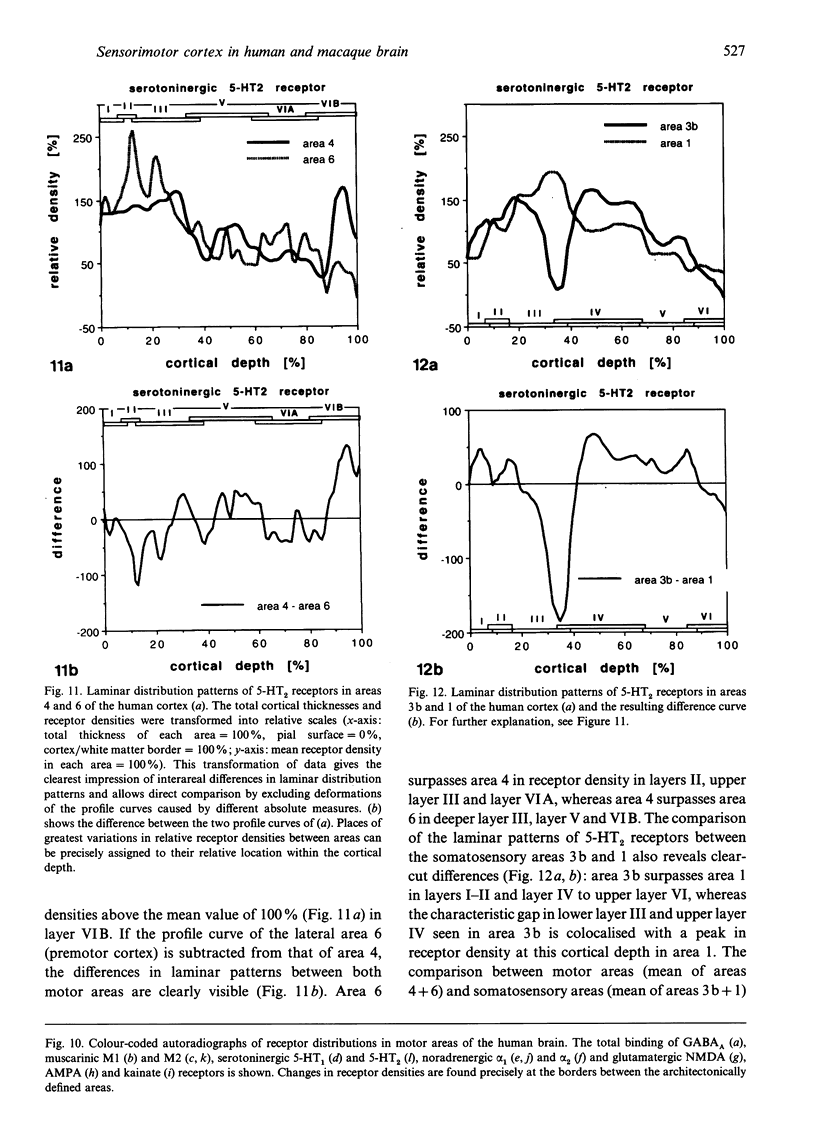
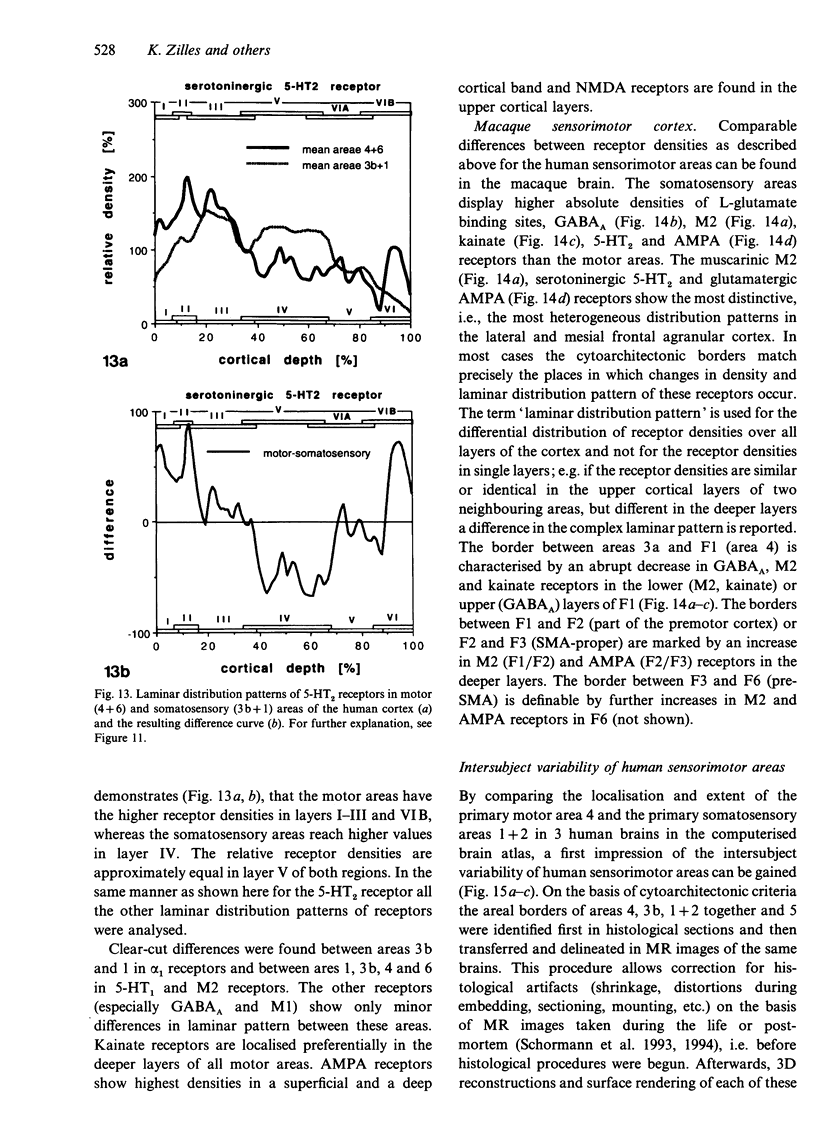
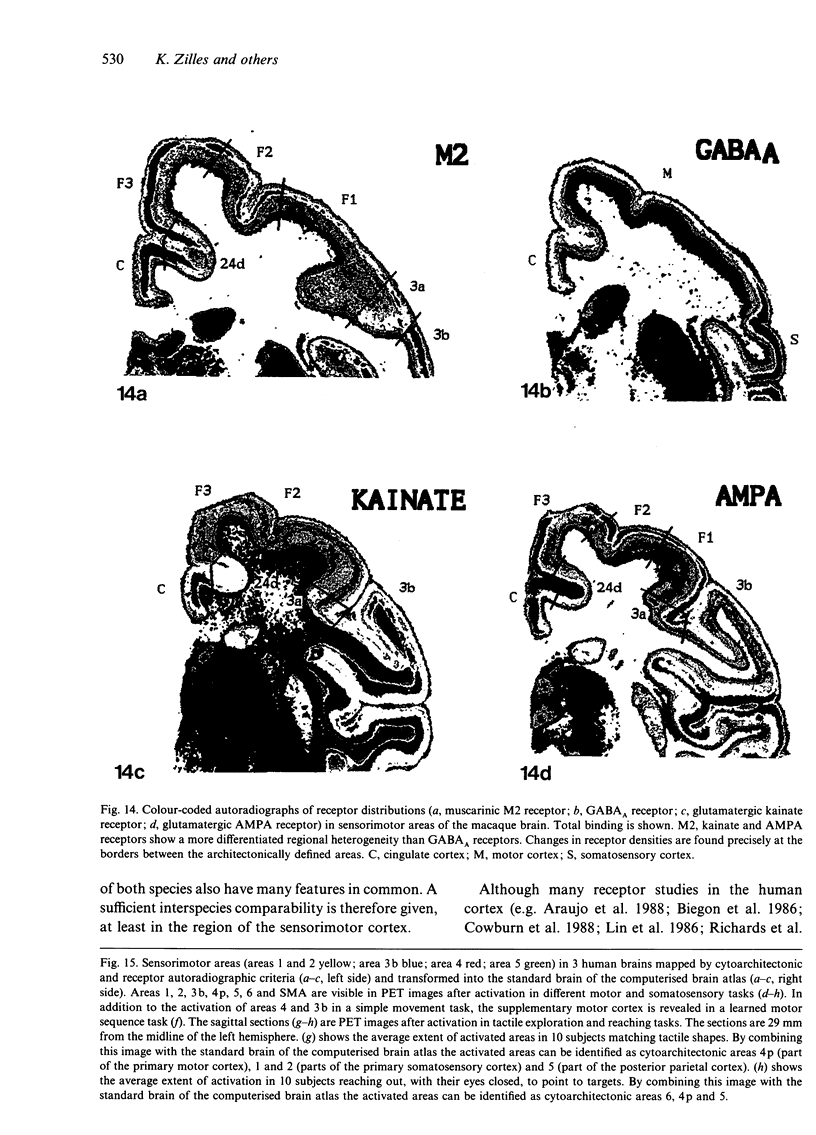
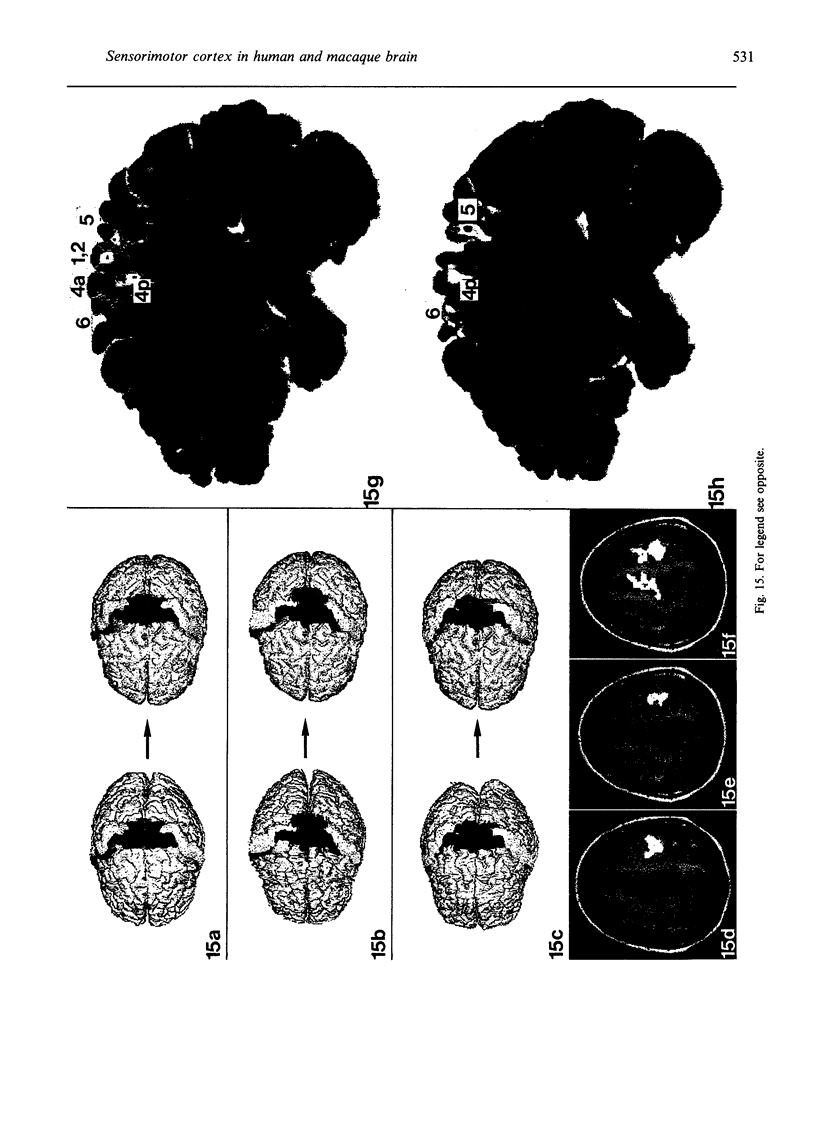
Abstract
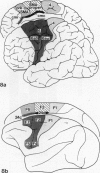
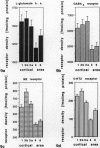
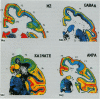
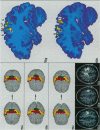
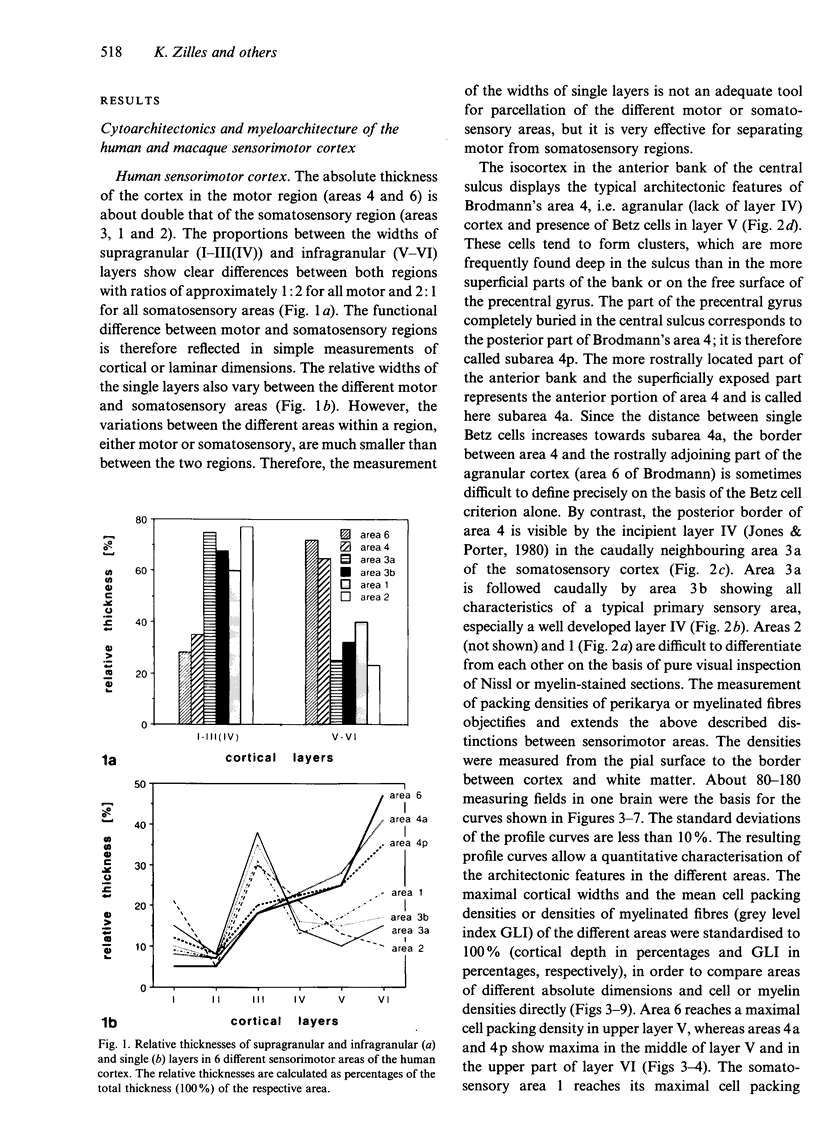
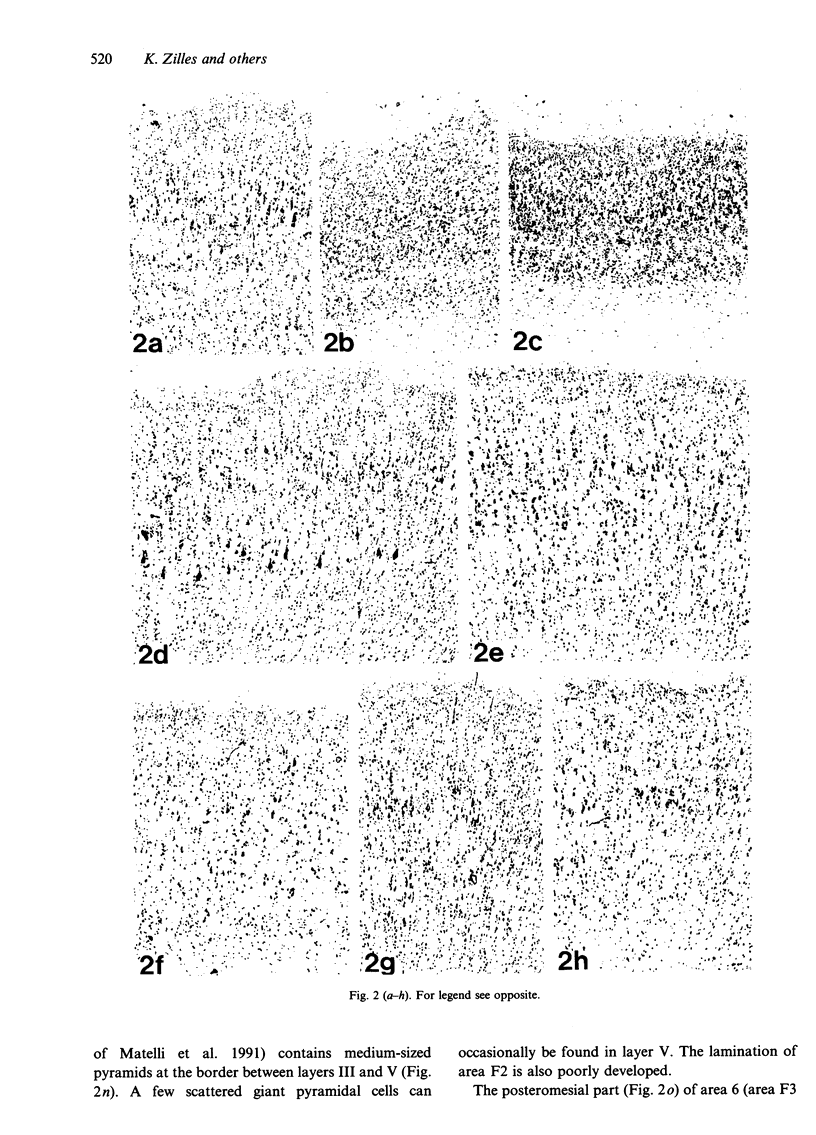
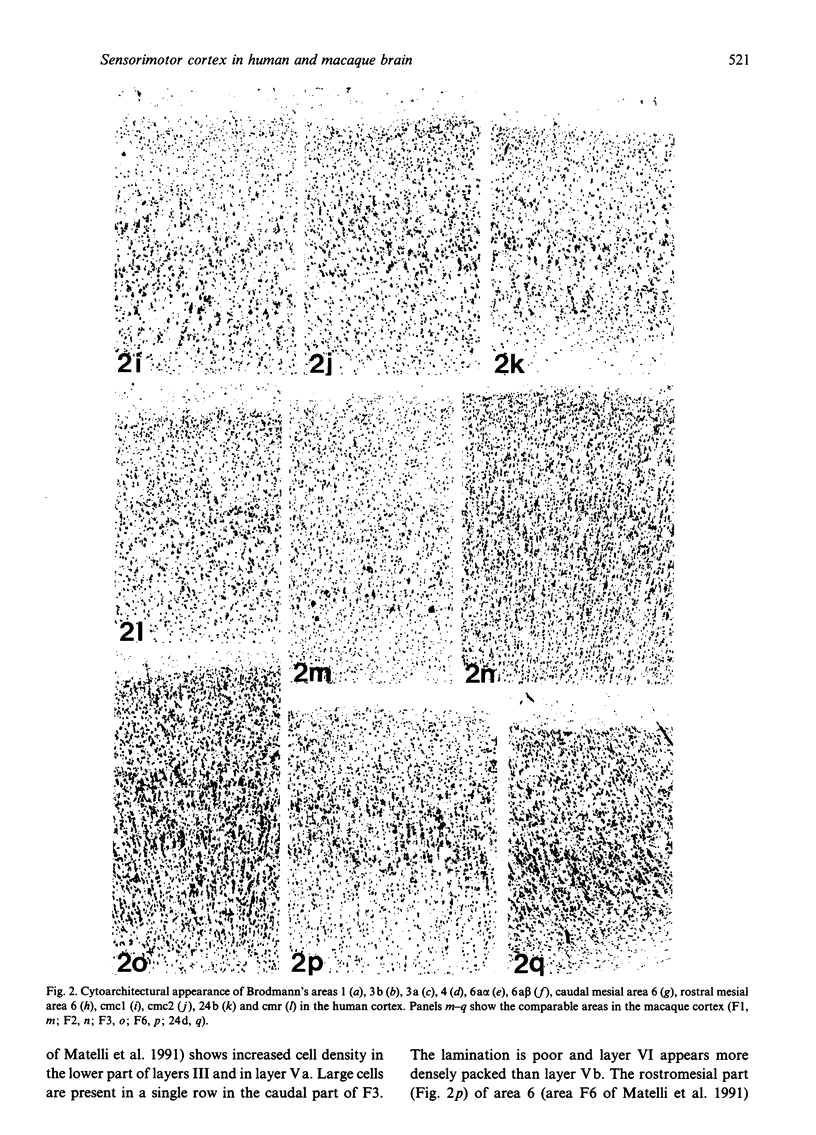
The human and macaque sensorimotor cortex was subdivided into numerous areas by a correlative analysis based on cytoarchitectonics, myeloarchitecture and the distribution of transmitter receptors. Receptor densities and laminar distribution patterns differ not only between motor and somatosensory regions, but also between different areas within these regions of the cortex. Changes in receptor distribution often match architectonically defined borders. Receptor findings provide new criteria for a more detailed mapping in the human brain which cannot be achieved by cytoarchitectonic analysis alone. Morphological data on these areas were integrated with functional data from positron emission tomography (PET) on the basis of a recently developed computerised brain atlas. The central sulcus marks the border between (1) the agranular motor cortex with a generally low density of glutamatergic, muscarinic, GABAergic and serotoninergic receptors, and (2) the granular somatosensory cortex with higher densities of these receptors. Rostral to the primary motor cortex, 2 isocortical areas are found on the mesial cortex which probably represent the functionally defined supplementary motor areas (SMA) SMA-proper (caudally) and pre-SMA (rostrally). Below SMA-proper the areas 24d (macaque) and the caudal cingulate motor area cmc (human) are located in the cingulate sulcus. Both regions correspond to the 'posterior cingulate motor areas' of recent PET studies and to the posterior part of the agranular cingulate cortex of architectonic studies. Below pre-SMA the area 24c (macaque) and the rostral cingulate motor area cmr (human) are located in the cingulate sulcus; they correspond to the 'anterior cingulate motor areas' of recent PET observations and to the anterior part of the agranular cingulate cortex of architectonic studies. Homologous sensorimotor areas can be defined in both species on the basis of common architectonic features.
Full text
PDF



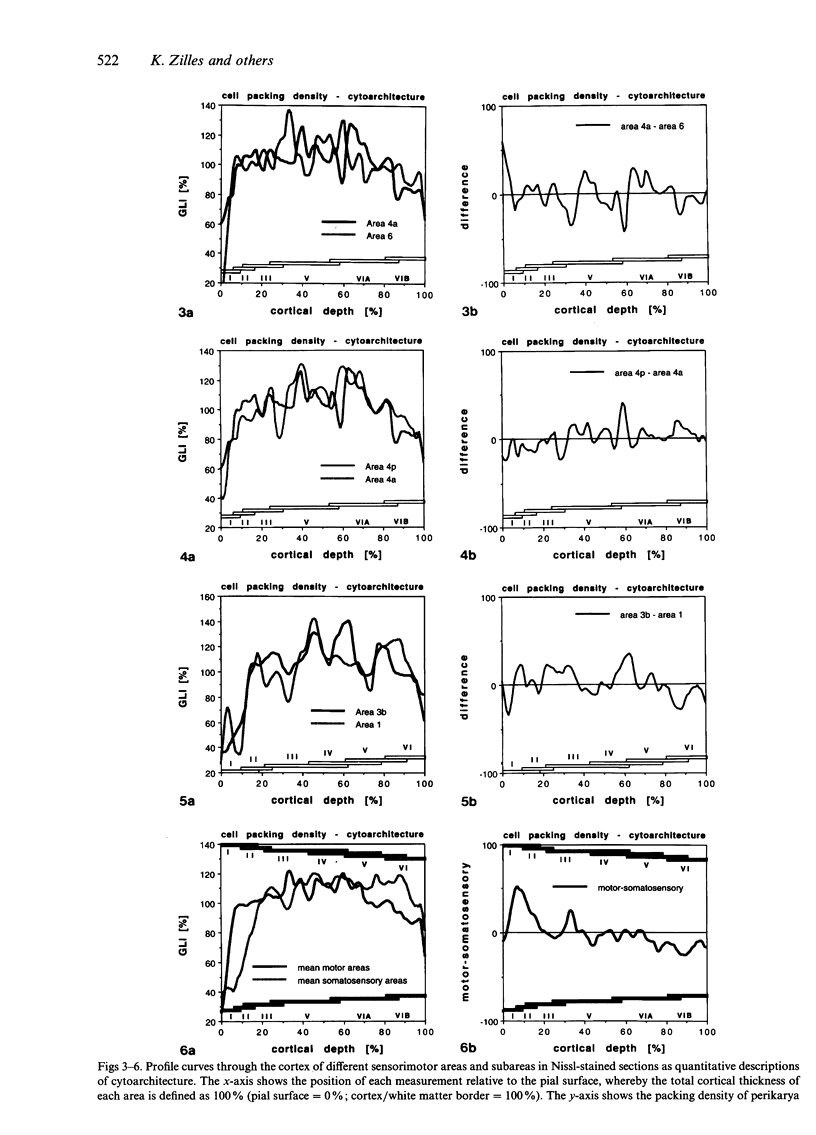
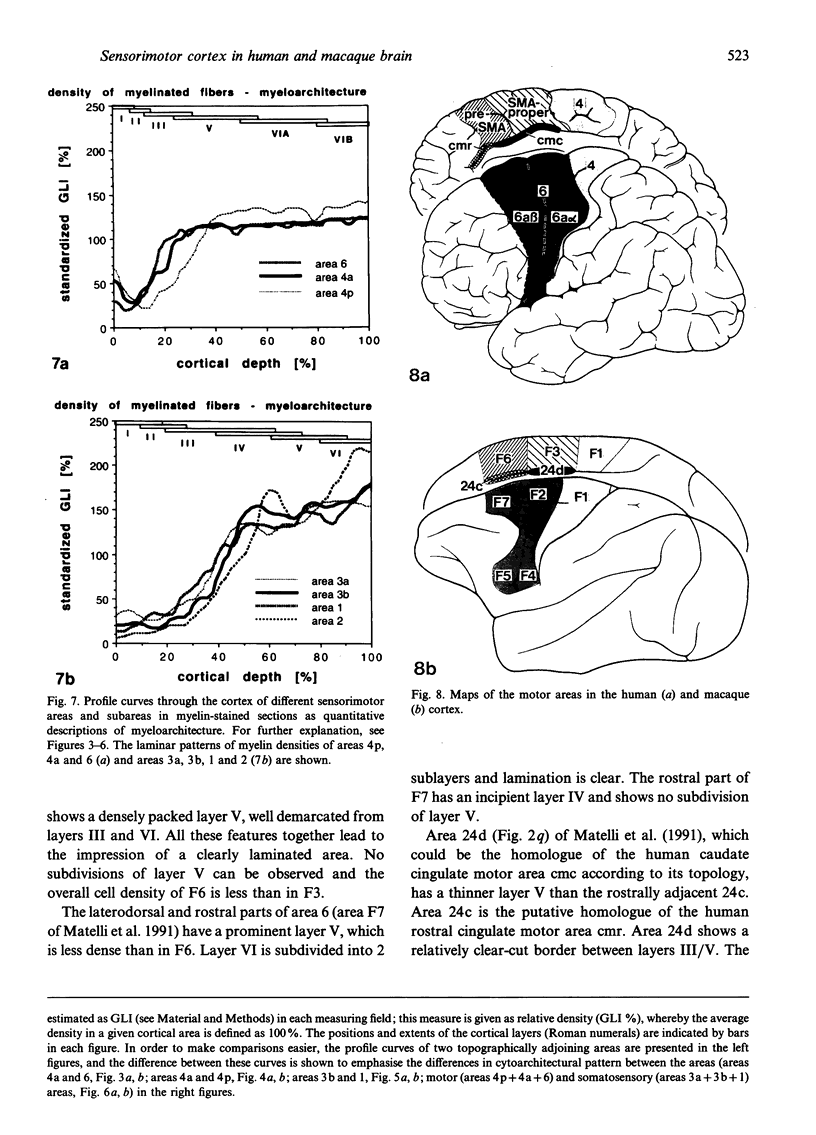
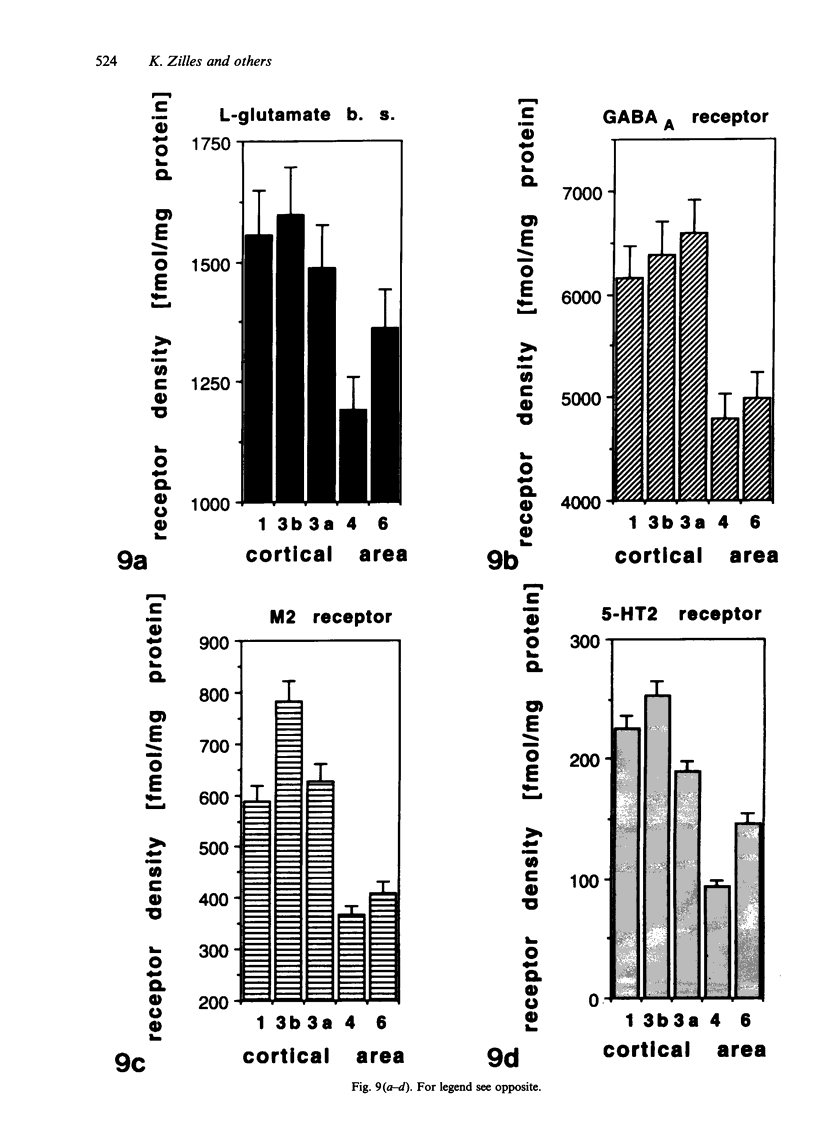

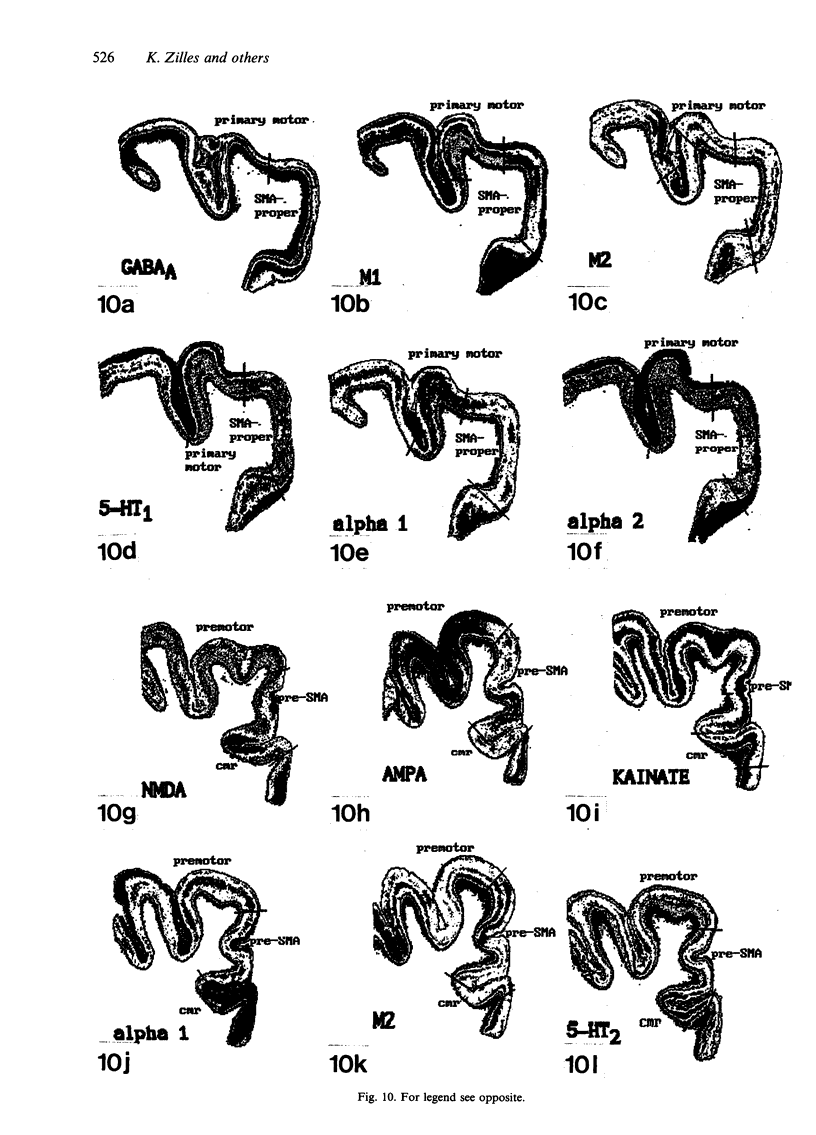






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alloway K. D., Burton H. Differential effects of GABA and bicuculline on rapidly- and slowly-adapting neurons in primary somatosensory cortex of primates. Exp Brain Res. 1991;85(3):598–610. doi: 10.1007/BF00231744. [DOI] [PubMed] [Google Scholar]
- Araujo D. M., Lapchak P. A., Robitaille Y., Gauthier S., Quirion R. Differential alteration of various cholinergic markers in cortical and subcortical regions of human brain in Alzheimer's disease. J Neurochem. 1988 Jun;50(6):1914–1923. doi: 10.1111/j.1471-4159.1988.tb02497.x. [DOI] [PubMed] [Google Scholar]
- BRAIN W. R. Malnutrition of the nervous system. Br Med J. 1947 Nov 15;2(4532):763–766. doi: 10.1136/bmj.2.4532.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H., Pandya D. N. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol. 1987 Feb 8;256(2):211–228. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- Berger B., Trottier S., Verney C., Gaspar P., Alvarez C. Regional and laminar distribution of the dopamine and serotonin innervation in the macaque cerebral cortex: a radioautographic study. J Comp Neurol. 1988 Jul 1;273(1):99–119. doi: 10.1002/cne.902730109. [DOI] [PubMed] [Google Scholar]
- Biegon A., Kargman S., Snyder L., McEwen B. S. Characterization and localization of serotonin receptors in human brain postmortem. Brain Res. 1986 Jan 15;363(1):91–98. doi: 10.1016/0006-8993(86)90661-x. [DOI] [PubMed] [Google Scholar]
- Braak H. A primitive gigantopyramidal field buried in the depth of the cingulate sulcus of the human brain. Brain Res. 1976 Jun 11;109(2):219–223. doi: 10.1016/0006-8993(76)90526-6. [DOI] [PubMed] [Google Scholar]
- Braak H. The pigment architecture of the human frontal lobe. I. Precentral, subcentral and frontal region. Anat Embryol (Berl) 1979;157(1):35–68. doi: 10.1007/BF00315640. [DOI] [PubMed] [Google Scholar]
- Chudler E. H., Pretel S., Kenshalo D. R., Jr Distribution of GAD-immunoreactive neurons in the first (SI) and second (SII) somatosensory cortex of the monkey. Brain Res. 1988 Jul 19;456(1):57–63. doi: 10.1016/0006-8993(88)90346-0. [DOI] [PubMed] [Google Scholar]
- Colebatch J. G., Deiber M. P., Passingham R. E., Friston K. J., Frackowiak R. S. Regional cerebral blood flow during voluntary arm and hand movements in human subjects. J Neurophysiol. 1991 Jun;65(6):1392–1401. doi: 10.1152/jn.1991.65.6.1392. [DOI] [PubMed] [Google Scholar]
- Cowburn R., Hardy J., Roberts P., Briggs R. Regional distribution of pre- and postsynaptic glutamatergic function in Alzheimer's disease. Brain Res. 1988 Jun 14;452(1-2):403–407. doi: 10.1016/0006-8993(88)90048-0. [DOI] [PubMed] [Google Scholar]
- DeFelipe J., Jones E. G. A light and electron microscopic study of serotonin-immunoreactive fibers and terminals in the monkey sensory-motor cortex. Exp Brain Res. 1988;71(1):171–182. doi: 10.1007/BF00247532. [DOI] [PubMed] [Google Scholar]
- Deiber M. P., Passingham R. E., Colebatch J. G., Friston K. J., Nixon P. D., Frackowiak R. S. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84(2):393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- Dum R. P., Strick P. L. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991 Mar;11(3):667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried I., Katz A., McCarthy G., Sass K. J., Williamson P., Spencer S. S., Spencer D. D. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci. 1991 Nov;11(11):3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries W. Inputs from motor and premotor cortex to the superior colliculus of the macaque monkey. Behav Brain Res. 1985 Nov-Dec;18(2):95–105. doi: 10.1016/0166-4328(85)90066-x. [DOI] [PubMed] [Google Scholar]
- Frith C. D., Friston K., Liddle P. F., Frackowiak R. S. Willed action and the prefrontal cortex in man: a study with PET. Proc Biol Sci. 1991 Jun 22;244(1311):241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- Gaspar P., Berger B., Febvret A., Vigny A., Henry J. P. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J Comp Neurol. 1989 Jan 8;279(2):249–271. doi: 10.1002/cne.902790208. [DOI] [PubMed] [Google Scholar]
- Grafton S. T., Mazziotta J. C., Presty S., Friston K. J., Frackowiak R. S., Phelps M. E. Functional anatomy of human procedural learning determined with regional cerebral blood flow and PET. J Neurosci. 1992 Jul;12(7):2542–2548. doi: 10.1523/JNEUROSCI.12-07-02542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Isseroff R., Dillon K. A., Fieldust S. J., Biegon A. Autoradiographic analysis of alpha 1-noradrenergic receptors in the human brain postmortem. Effect of suicide. Arch Gen Psychiatry. 1990 Nov;47(11):1049–1053. doi: 10.1001/archpsyc.1990.01810230065010. [DOI] [PubMed] [Google Scholar]
- Gross-Isseroff R., Salama D., Israeli M., Biegon A. Autoradiographic analysis of age-dependent changes in serotonin 5-HT2 receptors of the human brain postmortem. Brain Res. 1990 Jun 11;519(1-2):223–227. doi: 10.1016/0006-8993(90)90081-l. [DOI] [PubMed] [Google Scholar]
- Halsband U., Freund H. J. Premotor cortex and conditional motor learning in man. Brain. 1990 Feb;113(Pt 1):207–222. doi: 10.1093/brain/113.1.207. [DOI] [PubMed] [Google Scholar]
- He S. Q., Dum R. P., Strick P. L. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci. 1993 Mar;13(3):952–980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry S. H., Schwark H. D., Jones E. G., Yan J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 1987 May;7(5):1503–1519. doi: 10.1523/JNEUROSCI.07-05-01503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herregodts P., Ebinger G., Michotte Y. Distribution of monoamines in human brain: evidence for neurochemical heterogeneity in subcortical as well as in cortical areas. Brain Res. 1991 Mar 1;542(2):300–306. doi: 10.1016/0006-8993(91)91582-l. [DOI] [PubMed] [Google Scholar]
- Jansen K. L., Faull R. L., Dragunow M. Excitatory amino acid receptors in the human cerebral cortex: a quantitative autoradiographic study comparing the distributions of [3H]TCP, [3H]glycine, L-[3H]glutamate, [3H]AMPA and [3H]kainic acid binding sites. Neuroscience. 1989;32(3):587–607. doi: 10.1016/0306-4522(89)90282-0. [DOI] [PubMed] [Google Scholar]
- Jansen K. L., Faull R. L., Dragunow M., Leslie R. A. Distribution of excitatory and inhibitory amino acid, sigma, monoamine, catecholamine, acetylcholine, opioid, neurotensin, substance P, adenosine and neuropeptide Y receptors in human motor and somatosensory cortex. Brain Res. 1991 Dec 6;566(1-2):225–238. doi: 10.1016/0006-8993(91)91703-4. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Porter R. What is area 3a? Brain Res. 1980 May;203(1):1–43. doi: 10.1016/0165-0173(80)90002-8. [DOI] [PubMed] [Google Scholar]
- Kawashima R., Roland P. E., O'Sullivan B. T. Fields in human motor areas involved in preparation for reaching, actual reaching, and visuomotor learning: a positron emission tomography study. J Neurosci. 1994 Jun;14(6):3462–3474. doi: 10.1523/JNEUROSCI.14-06-03462.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata K. Somatotopy in the human supplementary motor area. Trends Neurosci. 1992 May;15(5):159–160. doi: 10.1016/0166-2236(92)90164-4. [DOI] [PubMed] [Google Scholar]
- Lemon R. The output map of the primate motor cortex. Trends Neurosci. 1988 Nov;11(11):501–506. doi: 10.1016/0166-2236(88)90012-4. [DOI] [PubMed] [Google Scholar]
- Lin S. C., Olson K. C., Okazaki H., Richelson E. Studies on muscarinic binding sites in human brain identified with [3H]pirenzepine. J Neurochem. 1986 Jan;46(1):274–279. doi: 10.1111/j.1471-4159.1986.tb12958.x. [DOI] [PubMed] [Google Scholar]
- Luppino G., Matelli M., Camarda R. M., Gallese V., Rizzolatti G. Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: an intracortical microstimulation study in the macaque monkey. J Comp Neurol. 1991 Sep 22;311(4):463–482. doi: 10.1002/cne.903110403. [DOI] [PubMed] [Google Scholar]
- Luppino G., Matelli M., Camarda R., Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol. 1993 Dec 1;338(1):114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- Luppino G., Matelli M., Rizzolatti G. Cortico-cortical connections of two electrophysiologically identified arm representations in the mesial agranular frontal cortex. Exp Brain Res. 1990;82(1):214–218. doi: 10.1007/BF00230855. [DOI] [PubMed] [Google Scholar]
- Macpherson J. M., Marangoz C., Miles T. S., Wiesendanger M. Microstimulation of the supplementary motor area (SMA) in the awake monkey. Exp Brain Res. 1982;45(3):410–416. doi: 10.1007/BF01208601. [DOI] [PubMed] [Google Scholar]
- Matelli M., Camarda R., Glickstein M., Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the macaque monkey. J Comp Neurol. 1986 Sep 15;251(3):281–298. doi: 10.1002/cne.902510302. [DOI] [PubMed] [Google Scholar]
- Matelli M., Luppino G., Fogassi L., Rizzolatti G. Thalamic input to inferior area 6 and area 4 in the macaque monkey. J Comp Neurol. 1989 Feb 15;280(3):468–488. doi: 10.1002/cne.902800311. [DOI] [PubMed] [Google Scholar]
- Matelli M., Luppino G., Rizzolatti G. Architecture of superior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. J Comp Neurol. 1991 Sep 22;311(4):445–462. doi: 10.1002/cne.903110402. [DOI] [PubMed] [Google Scholar]
- Matelli M., Luppino G., Rizzolatti G. Patterns of cytochrome oxidase activity in the frontal agranular cortex of the macaque monkey. Behav Brain Res. 1985 Nov-Dec;18(2):125–136. doi: 10.1016/0166-4328(85)90068-3. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Sawaguchi T., Oishi T., Ueki K., Kubota K. Behavioral deficits induced by local injection of bicuculline and muscimol into the primate motor and premotor cortex. J Neurophysiol. 1991 Jun;65(6):1542–1553. doi: 10.1152/jn.1991.65.6.1542. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y., Aizawa H., Tanji J. A motor area rostral to the supplementary motor area (presupplementary motor area) in the monkey: neuronal activity during a learned motor task. J Neurophysiol. 1992 Sep;68(3):653–662. doi: 10.1152/jn.1992.68.3.653. [DOI] [PubMed] [Google Scholar]
- Mitz A. R., Wise S. P. The somatotopic organization of the supplementary motor area: intracortical microstimulation mapping. J Neurosci. 1987 Apr;7(4):1010–1021. doi: 10.1523/JNEUROSCI.07-04-01010.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muakkassa K. F., Strick P. L. Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized 'premotor' areas. Brain Res. 1979 Nov 9;177(1):176–182. doi: 10.1016/0006-8993(79)90928-4. [DOI] [PubMed] [Google Scholar]
- Mushiake H., Inase M., Tanji J. Selective coding of motor sequence in the supplementary motor area of the monkey cerebral cortex. Exp Brain Res. 1990;82(1):208–210. doi: 10.1007/BF00230853. [DOI] [PubMed] [Google Scholar]
- Okano K., Tanji J. Neuronal activities in the primate motor fields of the agranular frontal cortex preceding visually triggered and self-paced movement. Exp Brain Res. 1987;66(1):155–166. doi: 10.1007/BF00236211. [DOI] [PubMed] [Google Scholar]
- PENFIELD W., WELCH K. The supplementary motor area of the cerebral cortex; a clinical and experimental study. AMA Arch Neurol Psychiatry. 1951 Sep;66(3):289–317. doi: 10.1001/archneurpsyc.1951.02320090038004. [DOI] [PubMed] [Google Scholar]
- Paus T., Petrides M., Evans A. C., Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol. 1993 Aug;70(2):453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- Pazos A., Probst A., Palacios J. M. Serotonin receptors in the human brain--III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience. 1987 Apr;21(1):97–122. doi: 10.1016/0306-4522(87)90326-5. [DOI] [PubMed] [Google Scholar]
- Pazos A., Probst A., Palacios J. M. Serotonin receptors in the human brain--IV. Autoradiographic mapping of serotonin-2 receptors. Neuroscience. 1987 Apr;21(1):123–139. doi: 10.1016/0306-4522(87)90327-7. [DOI] [PubMed] [Google Scholar]
- Richards J. G., Schoch P., Häring P., Takacs B., Möhler H. Resolving GABAA/benzodiazepine receptors: cellular and subcellular localization in the CNS with monoclonal antibodies. J Neurosci. 1987 Jun;7(6):1866–1886. doi: 10.1523/JNEUROSCI.07-06-01866.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G., Gentilucci M., Camarda R. M., Gallese V., Luppino G., Matelli M., Fogassi L. Neurons related to reaching-grasping arm movements in the rostral part of area 6 (area 6a beta). Exp Brain Res. 1990;82(2):337–350. doi: 10.1007/BF00231253. [DOI] [PubMed] [Google Scholar]
- Roland P. E., Larsen B., Lassen N. A., Skinhøj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980 Jan;43(1):118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- Roland P. E., Meyer E., Shibasaki T., Yamamoto Y. L., Thompson C. J. Regional cerebral blood flow changes in cortex and basal ganglia during voluntary movements in normal human volunteers. J Neurophysiol. 1982 Aug;48(2):467–480. doi: 10.1152/jn.1982.48.2.467. [DOI] [PubMed] [Google Scholar]
- Roland P. E., Skinhøj E., Lassen N. A., Larsen B. Different cortical areas in man in organization of voluntary movements in extrapersonal space. J Neurophysiol. 1980 Jan;43(1):137–150. doi: 10.1152/jn.1980.43.1.137. [DOI] [PubMed] [Google Scholar]
- Roland P. E., Zilles K. Brain atlases--a new research tool. Trends Neurosci. 1994 Nov;17(11):458–467. doi: 10.1016/0166-2236(94)90131-7. [DOI] [PubMed] [Google Scholar]
- SANIDES F. THE CYTO-MYELOARCHITECTURE OF THE HUMAN FRONTAL LOBE AND ITS RELATION TO PHYLOGENETIC DIFFERENTIATION OF THE CEREBRAL CORTEX. J Hirnforsch. 1964;7:269–282. [PubMed] [Google Scholar]
- Schieber M. H. How might the motor cortex individuate movements? Trends Neurosci. 1990 Nov;13(11):440–445. doi: 10.1016/0166-2236(90)90093-p. [DOI] [PubMed] [Google Scholar]
- Schlaug G., Knorr U., Seitz R. Inter-subject variability of cerebral activations in acquiring a motor skill: a study with positron emission tomography. Exp Brain Res. 1994;98(3):523–534. doi: 10.1007/BF00233989. [DOI] [PubMed] [Google Scholar]
- Schleicher A., Zilles K., Wree A. A quantitative approach to cytoarchitectonics: software and hardware aspects of a system for the evaluation and analysis of structural inhomogeneities in nervous tissue. J Neurosci Methods. 1986 Oct;18(1-2):221–235. doi: 10.1016/0165-0270(86)90121-4. [DOI] [PubMed] [Google Scholar]
- Seitz R. J., Roland E., Bohm C., Greitz T., Stone-Elander S. Motor learning in man: a positron emission tomographic study. Neuroreport. 1990 Sep;1(1):57–60. doi: 10.1097/00001756-199009000-00016. [DOI] [PubMed] [Google Scholar]
- Seitz Rüdiger J., Roland Per E., Bohm Christian, Greitz Torgny, Stone-Elander Sharon. Somatosensory Discrimination of Shape: Tactile Exploration and Cerebral Activation. Eur J Neurosci. 1991 Jun;3(6):481–492. doi: 10.1111/j.1460-9568.1991.tb00835.x. [DOI] [PubMed] [Google Scholar]
- Seitz Rüdiger J., Roland Per E. Learning of Sequential Finger Movements in Man: A Combined Kinematic and Positron Emission Tomography (PET) Study. Eur J Neurosci. 1992;4(2):154–165. doi: 10.1111/j.1460-9568.1992.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Sessle B. J., Wiesendanger M. Structural and functional definition of the motor cortex in the monkey (Macaca fascicularis). J Physiol. 1982 Feb;323:245–265. doi: 10.1113/jphysiol.1982.sp014071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K., Tanji J. Involvement of NMDA and non-NMDA receptors in motor task-related activity in the primary and secondary cortical motor areas of the monkey. Cereb Cortex. 1993 Jul-Aug;3(4):330–347. doi: 10.1093/cercor/3.4.330. [DOI] [PubMed] [Google Scholar]
- Strick P. L. Anatomical organization of multiple motor areas in the frontal lobe: implications for recovery of function. Adv Neurol. 1988;47:293–312. [PubMed] [Google Scholar]
- Tanji J., Kurata K. Changing concepts of motor areas of the cerebral cortex. Brain Dev. 1989;11(6):374–377. doi: 10.1016/s0387-7604(89)80019-1. [DOI] [PubMed] [Google Scholar]
- Tanji J., Taniguchi K., Saga T. Supplementary motor area: neuronal response to motor instructions. J Neurophysiol. 1980 Jan;43(1):60–68. doi: 10.1152/jn.1980.43.1.60. [DOI] [PubMed] [Google Scholar]
- Wiesendanger R., Wiesendanger M. The thalamic connections with medial area 6 (supplementary motor cortex) in the monkey (macaca fascicularis). Exp Brain Res. 1985;59(1):91–104. doi: 10.1007/BF00237670. [DOI] [PubMed] [Google Scholar]
- Wise J. K. Employment of 1986 male and female graduates of US veterinary medical colleges. J Am Vet Med Assoc. 1987 Feb 15;190(4):449–450. [PubMed] [Google Scholar]
- Wise S. P. The primate premotor cortex fifty years after Fulton. Behav Brain Res. 1985 Nov-Dec;18(2):79–88. doi: 10.1016/0166-4328(85)90064-6. [DOI] [PubMed] [Google Scholar]
- Wise S. P. The primate premotor cortex: past, present, and preparatory. Annu Rev Neurosci. 1985;8:1–19. doi: 10.1146/annurev.ne.08.030185.000245. [DOI] [PubMed] [Google Scholar]
- Zezula J., Cortés R., Probst A., Palacios J. M. Benzodiazepine receptor sites in the human brain: autoradiographic mapping. Neuroscience. 1988 Jun;25(3):771–795. doi: 10.1016/0306-4522(88)90036-x. [DOI] [PubMed] [Google Scholar]
- Zilles K., Gross G., Schleicher A., Schildgen S., Bauer A., Bahro M., Schwendemann G., Zech K., Kolassa N. Regional and laminar distributions of alpha 1-adrenoceptors and their subtypes in human and rat hippocampus. Neuroscience. 1991;40(2):307–320. doi: 10.1016/0306-4522(91)90122-5. [DOI] [PubMed] [Google Scholar]
- Zilles K., Qü M., Schleicher A. Regional distribution and heterogeneity of alpha-adrenoceptors in the rat and human central nervous system. J Hirnforsch. 1993;34(2):123–132. [PubMed] [Google Scholar]
- Zilles K., Schleicher A., Rath M., Bauer A. Quantitative receptor autoradiography in the human brain. Methodical aspects. Histochemistry. 1988;90(2):129–137. doi: 10.1007/BF00500977. [DOI] [PubMed] [Google Scholar]
- Zilles K., Werner L., Qü M., Schleicher A., Gross G. Quantitative autoradiography of 11 different transmitter binding sites in the basal forebrain region of the rat--evidence of heterogeneity in distribution patterns. Neuroscience. 1991;42(2):473–481. doi: 10.1016/0306-4522(91)90390-a. [DOI] [PubMed] [Google Scholar]