Abstract
Tendons are parallel arrays of collagenous fibres which are specialised to resist and transmit tensile stresses. The response of tendon fibres to tensile stress is age-dependent and complex. Elastic elongation at low stress is accompanied by the disappearance of alternate light and dark bands seen in transmitted polarised light. This region of the stress/strain curve is associated with straightening of fibre 'crimps'. At higher stress, elongation is still elastic and reversible until break point is reached. This behaviour may be associated with straightening of a helical arrangement of collagen fibrils. In addition to the collagen fibrils, there are transverse and longitudinal proteoglycan filaments, many of which bridge and link between the fibrils. We have investigated the effect of various levels of stress from very low up to breaking point on the appearance of the proteoglycan filaments and their relationships with the collagen fibrils. Proteoglycan-collagen fibril interactions in rat and mouse tail and flexor digitorum tendons were visualised by Cupromeronic blue staining, applied to dissected fibres in the resting state and at stresses up to breaking. Proteoglycan filaments were seen to be orthogonally arranged in every D period, probably at the d band in mature tendons. In immature tendons proteoglycan filaments took up more varied orientations, but were mainly orthogonal or axially arranged with respect to the collagen fibrils. Both pictures appeared unchanged after application of stress of any level up to breaking point. Young tendons ruptured at lower stresses than mature tendons. It is suggested that PG bridges between collagen fibrils play a part in transmitting and resisting tensile stresses in tendons, contributing to the strength of the tissue.
Full text
PDF


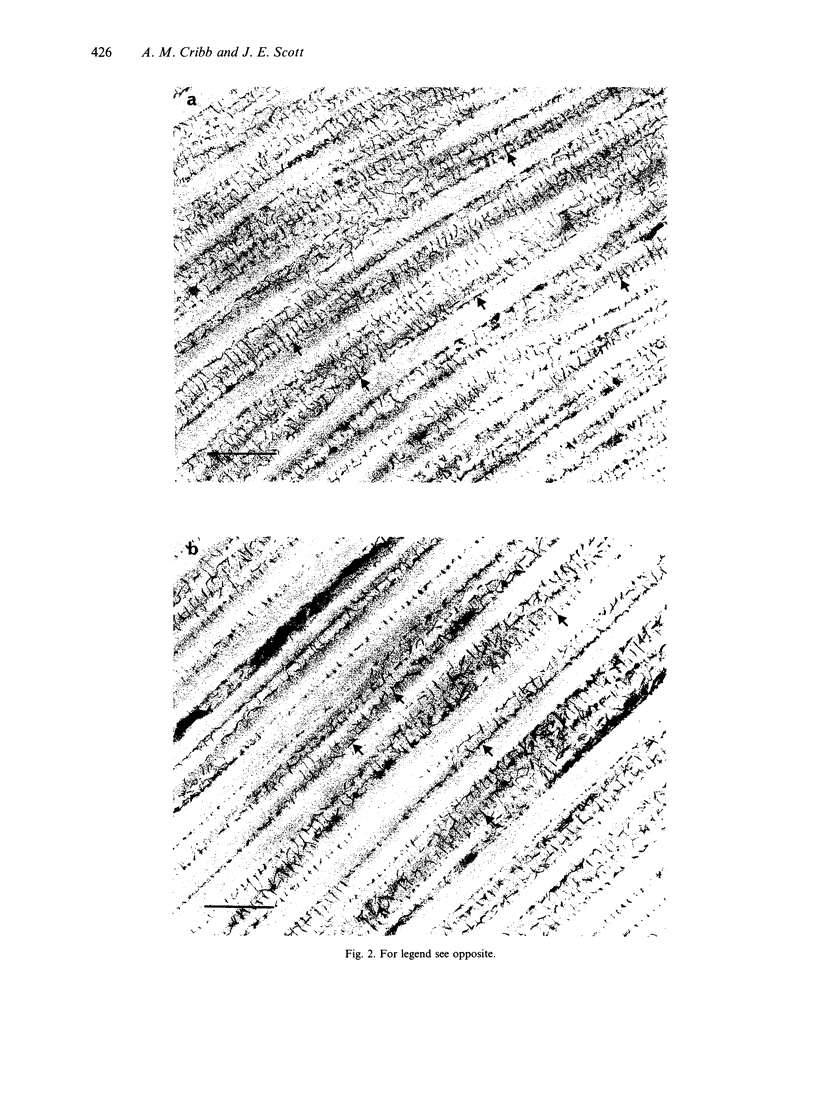
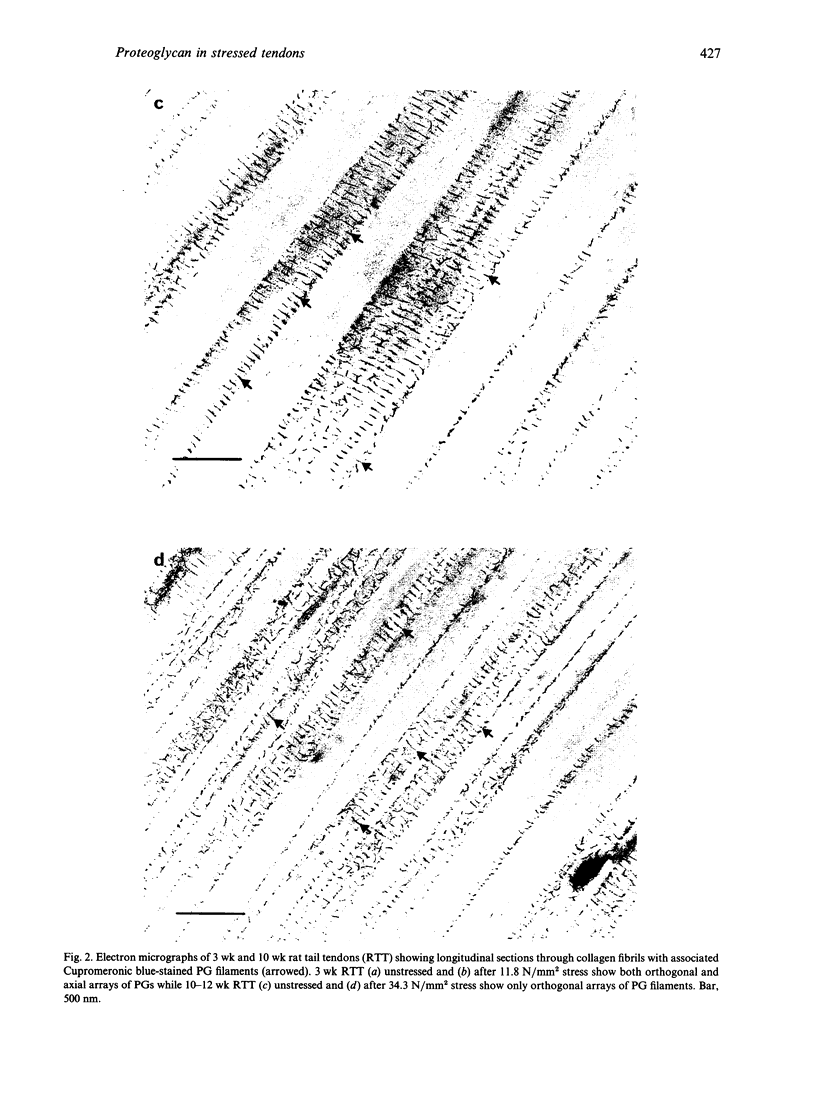

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castellani P. P., Morocutti M., Franchi M., Ruggeri A., Bigi A., Roveri N. Arrangement of microfibrils in collagen fibrils of tendons in the rat tail. Ultrastructural and x-ray diffraction investigation. Cell Tissue Res. 1983;234(3):735–743. doi: 10.1007/BF00218664. [DOI] [PubMed] [Google Scholar]
- Gathercole L. J., Keller A. Crimp morphology in the fibre-forming collagens. Matrix. 1991 Jun;11(3):214–234. doi: 10.1016/s0934-8832(11)80161-7. [DOI] [PubMed] [Google Scholar]
- Haigh M., Scott J. E. A method of processing tissue sections for staining with cu-promeronic blue and other dyes, using CEC techniques, for light and electron microscopy. Basic Appl Histochem. 1986;30(4):479–486. [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R., Hughes E. W. Proteoglycan-collagen arrangements in developing rat tail tendon. An electron microscopical and biochemical investigation. Biochem J. 1981 Jun 1;195(3):573–581. doi: 10.1042/bj1950573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Parry D. A. Control of collagen fibril diameters in tissues. Int J Biol Macromol. 1992 Oct;14(5):292–293. doi: 10.1016/s0141-8130(05)80043-1. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan histochemistry--a valuable tool for connective tissue biochemists. Coll Relat Res. 1985 Dec;5(6):541–575. doi: 10.1016/s0174-173x(85)80008-x. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan:collagen interactions and subfibrillar structure in collagen fibrils. Implications in the development and ageing of connective tissues. J Anat. 1990 Apr;169:23–35. [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992 Jun;6(9):2639–2645. [PubMed] [Google Scholar]
- Scott J. E. The nomenclature of glycosaminoglycans and proteoglycans. Glycoconj J. 1993 Dec;10(6):419–421. doi: 10.1007/BF00737960. [DOI] [PubMed] [Google Scholar]




