Abstract
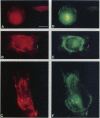
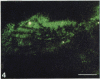
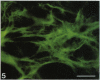
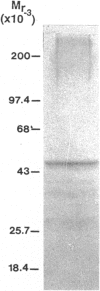
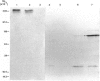
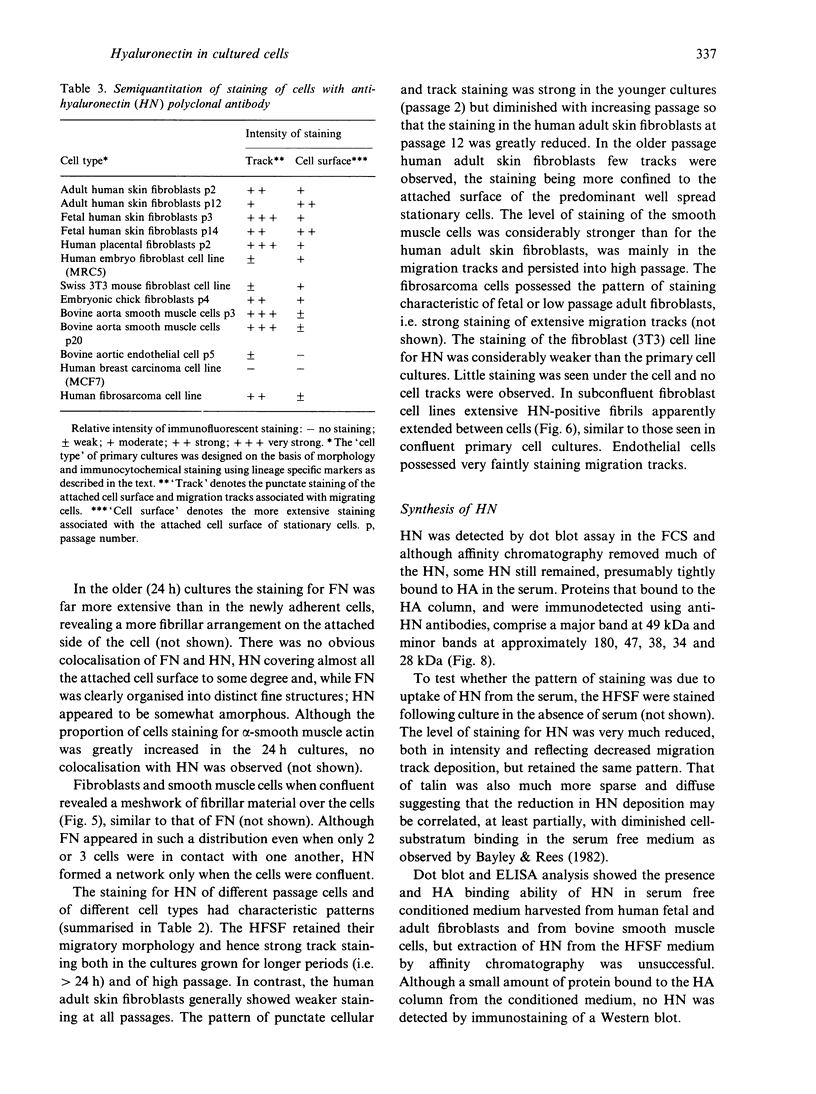
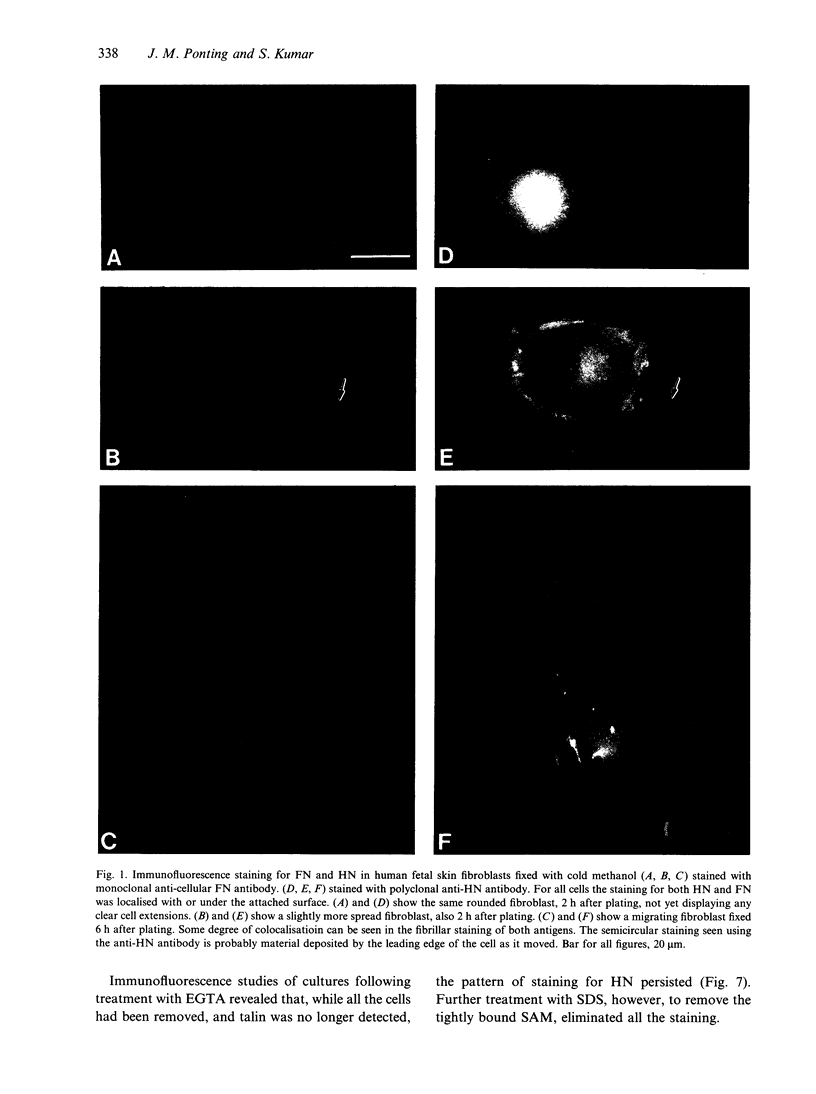
Hyaluronectin is an extracellular matrix glycoprotein which specifically binds to hyaluronan. Isoforms of hyaluronectin are present in nervous and mesenchymal tissues but, while the nervous tissue isoform has been characterised in some detail, less is known about the mesenchymal isoform. Although its tissue localisation suggests a role in tumour development, neither its cellular origin nor its exact function are known. In this study we demonstrate hyaluronectin synthesis in fibroblasts and smooth muscle cells in vitro. The pattern of immunolocalisation of hyaluronectin in fibroblasts depended on the cell type, length of time spent by the cells in culture and cell density. Immunoreactivity in sparsely plated migratory cells was seen mainly in a patchy distribution at the attached cell surface and in the migration tracks left by the cells on the subtratum. In stationary cells a more uniform distribution associated with the attached cell surface was observed, while in confluent cultures hyaluronectin immunoreactivity was mainly seen as a network of fibrillar material above the cell. The pattern of staining was distinct from that of other hyaluronan-binding proteins. Immunoprecipitation, using antihyaluronectin antibodies, of the substratum-attached material deposited by human fetal fibroblasts revealed a family of proteins ranging from 22 to 90 kDa, the major protein being of approximately 60 kDa. These results lead us to propose that hyaluronectin plays an important role in cell migration, probably by regulation of hyaluronan distribution and binding.
Full text
PDF





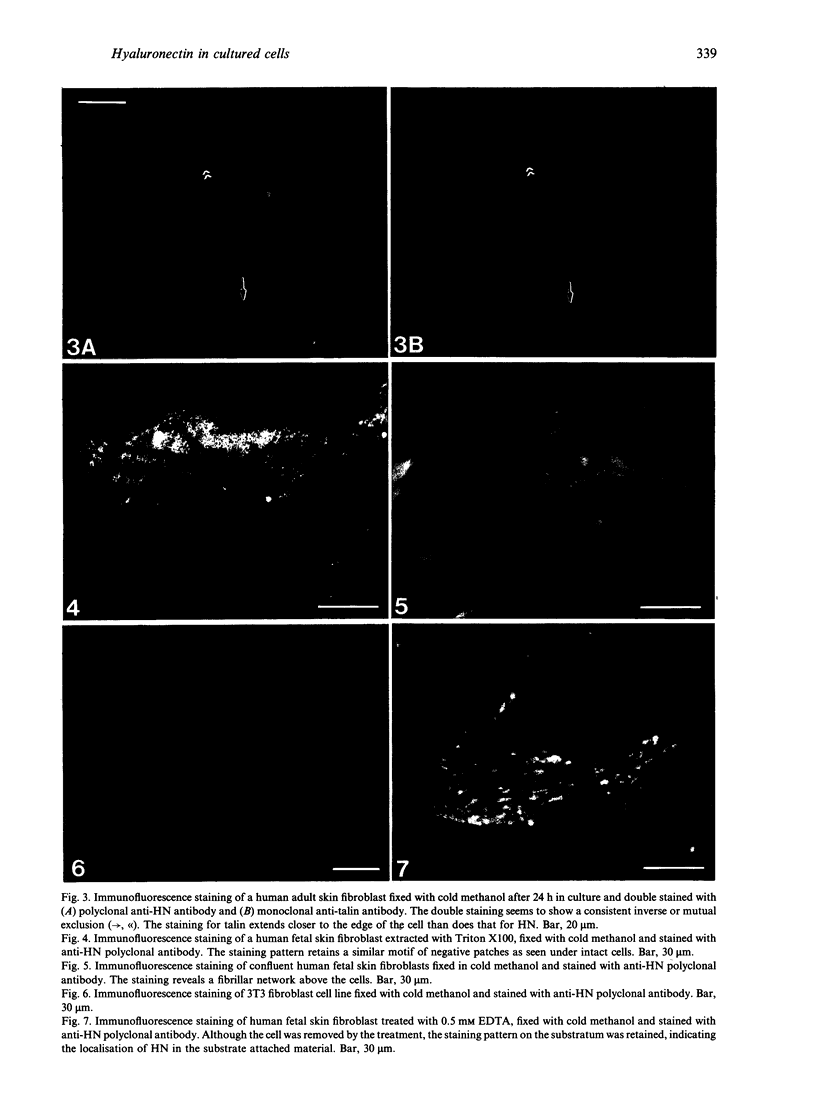
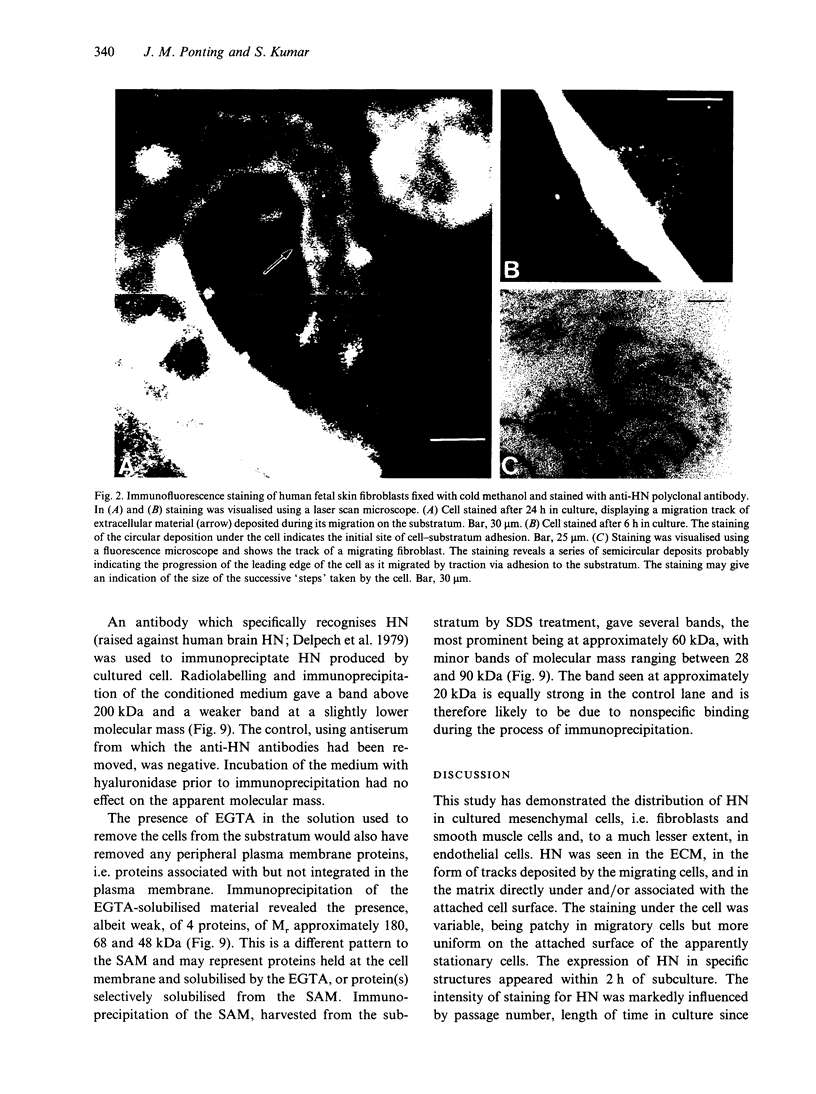






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abatangelo G., Cortivo R., Martelli M., Vecchia P. Cell detachment mediated by hyaluronic acid. Exp Cell Res. 1982 Jan;137(1):73–78. doi: 10.1016/0014-4827(82)90009-x. [DOI] [PubMed] [Google Scholar]
- Asou H., Brunngraber E. G., Delpech B. Localization of hyaluronectin in oligodendroglial cells. J Neurochem. 1983 Feb;40(2):589–591. doi: 10.1111/j.1471-4159.1983.tb11324.x. [DOI] [PubMed] [Google Scholar]
- Atherly A. G., Barnhart B. J., Kraemer P. M. Growth and biochemical characteristics of a detachment variant of CHO cells. J Cell Physiol. 1977 Mar;90(3):375–385. doi: 10.1002/jcp.1040900302. [DOI] [PubMed] [Google Scholar]
- Barnhart B. J., Cox S. H., Kraemer P. M. Detachment variants of Chinese hamster cells. Hyaluronic acid as a modulator of cell detachment. Exp Cell Res. 1979 Mar 15;119(2):327–332. doi: 10.1016/0014-4827(79)90360-4. [DOI] [PubMed] [Google Scholar]
- Bayley S. A., Rees D. A. Analysis of the proteins, glycoproteins and glycosaminoglycans of fibroblast adhesions to substratum. Biochim Biophys Acta. 1982 Jul 28;689(2):351–362. doi: 10.1016/0005-2736(82)90269-3. [DOI] [PubMed] [Google Scholar]
- Boudreau N., Turley E., Rabinovitch M. Fibronectin, hyaluronan, and a hyaluronan binding protein contribute to increased ductus arteriosus smooth muscle cell migration. Dev Biol. 1991 Feb;143(2):235–247. doi: 10.1016/0012-1606(91)90074-d. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Burridge K., Connell L. A new protein of adhesion plaques and ruffling membranes. J Cell Biol. 1983 Aug;97(2):359–367. doi: 10.1083/jcb.97.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. T., Singer S. J. Immunoelectron microscopic studies of the sites of cell-substratum and cell-cell contacts in cultured fibroblasts. J Cell Biol. 1982 Oct;95(1):205–222. doi: 10.1083/jcb.95.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. T., Wang J., Hasegawa T., Yamada S. S., Yamada K. M. Regulation of fibronectin receptor distribution by transformation, exogenous fibronectin, and synthetic peptides. J Cell Biol. 1986 Nov;103(5):1649–1661. doi: 10.1083/jcb.103.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. Anti-adhesive molecules of the extracellular matrix. Curr Opin Cell Biol. 1991 Oct;3(5):800–804. doi: 10.1016/0955-0674(91)90053-2. [DOI] [PubMed] [Google Scholar]
- Cooper A. R., Kurkinen M., Taylor A., Hogan B. L. Studies on the biosynthesis of laminin by murine parietal endoderm cells. Eur J Biochem. 1981 Sep;119(1):189–197. doi: 10.1111/j.1432-1033.1981.tb05593.x. [DOI] [PubMed] [Google Scholar]
- Couchman J. R., Rees D. A., Green M. R., Smith C. G. Fibronectin has a dual role in locomotion and anchorage of primary chick fibroblasts and can promote entry into the division cycle. J Cell Biol. 1982 May;93(2):402–410. doi: 10.1083/jcb.93.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman J. R., Rees D. A. The behaviour of fibroblasts migrating from chick heart explants: changes in adhesion, locomotion and growth, and in the distribution of actomyosin and fibronectin. J Cell Sci. 1979 Oct;39:149–165. doi: 10.1242/jcs.39.1.149. [DOI] [PubMed] [Google Scholar]
- Culp L. A. Electrophoretic analysis of substrate-attached proteins from normal and virus-transformed cells. Biochemistry. 1976 Sep 7;15(18):4094–4104. doi: 10.1021/bi00663a028. [DOI] [PubMed] [Google Scholar]
- DePasquale J. A., Izzard C. S. Accumulation of talin in nodes at the edge of the lamellipodium and separate incorporation into adhesion plaques at focal contacts in fibroblasts. J Cell Biol. 1991 Jun;113(6):1351–1359. doi: 10.1083/jcb.113.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpech A., Delpech B. Expression of hyaluronic acid-binding glycoprotein, hyaluronectin, in the developing rat embryo. Dev Biol. 1984 Feb;101(2):391–400. doi: 10.1016/0012-1606(84)90153-2. [DOI] [PubMed] [Google Scholar]
- Delpech A., Girard N., Delpech B. Localization of hyaluronectin in the nervous system. Brain Res. 1982 Aug 12;245(2):251–257. doi: 10.1016/0006-8993(82)90807-1. [DOI] [PubMed] [Google Scholar]
- Delpech B., Delpech A., Girard N., Chauzy C., Laumonier R. An antigen associated with mesenchyme in human tumours that cross-reacts with brain glycoprotein. Br J Cancer. 1979 Jul;40(1):123–133. doi: 10.1038/bjc.1979.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpech B., Girard N., Vannier J. P., Tilly H., Piguet H. Production d'une glycoprotéine affine pour l'hyaluronane par les monocytes du sang humain. Son utilisation comme marqueur dans les leucémies myéloïdes. C R Acad Sci III. 1992;314(13):579–585. [PubMed] [Google Scholar]
- Delpech B., Maingonnat C., Delpech A., Maes P., Girard N., Bertrand P. Characterization of a hyaluronic acid-binding protein from sheep brain comparison with human brain hyaluronectin. Int J Biochem. 1991;23(3):329–337. doi: 10.1016/0020-711x(91)90115-4. [DOI] [PubMed] [Google Scholar]
- Elstad C. A., Hosick H. L. Contribution of the extracellular matrix to growth properties of cells from a preneoplastic outgrowth: possible role of hyaluronic acid. Exp Cell Biol. 1987;55(6):313–321. doi: 10.1159/000163434. [DOI] [PubMed] [Google Scholar]
- Grey A. M., Schor A. M., Rushton G., Ellis I., Schor S. L. Purification of the migration stimulating factor produced by fetal and breast cancer patient fibroblasts. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2438–2442. doi: 10.1073/pnas.86.7.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Batchu R. B., Datta K. Purification, partial characterization of rat kidney hyaluronic acid binding protein and its localization on the cell surface. Eur J Cell Biol. 1991 Oct;56(1):58–67. [PubMed] [Google Scholar]
- Gupta S., Datta K. Possible role of hyaluronectin on cell adhesion in rat histiocytoma. Exp Cell Res. 1991 Aug;195(2):386–394. doi: 10.1016/0014-4827(91)90388-b. [DOI] [PubMed] [Google Scholar]
- Halfter W., Liverani D., Vigny M., Monard D. Deposition of extracellular matrix along the pathways of migrating fibroblasts. Cell Tissue Res. 1990 Dec;262(3):467–481. doi: 10.1007/BF00305243. [DOI] [PubMed] [Google Scholar]
- Izzard C. S., Lochner L. R. Formation of cell-to-substrate contacts during fibroblast motility: an interference-reflexion study. J Cell Sci. 1980 Apr;42:81–116. doi: 10.1242/jcs.42.1.81. [DOI] [PubMed] [Google Scholar]
- Knudson C. B., Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993 Oct;7(13):1233–1241. [PubMed] [Google Scholar]
- Kolega J., Shure M. S., Chen W. T., Young N. D. Rapid cellular translocation is related to close contacts formed between various cultured cells and their substrata. J Cell Sci. 1982 Apr;54:23–34. doi: 10.1242/jcs.54.1.23. [DOI] [PubMed] [Google Scholar]
- Kujawa M. J., Carrino D. A., Caplan A. I. Substrate-bonded hyaluronic acid exhibits a size-dependent stimulation of chondrogenic differentiation of stage 24 limb mesenchymal cells in culture. Dev Biol. 1986 Apr;114(2):519–528. doi: 10.1016/0012-1606(86)90215-0. [DOI] [PubMed] [Google Scholar]
- Kujawa M. J., Pechak D. G., Fiszman M. Y., Caplan A. I. Hyaluronic acid bonded to cell culture surfaces inhibits the program of myogenesis. Dev Biol. 1986 Jan;113(1):10–16. doi: 10.1016/0012-1606(86)90103-x. [DOI] [PubMed] [Google Scholar]
- Lark M. W., Culp L. A. Selective solubilization of hyaluronic acid from fibroblast substratum adhesion sites. J Biol Chem. 1982 Dec 10;257(23):14073–14080. [PubMed] [Google Scholar]
- Laterra J., Culp L. A. Differences in hyaluronate binding to plasma and cell surface fibronectins. Requirement for aggregation. J Biol Chem. 1982 Jan 25;257(2):719–726. [PubMed] [Google Scholar]
- Lesley J., Schulte R., Hyman R. Binding of hyaluronic acid to lymphoid cell lines is inhibited by monoclonal antibodies against Pgp-1. Exp Cell Res. 1990 Apr;187(2):224–233. doi: 10.1016/0014-4827(90)90085-o. [DOI] [PubMed] [Google Scholar]
- Lévesque H., Girard N., Maingonnat C., Delpech A., Chauzy C., Tayot J., Courtois H., Delpech B. Localization and solubilization of hyaluronan and of the hyaluronan-binding protein hyaluronectin in human normal and arteriosclerotic arterial walls. Atherosclerosis. 1994 Jan;105(1):51–62. doi: 10.1016/0021-9150(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Neyfakh A. A., Jr, Tint I. S., Svitkina T. M., Bershadsky A. D., Gelfand V. I. Visualization of cellular focal contacts using a monoclonal antibody to 80 kD serum protein adsorbed on the substratum. Exp Cell Res. 1983 Dec;149(2):387–396. doi: 10.1016/0014-4827(83)90351-8. [DOI] [PubMed] [Google Scholar]
- Ponting J. M., Kumar S. Isolation and characterisation of a hyaluronan binding protein, hyaluronectin, from human placenta and its colocalisation with hyaluronan. J Anat. 1995 Feb;186(Pt 1):131–142. [PMC free article] [PubMed] [Google Scholar]
- Pulleyblank D. E., Booth G. M. Improved methods for the fluorographic detection of weak beta-emitting radioisotopes in Agarose and acrylamide gel electrophoresis media. J Biochem Biophys Methods. 1981 Jun;4(5-6):339–346. doi: 10.1016/0165-022x(81)90074-9. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Culp L. A. Preliminary characterization of the proteoglycans in the substrate adhesion sites of normal and virus-transformed murine cells. Biochemistry. 1979 Dec 11;18(25):5621–5629. doi: 10.1021/bi00592a016. [DOI] [PubMed] [Google Scholar]
- Roman J., LaChance R. M., Broekelmann T. J., Kennedy C. J., Wayner E. A., Carter W. G., McDonald J. A. The fibronectin receptor is organized by extracellular matrix fibronectin: implications for oncogenic transformation and for cell recognition of fibronectin matrices. J Cell Biol. 1989 Jun;108(6):2529–2543. doi: 10.1083/jcb.108.6.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Scott S., Kawka D. W., Kazazis D. M., Gailit J., Ruoslahti E. Cell surface distribution of fibronectin and vitronectin receptors depends on substrate composition and extracellular matrix accumulation. J Cell Biol. 1988 Jun;106(6):2171–2182. doi: 10.1083/jcb.106.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengblad A. Affinity chromatography on immobilized hyaluronate and its application to the isolation of hyaluronate binding properties from cartilage. Biochim Biophys Acta. 1979 Jun 19;578(2):281–289. doi: 10.1016/0005-2795(79)90158-2. [DOI] [PubMed] [Google Scholar]
- Terry A. H., Culp L. A. Substrate-attached glycoproteins from normal and virus-transformed cells. Biochemistry. 1974 Jan 29;13(3):414–425. doi: 10.1021/bi00700a004. [DOI] [PubMed] [Google Scholar]
- Toole B. P. Developmental role of hyaluronate. Connect Tissue Res. 1982;10(1):93–100. doi: 10.3109/03008208209034409. [DOI] [PubMed] [Google Scholar]
- Toole B. P. Hyaluronan and its binding proteins, the hyaladherins. Curr Opin Cell Biol. 1990 Oct;2(5):839–844. doi: 10.1016/0955-0674(90)90081-o. [DOI] [PubMed] [Google Scholar]
- Turley E. A., Bowman P., Kytryk M. A. Effects of hyaluronate and hyaluronate binding proteins on cell motile and contact behaviour. J Cell Sci. 1985 Oct;78:133–145. doi: 10.1242/jcs.78.1.133. [DOI] [PubMed] [Google Scholar]
- Turley E. A., Brassel P., Moore D. A hyaluronan-binding protein shows a partial and temporally regulated codistribution with actin on locomoting chick heart fibroblasts. Exp Cell Res. 1990 Apr;187(2):243–249. doi: 10.1016/0014-4827(90)90087-q. [DOI] [PubMed] [Google Scholar]
- Turley E. A. Hyaluronan and cell locomotion. Cancer Metastasis Rev. 1992 Mar;11(1):21–30. doi: 10.1007/BF00047600. [DOI] [PubMed] [Google Scholar]
- Turley E. A., Torrance J. Localization of hyaluronate and hyaluronate-binding protein on motile and non-motile fibroblasts. Exp Cell Res. 1985 Nov;161(1):17–28. doi: 10.1016/0014-4827(85)90486-0. [DOI] [PubMed] [Google Scholar]
- Underhill C. CD44: the hyaluronan receptor. J Cell Sci. 1992 Oct;103(Pt 2):293–298. doi: 10.1242/jcs.103.2.293. [DOI] [PubMed] [Google Scholar]
- Yamagata M., Saga S., Kato M., Bernfield M., Kimata K. Selective distributions of proteoglycans and their ligands in pericellular matrix of cultured fibroblasts. Implications for their roles in cell-substratum adhesion. J Cell Sci. 1993 Sep;106(Pt 1):55–65. doi: 10.1242/jcs.106.1.55. [DOI] [PubMed] [Google Scholar]
- Yang B., Yang B. L., Savani R. C., Turley E. A. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994 Jan 15;13(2):286–296. doi: 10.1002/j.1460-2075.1994.tb06261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda M., Shimizu S., Nishi Y., Yamagata M., Suzuki S., Kimata K. Hyaluronic acid-dependent change in the extracellular matrix of mouse dermal fibroblasts that is conducive to cell proliferation. J Cell Sci. 1988 Jun;90(Pt 2):275–286. doi: 10.1242/jcs.90.2.275. [DOI] [PubMed] [Google Scholar]
- Yoneda M., Yamagata M., Suzuki S., Kimata K. Hyaluronic acid modulates proliferation of mouse dermal fibroblasts in culture. J Cell Sci. 1988 Jun;90(Pt 2):265–273. doi: 10.1242/jcs.90.2.265. [DOI] [PubMed] [Google Scholar]
- Zimmermann D. R., Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989 Oct;8(10):2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]