56(6):e01626-e1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christopher-Hennings J, Araujo KPC, Souza CJH, Fang Y, Lawson S, Nelson EA, et al. Opportunities for bead-based multiplex assays in veterinary diagnostic laboratories. J VET Diagn Invest. 2013;25(6):671–91. [DOI] [PubMed] [Google Scholar]
- 26.Raulino R, Thaurignac G, Butel C, Villabona-Arenas CJ, Foe T, Loul S, et al. Multiplex detection of antibodies to Chikungunya, O’nyong-nyong, Zika, dengue, west nile and usutu viruses in diverse non-human primate species from cameroon and the democratic republic of congo. PLoS Negl Trop Dis. 2021;15(1): e0009028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayouba A, Touré A, Butel C, Keita AK, Binetruy F, Sow MS, et al. Development of a sensitive and specific serological assay based on Luminex technology for detection of antibodies to Zaire Ebola virus. J Clin Microbiol. 2017;55(1):165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berguido FJ, Gelaye E, Liu Y, Davaasuren B, Krstevski K, Djadjovski I, et al. Development and optimization of indirect ELISAs for the detection of anti-capripoxvirus antibodies in cattle, sheep, and goat sera. Microorganisms. 2022;10(10):1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelaye E, Lamien CE, Silber R, Tuppurainen ESM, Grabherr R, Diallo A. Development of a cost-effective method for capripoxvirus genotyping using snapback primer and dsDNA intercalating dye. PLoS ONE. 2013;8(10):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caufour P, Rufael T, Lamien CE, Lancelot R, Kidane M, Awel D, et al. Protective efficacy of a single immunization with capripoxvirus-vectored recombinant peste des petits ruminants vaccines in presence of pre-existing immunity. Vaccine. 2014;32(30):3772–9. [DOI] [PubMed] [Google Scholar]
- 31.Carn V, Kitching R. An investigation of possible routes of transmission of lumpy skin disease virus (Neethling). Epidemiol Infect. 1995;114(1):219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson S, Wakeley P, Wibberley G, Webster K, Sawyer J. Development and evaluation of a Luminex multiplex serology assay to detect antibodies to bovine herpes virus 1, parainfluenza 3 virus, bovine viral diarrhoea virus, and bovine respiratory syncytial virus, with comparison to existing ELISA detection methods. J Immunol Methods. 2011;366(1):79–88. [DOI] [PubMed] [Google Scholar]
- 33.Carn VM, Kitching RP, Hammond JM, Chand P, Anderson J, Black DN. Use of a recombinant antigen in an indirect ELISA for detecting bovine antibody to capripoxvirus. J Virol Methods. 1995;53(2–3):273–273. [DOI] [PubMed] [Google Scholar]
- 34.Heine HG, Stevens MP, Foord AJ, Boyle DB. A capripoxvirus detection PCR and antibody ELISA based on the major antigen P32, the homolog of the vaccinia virus H3L gene. J Immunol Methods. 1999;227(1–2):187–96. [DOI] [PubMed] [Google Scholar]
- 35.Babiuk S, Wallace DB, Smith SJ, Bowden TR, Dalman B, Parkyn G, et al. Detection of antibodies against capripoxviruses using an inactivated sheeppox virus ELISA. Transbound Emerg Dis. 2009;56(4):132–41. [DOI] [PubMed] [Google Scholar]
- 36.Tian H, Chen Y, Wu J, Shang Y, Liu X. Serodiagnosis of sheeppox and goatpox using an indirect ELISA based on synthetic peptide targeting for the major antigen P32. Virol J. 2010;7(1):245–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowden TR, Coupar BE, Babiuk SL, White JR, Boyd V, Duch CJ, et al. Detection of antibodies specific for sheeppox and goatpox viruses using recombinant capripoxvirus antigens in an indirect enzyme-linked immunosorbent assay. J Virol Methods. 2009;161(1):19–29. [DOI] [PubMed] [Google Scholar]
- 38.Venkatesan G, Teli MK, Sankar M, Kumar A, Dashprakash M, Arya S, et al. Expression and evaluation of recombinant P32 protein based ELISA for sero-diagnostic potential of capripox in sheep and goats. Mol Cell Probes. 2017;2018(37):48–54. [DOI] [PubMed] [Google Scholar]
- 39.Innovative diagnostics. Validation report ID screen® Capripox double antigen multi-species [Internet]. 2017 Mar p. 16. Available from: https://www.ambifood.com/catalogo/download.php?id=135&fich=1
- 40.World organisation for animal health. Manual of diagnostic tests and vaccines for terrestrial animals 2022 CHAPTER 3.1.19. RIFT VALLEY FEVER [Internet]. 2022. Available from: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.01.19_RVF.pdf
- 41.Eric R, Mateusz P, Baltazar PC, Delynn MM, Aridth G, James C, et al. Adaptation to a multiplex bead assay and seroprevalence to rift valley fever n protein: Nampula province, Mozambique. J Virol. 2022;96(16):e00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kading RC, Abworo EO, Hamer GL. Rift valley fever virus, Japanese encephalitis virus, and African swine fever virus: three transboundary, vector-borne, veterinary biothreats with diverse surveillance, and response capacity needs. Front Vet Sci. 2019;6:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wernike K, Beer M. Re-circulation of Schmallenberg virus, Germany, 2019. Transbound Emerg Dis. 2020;67(6):2290–5. [DOI] [PubMed] [Google Scholar]
- 44.Ayhan N, Charrel RN. An update on Toscana virus distribution, genetics, medical and diagnostic aspects. Clin Microbiol Infect. 2020;26(8):1017–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.