Abstract
BACKGROUND:
Cardiometabolic diseases are risk factors for COVID-19 severity. The extent that cardiometabolic health represents a modifiable factor to mitigate the short- and long-term consequences from SARS-CoV-2 remains unclear. Our objective was to evaluate the associations between intraindividual variability of cardiometabolic health indicators and COVID-19 related hospitalizations and post-COVID conditions (PCC) among a relatively healthy population.
METHODS:
This retrospective, multi-site cohort study was a post-hoc analysis among individuals with cardiometabolic health data collected during routine blood donation visits in 24 US states (2009–2018) and who responded to COVID-19 questionnaires (2021–2023). Intraindividual variability of blood pressure (systolic, diastolic), total circulating cholesterol, and body mass index (BMI) were defined as the coefficient of variation (CV) across all available donation timepoints (ranging from 3 to 74); participants were categorized into CV quartiles. Associations were evaluated by multivariable binomial regressions.
RESULTS:
Overall, 3344 participants provided 42,090 donations (median 9 [IQR 5, 17]). The median age was 48 years (38, 56) at the first study donation. 1.2% (N = 40) were hospitalized due to COVID-19 and 15.5% (N = 519) had PCC. Higher BMI variability was associated with greater risk of COVID-19 hospitalization (4th quartile aRR 4.15 [95% CI 1.31, 13.11], p = 0.02; 3rd quartile aRR 3.41 [95% CI 1.09, 10.69], p = 0.04). Participants with higher variability of BMI had greater risk of PCC (4th quartile aRR 1.29 [95% CI 1.02, 1.64]; p = 0.04). Intraindividual variability of blood pressure (systolic, diastolic) and total circulating cholesterol were not associated with COVID-19 hospitalization or PCC risk (all p > 0.05). From causal mediation analysis, the association between the highest quartiles of BMI variability and PCC was not mediated by hospitalization (p > 0.05).
CONCLUSIONS:
Higher intraindividual variability of BMI was associated with COVID-19 hospitalization and PCC risk. Our findings underscore the need for further elucidating mechanisms that explain these associations and importance for consistent maintenance of body weight.
INTRODUCTION
Globally, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused 760 million coronavirus disease 2019 (COVID-19) cases and 7 million COVID-19 related deaths, as of August 16, 2023 [1]. The mitigation of population-level consequences of acute SARS-CoV-2 infection and long-term sequelae remain unclear due to substantial knowledge gaps, despite laudable development of COVID-19 vaccines and therapeutics [2]. One major challenge is elucidating how to tailor current prevention and treatment strategies for specific higher-risk subgroups of individuals [3], thereby potentially improving their effectiveness. Over 10% of individuals are estimated to experience post-COVID conditions (PCC), which is also referred to as post-acute sequelae of COVID-19, long COVID, post-COVID syndrome, after SARS-CoV-2 infection [4, 5]. PCC is a multi-system and multi-organ syndrome encompassing diverse signs, symptoms, and conditions during the weeks, months, and years after initial SARS-CoV-2 infection [6]. Another key unresolved question is determining the extent that modifiable risk factors, in addition to vaccines, influence the incidence and wide-ranging severity of PCC [7].
Cardiometabolic disease comorbidities and adiposity are risk factors of greater severity of acute SARS-CoV-2 infections, including COVID-19 hospitalizations [8–11]. SARS-CoV-2 has been shown to infect adipocytes [12] and inflammatory adipose tissue resident macrophages without dependence on the expression of angiotensin-converting enzyme 2 (ACE2) receptor [13]. SARS-CoV-2 infection in adipose tissues is hypothesized to cause greater COVID-19 severity via viral replication in adipocytes and inflammation responses of infected adipose tissue-resident macrophage [13], and insulin resistance via adipose tissue dysfunction [12]. Mendelian randomization [14] and mechanistic studies provide strong preliminary evidence of a link between suboptimal cardiometabolic health and COVID severity. Moreover, obesity and diabetes have been associated with an elevated risk of PCC [15, 16]. The underlying pathobiological mechanisms and etiology remain foundational questions of interest [17], given the potential clinical and public health implications of cardiometabolic comorbidities of COVID-19 severity and PCC.
High intraindividual variability of adiposity and metabolic health are associated with increased risk of all-cause mortality [18–21], cardiovascular mortality and diseases [22–26], and diabetes [15]. Elevated blood pressure variability is closely linked with increased arterial stiffness, vascular remodeling and injury, endothelial alterations, inflammation activation, which can result in micro-circulation alterations, lung damage, and reduced lung function [27, 28]. Obesity and adipose tissue expansion are associated with systemic, low-grade metabolic inflammation [29, 30] and greater senescent cell burden [31]. Post-infection inflammatory syndromes are observed among some individuals with severe manifestations of COVID-19 [32]; if unrestrained, excessive pro-inflammation can result in lung tissue injury [33]. One unresolved question is determining the extent that intraindividual variability of cardiometabolic health influences the risk of COVID-19 severity and long-term adverse consequences. Therefore, our study objective was to assess the associations between intraindividual variability of cardiometabolic health indicators (body mass index [BMI], total circulating cholesterol, systolic and diastolic blood pressure) and COVID-19 related hospitalizations or post-COVID conditions (PCC) among adolescents and adults in the US.
METHODS
We conducted a multi-site retrospective cohort study, which as a secondary analysis among individuals who donated blood in the US and participated in COVID-19 surveys [34, 35].
Participants
The study inclusion criteria were any individual who: (1) donated allogeneic whole blood or plasma (2009–2018) at a Vitalant blood collection location that had implemented the current electronic database system; and (2) completed ≥1 COVID-19 survey (2021–2023). At each blood donation visit, every individual met all Food and Drug Administration (FDA) eligibility requirements for routine blood donations, such as age (≥16 years) and weight (≥110 lb). We excluded participants who had missing key variables, specifically: (1) survey responses regarding COVID-19 hospitalization and PCC; (2) cardiometabolic indicators (systolic and diastolic blood pressure, total circulating cholesterol, BMI) at ≥3 donation timepoints; and (3) other key covariates. Based on these eligibility criteria, the study participant flow diagram is in Supplementary Fig. 1.
Data collection
During every routine blood donation, demographic and cardiometabolic indicators are collected by donor health questionnaire, donation site staff, and blood assays. Self-reported information included demographics (e.g., age, gender, race-ethnicity, height [in], and weight [lb], geographic location). During each donation visit, donation site staff measured the donor’s diastolic and systolic blood pressure (millimeters of mercury [mm Hg]) by automated digital sphygmomanometer. If the first blood pressure measurement was within prespecified ranges (90–180 mm Hg for systolic, 50–100 for diastolic), this measurement was recorded. If the first blood pressure measurement was outside these ranges, a second measurement was recorded and included in this analysis. Following blood collection organization donation eligibility criteria, donors with blood pressure outside of the prespecified ranges were deferred from donating. As exceptions, a medical director can approve allogeneic donors based on in person assessment of the donor and their completed health questionnaire; donors that are otherwise healthy and at low risk for adverse consequences caused by blood donation can be cleared for donations. Non-fasting blood samples were assayed for total cholesterol concentrations (mg/dL) by automated clinical chemistry analyzers (e.g., Beckman Coulter AU analyzer [Brea, CA]) by laboratory staff at Creative Testing Solutions.
As part of a larger CDC-funded COVID-19 serosurveillance study [34, 35], blood donors were invited to complete electronic COVID-19 surveys in multiple waves between 2021–2023. Surveys included questions regarding COVID-19 symptomology and hospitalizations as well as PCC.
Definitions
PCC was defined as any health consequences (e.g., signs, symptoms, conditions) present ≥4 weeks after initial acute SARS-CoV-2 infection, per the US Department of Health and Human Services (DHHS) definition [36]. BMI was calculated as weight (kg) divided by height squared (m2) and categorized with standard World Health Organization (WHO) categories [37]. For each participant, we defined a baseline visit as the first donation date that was included in this analysis.
Intraindividual variability was considered as the coefficient of variation (CV) of a cardiometabolic indicator across all available timepoints for the participant. For example, if a donor had 14 donations during the study time period, their intraindividual variability of cholesterol was calculated as the CV across all 14 measurements of total cholesterol (mg/dL). Donors with the top 20 highest BMI CV are visually illustrated in Fig. 1. For each cardiometabolic indicator (BMI, systolic and diastolic blood pressure, total circulating cholesterol), we categorized participants into quartiles of CV (Fig. 1).
Fig. 1. Visual overview of study design, primary exposures and outcomes.
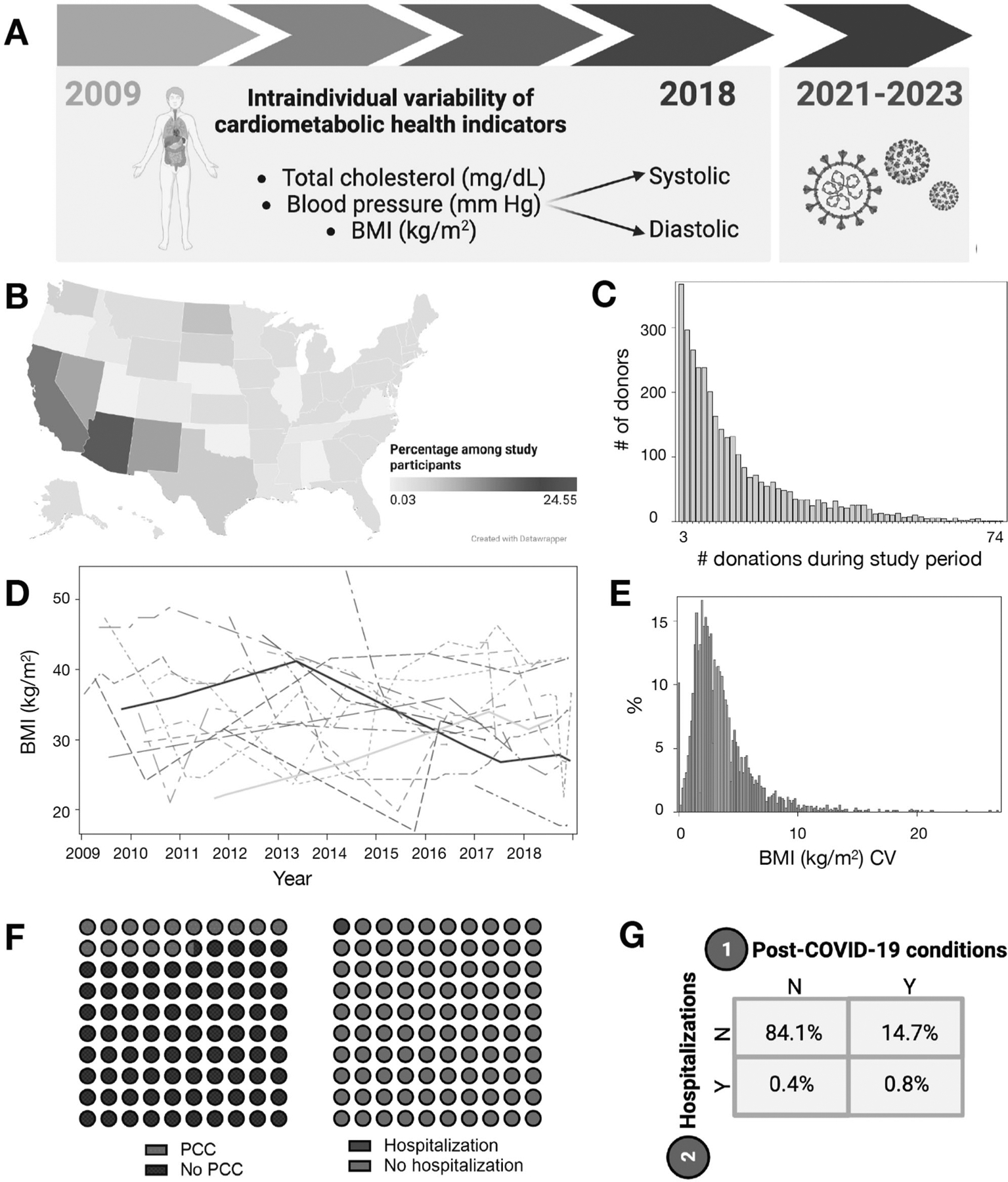
A In this retrospective cohort study, intraindividual variability in cardiometabolic indicators were the primary exposures of interest (2009–2018 data collection) and post-COVID conditions and hospitalizations (2021–2023 surveys) were the primary outcomes. Figure created with BioRender.com. B Donors visited blood collection sites in 24 US states. Figure created with Datawrapper. C Total donations per donor in final analytic dataset. Among study participants, 3–74 allogeneic blood donations (whole blood, plasma) per donor were provided during 2009–2018. D BMI (kg/m2) at each donation timepoint among top 20 participants with the highest intraindividual variability (CV). E Among all participants, the distribution of BMI CV (overall, stratified by BMI CV quartile) are in the histogram. F, G Percentages of participants with PCC and COVID-19 hospitalization, respectively and as a contingency table. BMI body mass index, CV coefficient of variation, PCC post-COVID conditions.
Statistical analysis
As descriptive statistics, we reported measures of central tendency as the mean (SD) or median (IQR) of continuous variables and N (%) for categorical variables. We compared differences between subgroups with Wilcoxon rank-sum and Mantel-Haenszel chi-squared test statistics. Statistical significance was based on two-sided tests and alpha values of 0.05. SAS (version 9.4; SAS Institute; Cary, North Carolina), GraphPad Prism (version 9.3.1.; GraphPad Software, LLC; San Diego, California), and BioRender.com (Toronto, Ontario) were used for statistical analysis and visualizations. We used a complete-case analysis approach to address missingness of key variables (Supplementary Fig. 1).
We assessed the associations between intraindividual variability of cardiometabolic health indicators and the risk of COVID-19 hospitalization or PCC by fitting binomial regressions. COVID-19 related hospitalization and PCC were the primary dependent variables of interest. Intraindividual variability of cardiometabolic indicators (CV quartiles of BMI, systolic and diastolic blood pressure, total cholesterol) were the independent variables of interest; in all models, the lowest quartile was the reference group. For our confounder selection approach, we initially selected a set of potential covariates a priori per previous literature [38]. We first reported all univariable regressions for bivariate associations between each independent variable and a primary dependent variable; subsequently, we evaluated fully adjusted multivariable regression results. To confirm the dose-response, we also evaluated the linear test for trend between cardiometabolic indicator variability quartiles and each COVID-19 outcome in the final multivariable models. As a subgroup analysis, we evaluated the prevalence of specific PCC symptoms among donors with PCC and available symptomology data. Among donors with a particular PCC symptom (e.g., brain fog), we compared the proportions in differing BMI CV quartiles. As a sensitivity analysis, we utilized a log-binomial causal mediation model framework [39] to evaluate COVID-19 hospitalization as a mediator of the association between high BMI variability (highest CV quartile vs other quartiles) and PCC. Specifically, we considered a single mediator model with the highest quartile of BMI CV as a binary variable, with the SAS CausalMed procedure and alpha values as 0.05. We calculated estimates of bootstrap-corrected standard errors and confidence intervals with 1000 replicates and an alpha value of 0.05 as the confidence level for constructing bootstrap intervals.
RESULTS
Our final analytic dataset included 3344 participants with 42,090 donations in 24 US states during the study period (Fig. 1). The number of donations per participant ranged widely (median 9 [IQR 5, 17]; 3–74 donations; Fig. 1). Among all participants, 1.2% (N = 40) were hospitalized due to COVID-19 and 15.5% (N = 519) had PCC (Fig. 1 and Table 1). Among participants that were hospitalized, 67.5% had PCC; among those not hospitalized, 14.9% had PCC (p < 0.01). Overall, 83.8% of donors reported ever having COVID-19 vaccination.
Table 1.
Demographic and clinical characteristics of participants during first donation visita.
| Overall | PCCb | COVID-19 related hospitalization | ||||||
|---|---|---|---|---|---|---|---|---|
| Y | N | p | Y | N | p | |||
| N donors | 3344 | 519 | 2825 | – | 40 | 3304 | – | |
| Age (median [IQR])c, d | Years | 48.0 (38.0, 56.0) | 48.0 (38.0, 55.0) | 49.0 (38.0, 56.0) | 0.09g | 53.5 (46.5, 61.0) | 48.0 (37.0, 56.0) | <0.01g |
| 16–29 | 11.2% | 11.2% | 11.3% | 0.15h | 2.4% | 11.4% | <0.01h | |
| 30–44 | 27.6% | 29.9% | 27.2% | 17.5% | 27.7% | |||
| 45–59 | 45.7% | 46.4% | 45.6% | 50.0% | 45.6% | |||
| 60+ | 15.5% | 12.5% | 16.0% | 30.0% | 15.3% | |||
| Gender (%) | Female | 59.2% | 66.1% | 57.9% | <0.01h | 35.0% | 59.4% | <0.01h |
| Male | 40.9% | 33.9% | 42.1% | 65.0% | 40.6% | |||
| Race-ethnicity (%) | White (non-Hispanic) | 87.6% | 84.6% | 88.1% | 0.04h | 87.5% | 87.6% | 0.88h |
| Black (non-Hispanic) | 0.8% | 1.4% | 0.7% | 2.5% | 0.8% | |||
| Other | 11.6% | 14.1% | 11.2% | 10.0% | 11.6% | |||
| Educational attainment (%) | <High school | 0.8% | 1.0% | 0.7% | <0.01h | 0.0% | 0.8% | 0.52h |
| High school diplomae | 37.5% | 44.5% | 36.2% | 42.5% | 37.4% | |||
| Bachelor’s degree | 37.1% | 34.9% | 37.6% | 37.5% | 37.1% | |||
| Graduate degree | 24.6% | 19.7% | 25.5% | 20.0% | 24.7% | |||
| Country of birth (%) | US | 95.4% | 94.8% | 95.5% | 0.50h | 95.0% | 95.4% | 0.91h |
| Outside US | 4.6% | 5.2% | 4.5% | 5.0% | 4.6% | |||
| Cholesterol (median [IQR]) | mg/dL | 183.0 (161.0, 209.0) | 186.0 (162.0, 209.0) | 183.0 (161.0, 208.0) | 0.57g | 193.5 (172.0, 221.5) | 183.0 (161.0, 208.0) | 0.06g |
| SBP (median [IQR]) | mm Hg | 121.0 (112.0, 132.0) | 122.0 (112.0, 133.0) | 121.0 (112.0, 132.0) | 0.19g | 126.5 (117.0, 140.0) | 121.0 (112.0, 132.0) | <0.05g |
| DBP (median [IQR]) | mm Hg | 76.0 (70.0, 83.0) | 78.0 (71.0, 83.0) | 76.0 (70.0, 82.0) | 0.02g | 78.5 (72.0, 85.0) | 76.0 (70.0, 82.0) | 0.11g |
| Height (median [IQR])d, f | in | 67.0 (64.0, 70.0) | 66.0 (64.0, 70.0) | 67.0 (64.0, 70.0) | <0.01g | 70.0 (66.5, 72.0) | 67.0 (64.0, 70.0) | <0.01g |
| Weight (median [IQR])d, f | lb | 175.0 (147.0, 200.0) | 180.0 (148.0, 210.0) | 173.0 (147.0, 200.0) | 0.02g | 210.0 (192.5, 230.0) | 174.0 (147.0, 200.0) | <0.01g |
| BMI (median [IQR])f | kg/m2 | 26.5 (23.7, 30.2) | 27.5 (24.2, 31.7) | 26.4 (23.6, 30.0) | <0.01g | 30.4 (27.5, 34.1) | 26.5 (23.6, 30.2) | <0.01g |
| <18.5 kg/m2 | 0.2% | 0.2% | 0.2% | <0.01h | 0.0% | 0.2% | <0.01h | |
| ≥18.5 and <25.0 kg/m2 | 36.5% | 32.6% | 37.2% | 15.0% | 36.8% | |||
| ≥25.0 and <30.0 kg/m2 | 36.9% | 32.2% | 37.8% | 32.5% | 37.0% | |||
| ≥30.0 kg/m2 | 26.4% | 35.1% | 24.8% | 52.5% | 26.1% | |||
| Geographic region | Mountain | 21.4% | 24.9% | 20.8% | <0.01h | 27.5% | 21.4% | 0.18h |
| South | 23.4% | 26.0% | 22.9% | 20.0% | 23.4% | |||
| Southwest | 29.6% | 32.4% | 29.1% | 42.5% | 29.5% | |||
| West | 25.6% | 16.8% | 27.2% | 10.0% | 25.8% | |||
BMI body mass index, COVID-19 coronavirus disease 2019, DBP diastolic blood pressure, SBP systolic blood pressure, PCC post-acute sequelae of COVID-19.
Cardiometabolic indicators from 42,090 donations collected between January 1, 2009, and December 31, 2018.
Self-report, based on the following survey question: “Do you describe yourself has having symptoms lasting at least 4 weeks and as having ‘long COVID’?”
Age was reported by calendar age as integer values. One blood donation eligibility criterion is age; therefore, participants are 16 years and older. We also considered observations with age >4 SD above the mean (>116.2 years) as biologically implausible values; these were excluded.
We considered age, height, or weight values >4 SD above or below the mean as biologically implausible values. These observations were considered as missing values and excluded in this analysis.
Includes some college and associate’s degrees.
Not normally distributed, based on p values of normality test statistic (Kolmogorov–Smirnov). Median (IQR) values reported.
p value from Wilcoxon rank sum test statistic.
p value from Mantel–Haenszel chi-square test statistic.
Baseline demographic and clinical characteristics
At baseline, the median age of participants was 48 years (IQR 38, 56; Table 1); ages ranged between 16–80 years. 59.2% of participants identified as female. The proportions of self-reported race-ethnicity were: White, non-Hispanic (87.6%); Black, non-Hispanic (0.8%), and other races and ethnicities (11.6%). In terms of highest educational attainment, 0.8% had not completed high school, 37.5% had a high school diploma, 37.1% had a bachelor’s degree, and 24.6% had a graduate degree. The baseline median values of cardiometabolic indicators were: 26.6 kg/m2 (IQR 23.7, 30.2) BMI, 183.0 mg/dL (IQR 161.0, 209.0) total circulating cholesterol, 121.0 mm Hg (IQR 112.0, 132.0) systolic blood pressure, 76.0 mm Hg (IQR 70.0, 83.0) diastolic blood pressure.
Based on WHO categories of BMI, there were: 0.2% of participants with underweight, 36.5% with normal weight, 36.9% with overweight, and 26.5% with obesity (Table 1). The percentages of participants in BMI categories (under- or normal weight, overweight, obesity) differed by their BMI CV quartile (p < 0.01; Fig. 2). Among participants with obesity, a greater proportion were in the highest variability subgroup (4th CV quartile; 35.8%) and a lower proportion were in the lowest variability subgroup (1st CV quartile 16.5%).
Fig. 2. Percentages of participants in BMI variability subgroups, and with PCC or COVID-19 related hospitalizationa.
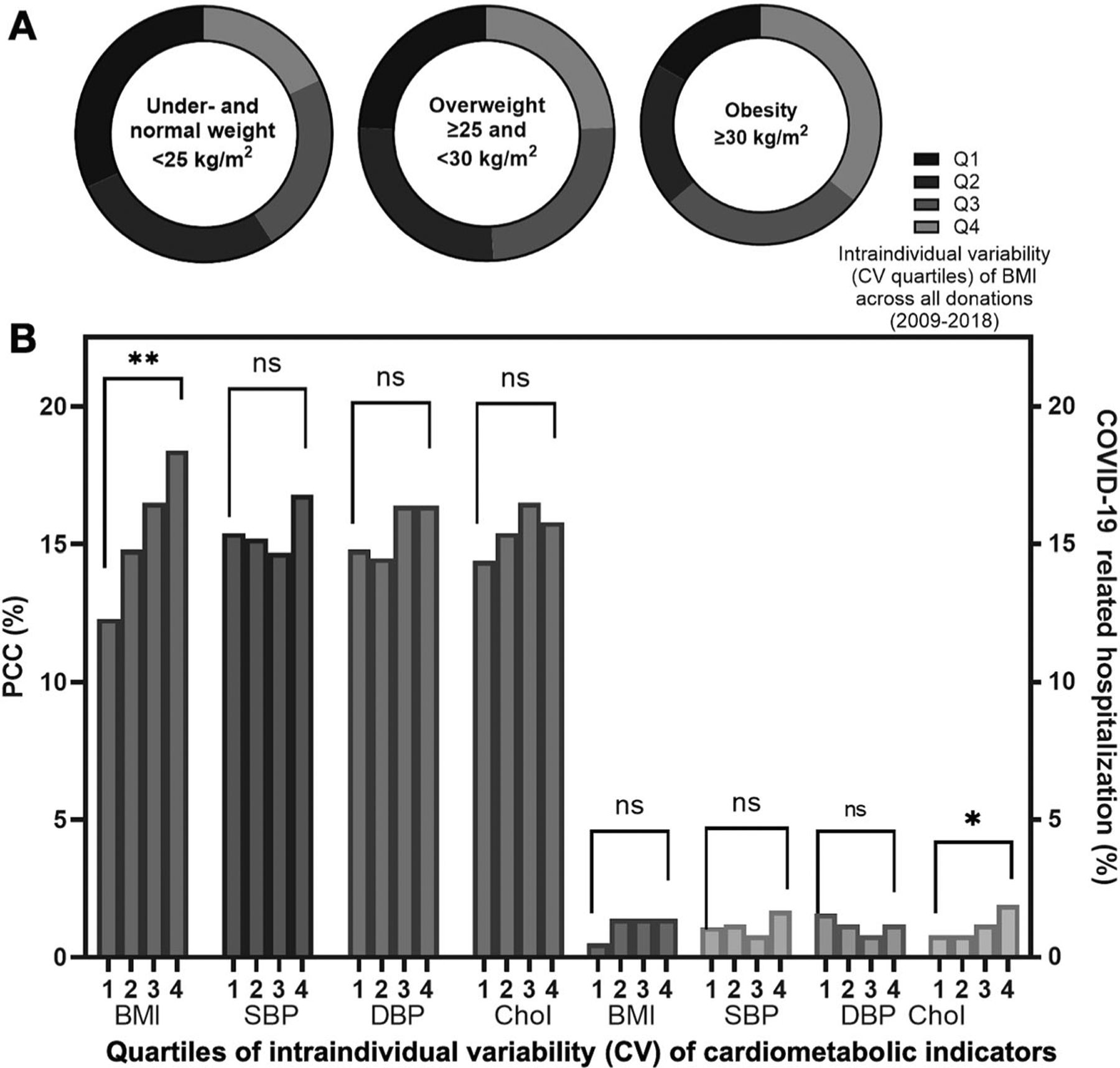
A The percentages of participants with underweight or normal weight, overweight, and obesity at their baseline study visit differed by BMI CV quartiles (p < 0.01), based on a Mantel-Haenszel chi-square test statistic. Stratified by WHO BMI categories, the proportion of donors in BMI variability quartiles are illustrated. B Proportions (%) of participants with PCC (left y-axis) and COVID-19 related hospitalization (right y-axis) were stratified by intraindividual variability of cardiometabolic indicators (BMI, SBP, DBP, cholesterol CV quartiles; x-axis). Subgroups were compared by Mantel-Haenszel chi-square or Fisher’s exact test statistics and associated p values. **p < 0.01. aPercentages not adjusted by covariates. BMI body mass index, CV coefficient of variation, DBP diastolic blood pressure, PCC post-COVID conditions, SBP systolic blood pressure.
Comparisons of cardiometabolic indicators, stratified by COVID-19 outcomes
At the first donation visit, systolic blood pressure (126.5 mm Hg [IQR 117.0, 140.0]) was higher among participants with hospitalization, compared to among those without (121.0 [112.0, 132.0]; p < 0.05). Median BMI was higher among donors with COVID-19 related hospitalization (30.4 kg/m2 [IQR 27.5, 34.1]), relative to those without PCC (26.5 [23.6, 30.2]; p < 0.01; Table 1). Median total cholesterol and diastolic blood pressure did not significantly differ by hospitalization (both p > 0.05).
At the first donation visit, median diastolic blood pressure (78.0 mm Hg [IQR 71.0, 83.0]) was higher among participants with PCC, compared to among those without PCC (76.0 [70.0, 82.0]; p = 0.02; Table 1). Median BMI was higher among donors with PCC (27.5 kg/m2 [IQR 24.2, 31.7]), relative to those without PCC (26.4 [23.6, 30.0]; p < 0.01). Systolic blood pressure and total cholesterol were similar by PCC status (both p > 0.05).
Comparing intraindividual variability of cardiometabolic health, the median CV of total cholesterol was higher among those hospitalized from COVID-19 (9.3 [IQR 7.3, 12.7]), compared to those without hospitalization (8.4 [IQR 6.3, 10.9]; p = 0.04; Supplementary Table 1). Median CV of BMI, systolic and diastolic blood pressure did not differ by hospitalization (all p > 0.05). The median CV of BMI was higher among those with PCC (3.1 [IQR 2.1, 4.8], relative to those without PCC (2.8 [IQR 1.8, 4.3]; p < 0.01). Median CV of blood pressure (systolic, diastolic) and cholesterol did not differ by PCC (all p > 0.05).
Adjusted associations between intraindividual cardiometabolic variability and COVID-19 hospitalization risk
BMI variability was positively associated with COVID-19 hospitalization risk (4th quartile aRR 4.15 [95% CI 1.31, 13.11], p = 0.02; 3rd quartile aRR 3.41 [95% CI 1.09, 10.69], p = 0.04) relative to the lowest [1st] quartile and accounting for age, gender, geographic region, and the total number of donations (Fig. 3B and Supplementary Table 2). In this multivariable regression, we also confirmed the dose-response (linear test of trend) of BMI quartiles in association with hospitalization risk (ptrend = 0.02). Total cholesterol, systolic and diastolic blood pressure variability were not significantly associated with COVID-19 hospitalization risk (all p > 0.05); none of the trend tests were significant (all ptrend > 0.05).
Fig. 3. Summary of adjusted risk ratios of associations between cardiometabolic health variability, COVID-19 hospitalization,a and PCCb probability.
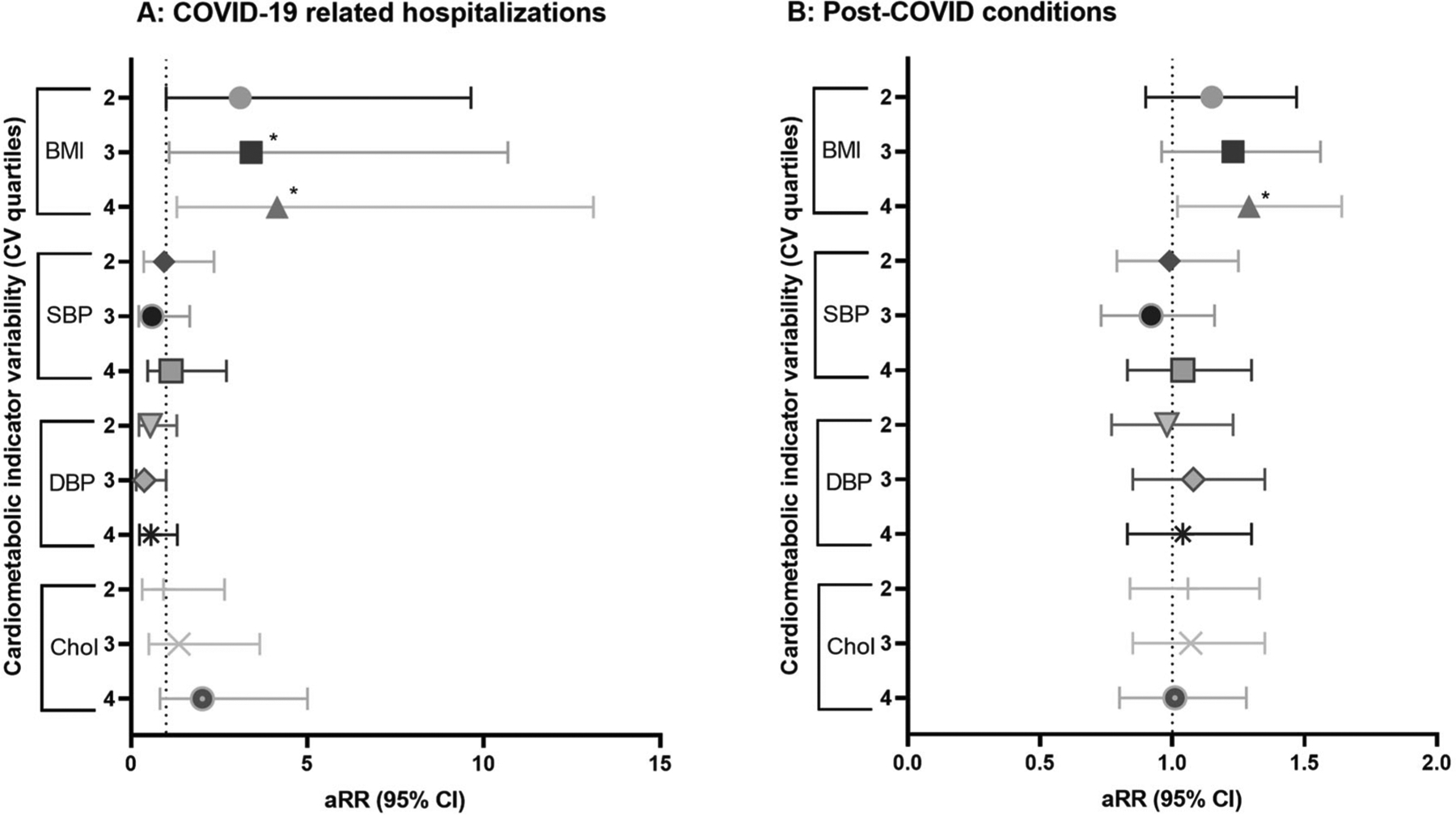
A The probability of COVID-19-related hospitalization was the primary dependent variable in this set of four multivariable regressions. B In this set of four multivariable regressions, each association evaluated PCC probability as the dependent variable. In all regressions, intraindividual variability of a cardiometabolic health indicator (CV quartiles of BMI, SBP, DBP, or cholesterol) was the key independent variable of interest. The lowest CV quartiles was considered the reference group. All values from final multivariable regressions, adjusted for gender, age, race-ethnicity, educational attainment, geographic region, COVID-19 vaccination, and the number of donations. See Supplementary Table 3. aRR adjusted risk ratio, BMI body mass index, CV coefficient of variation, DBP diastolic blood pressure, PCC post-COVID conditions, SBP systolic blood pressure.
Adjusted associations between intraindividual cardiometabolic variability and post-COVID conditions risk
Participants with higher variability of BMI had greater risk of PCC (4th CV quartile aRR 1.29 [95% CI 1.02, 1.64]; p = 0.04), relative to those with low variability (1st quartile), adjusting for age, gender, race-ethnicity, educational attainment, geographic region, COVID-19 vaccination, and total number of donations between 2009–2008 (Fig. 3A and Supplementary Table 3). In this multivariable regression, we also confirmed the dose-response (linear test of trend) of BMI quartiles in association with PCC risk (ptrend = 0.03). Intraindividual variability (CV quartiles) of blood pressure (systolic, diastolic) and total cholesterol were not significantly associated with PCC risk (all p > 0.05); tests for trend were also not significant (all ptrend > 0.05).
Prevalence of PCC symptoms, stratified by BMI variability
Among 519 participants who had PCC, 77.8% reported specific PCC symptomology. Prevalent PCC symptoms included fatigue or weakness (72.3%), difficulty thinking or concentrating (60.4%), change in taste (49.0%), and change in smell (48.4%), cough (46.0; Fig. 4A). Proportions of donors in BMI CV quartile subgroups differed by three of the top 10 PCC symptoms (fatigue or weakness, difficulty thinking or concentrating, difficulty sleeping; all p < 0.05; Fig. 4B).
Fig. 4. Comparing prevalence of post-COVID-19 symptoms by BMI intraindividual variability (CV) quartile subgroups, and sensitivity analysis via causal mediation modeling.
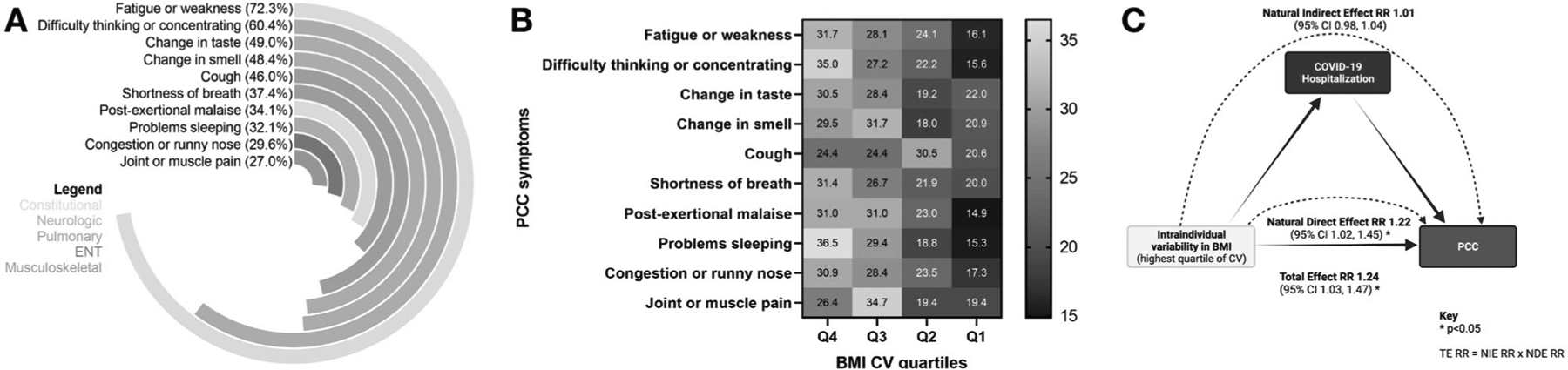
A Among participants with PCC during the study, percentages of the top 10 specific symptoms of PCC are illustrated. B For each PCC symptom, we compared of the proportion of donors across BMI CV quartiles. Among donors with fatigue or weakness, difficulty thinking or concentrating, and problems sleeping, higher proportions had elevated BMI variability (all three p < 0.05). C To confirm findings of the association between PCC and BMI variability (CV), we considered COVID-19 hospitalization as a potential mediator. The causal mediation model framework figure was created with BioRender.com. BMI body mass index, CV coefficient of variation, PCC post-COVID conditions.
COVID-19 severity as mediator of association between high BMI variability and post-COVID conditions risk
Based on causal mediation modeling framework and associated terminology, the total effect of the association between the highest quartiles of BMI CV, relative to all lower quartiles, and PCC risk was significant (aRR 1.24 [95% CI 1.03, 1.47; p < 0.05; Fig. 4C). Assessing COVID-19 hospitalization as a proxy for disease severity and mediator, the decomposed natural direct effect was aRR 1.22 (95% CI 1.02, 1.45; p < 0.05) and the natural indirect effect was aRR 1.01 (0.98, 1.04; p > 0.05; Fig. 4C).
DISCUSSION
Higher intraindividual variability of BMI was associated with greater risk of both COVID-19 related hospitalization and PCC among 3344 participants with 42,090 donations during a ten-year period (2009–2018) prior to the emergence of SARS-CoV-2. We found that variability in blood pressure (systolic, diastolic) and total cholesterol were not significantly associated with COVID-19 hospitalization or PCC risk. From a causal mediation model, we confirmed that high BMI variability remained associated with PCC risk; COVID-19 hospitalization did not mediate this association.
Obesity is a risk factor of severe COVID-19 [9, 10]. Higher BMI variability is associated with risk of obesity [40, 41]. Relatedly, among participants with the highest BMI variability (top 20 ranking), nearly all BMI measurements were in overweight and obesity categories (>25 kg/m2; Fig. 1). In one study among >34,000 children, BMI dynamics were evaluated as the annual change of BMI SD score; greater annual BMI acceleration was associated with elevated risk of overweight and obesity in adolescence [40].
Previous studies have shown that total cholesterol blood concentrations were associated with severe COVID-19, including mortality, among patients hospitalized with COVID-19 [42, 43]. As the most abundant membrane lipid, cholesterol is an essential component of host cell membranes, including immune cells with critical antiviral defense roles against SARS-CoV-2 infection. Following endocytosis into host cells, many viruses have been shown to metabolically reprogram the intracellular environment to specific replication-favorable conditions; altered lipid metabolism, including cholesterol, have been demonstrated following viral infection [44]. Future studies need to evaluate intracellular cholesterol metabolism in the pathogenesis of severe COVID-19 and PCC, particularly with more granular evaluation of cholesterol (HDL, LDL) as well as key cholesterol metabolites and receptors.
In agreement with our findings that systolic and diastolic blood pressure were not significantly associated with COVID-19 hospitalization, a large study of >45,000 adults with hypertension in the UK showed no association between blood pressure control and the probability of COVID-19 related hospital admission [45]. Among individuals hospitalized for COVID-19, smaller studies have reported that higher systolic and diastolic blood pressure variability were associated with elevated probability of COVID-19 severity [46, 47], including mortality [47]. Among COVID-19 patients with hypertension, systolic blood pressure variability was associated with mortality and acute respiratory syndrome distress, but diastolic blood pressure variability was not associated [48]. Separately, studies with wearable devices showed that intraindividual heart rate variability was higher among individuals not infected with SARS-CoV-2, relative to those with infection [49, 50].
In all four of our final multivariable regressions evaluating the associations between intraindividual variability of cardiometabolic indicators and COVID-19 hospitalization, every unit (year) increase of age and male gender were associated with higher hospitalization risk (Supplementary Table 2). These findings are consistent with prior studies showing that older individuals and male biological sex were associated with or predictive of greater COVID-19 severity, including mortality [51, 52].
Our finding demonstrating that intraindividual variability of BMI was positively associated with PCC was challenging to directly compare with the literature due to the limited previous evidence with longitudinal data of BMI prior to PCC. The underlying etiology remains unclear, however animal models demonstrated that weight cycling (persistent weight loss and regain) had similar adverse metabolic consequences as lifelong obesity [53]. Compared to animals with obesity onset later in life, animals with weight cycling had higher body weight, adipocyte size, fasting glucose, and impaired glucose tolerance [53]. To explain these observed longer-term influences of prior adiposity, even after an animal or individual has lost weight, putative mechanisms include neuroendocrine dysregulations (appetite and satiety hormones) [54], adipose tissue depot distributions and plasticity differences affecting energy homeostasis (e.g., brown fat thermogenesis) and sterile inflammation [55], and gut microbiota that affect energy metabolism [56]. Moreover, long-term metabolic dysregulations could reflect worse cardiometabolic health, and subsequently alter inflammation and immunity against PCC. Given the numerous research gaps regarding PCC, a multi-pronged effort with a wide range of study designs, including longitudinal epidemiologic and mechanistic studies, are needed to corroborate our findings. Future research directions include individuals experiencing in PCC symptoms by organ system subgroups.
Limitations
There were several key limitations of this study. First, there is no standard measurement of intraindividual variability. However, with respect to our primary outcomes, it is likely that unmeasured variability was non-differential, which would potentially bias results towards the null hypothesis. Second, COVID-19 hospitalizations and PCC were self-reported, and could have potential recall and missing data bias. There is no universal definition of PCC, including symptoms and duration of persistence (e.g., >4, >8, or >12 weeks); since this remains an active area of investigation, self-reporting of PCC is subjective [7, 57]. Heterogeneous definitions of PCC are currently used in the literature [58] and recommended by the WHO [59], US DHHS (CDC) [36], and UK National Institute for Health and Care Excellence (NICE) [60]. These multiple PCC definitions further exacerbate the adjacent challenges, which span from elucidating biological mechanisms of immunopathogenesis [61], prevalence, incidence, modifiable risk factors, etiology, and prevention and treatment guidelines. SARS-CoV-2 diagnostics (availability, accessibility, quality) was extensively variable in the US during the early phase of the COVID-19 pandemic, therefore the self-reporting of whether hospitalization was due to COVID-19 could be less accurate in this period. Third, we did not have viral strain sequencing data to account for the SARS-CoV-2 variants and sub-variants, in light of differing immune escape capacity [62], that caused infections resulting in severe COVID-19 and PCC. We also did not have complete data regarding other key factors, such as previous infection history, vaccination (e.g., COVID-19 vaccines [dosages, timing]), prior clinical diagnoses (e.g., Type 2 diabetes mellitus, ischemia heart disease), other routine clinical assessments (e.g., HDL- and LDL-cholesterol, waist circumference), medication history (e.g., statins, nirmatrelvir or COVID-19 antivirals, blood pressure and diabetes medications), socioeconomic status, and lifestyle behaviors (diet, physical activity) for all participants. Fourth, our causal mediation model was limited by the relatively small numbers of individuals with COVID-19 hospitalization and PCC. We were not able to comprehensively account for other potential confounders, given small sample cell sizes. We considered this a sensitivity analysis of our primary findings. Our findings emphasize the need to identify specific biological mechanisms underlying this association as well as larger observational studies to evaluate causal inference models. Fifth, blood donors meet the FDA and blood collection organization donor eligibility criteria at each successful donation timepoint; therefore, blood donors are not representative of the general population. Relatedly, there is potential competing risk bias, especially due to COVID-19 related mortality.
Strengths
One major strength of this study was the larger number of timepoints (median of 9 donations [IQR 5, 17] per participant) with complete cardiometabolic indicators over a 10-year exposure window (2009–2018) prior to first global detection of SARS-CoV-2 infection cases during 2019. This allowed for characterization of intraindividual variability of cardiometabolic health indicator data during period that was temporally antecedent of SARS-CoV-2 infections and COVID-19 consequences. Second, cardiometabolic indicators were collected during and assayed from routine blood donations, which minimizes the potential for recall bias of exposures. Third, the multi-site study design allowed for the inclusion of free-living individuals in their late adolescence and adulthood in 24 US states.
CONCLUSIONS
Higher intraindividual variability of BMI was associated with greater risk of COVID-19 related hospitalization and PCC. Our findings support the potential of BMI maintenance to have long-term health benefits and the need for further etiological studies.
Supplementary Material
ACKNOWLEDGEMENTS
We sincerely appreciate all team members of the Repeat Donor Cohort Study including at Vitalant Research Institute, the American Red Cross, Centers for Disease Control and Prevention, and Westat; Dr. Gustaf Edgren for discussion of an earlier iteration of the analysis; and Amber Morris for her assistance with initial exploratory statistics. Figures 1A and 4C were created with BioRender.com. Figure 1B was created with DataWrapper.com.
FUNDING
This project was supported by the Centers for Disease Control and Prevention (contract number 75D30120C08170) and the National Institute of General Medical Sciences of the National Institutes of Health (R25GM143298 for EAY).
Footnotes
COMPETING INTERESTS
The authors declare no competing interests.
ETHICAL APPROVAL
All donors provided informed consent for the use of their deidentified data and residual blood samples from routine blood donations for research as part of voluntarily consenting to donate (Advarra protocol # Pro00030878). The COVID-19 survey was approved by an IRB (Advarra protocol # Pro00056783); all individuals provided informed consent prior to participation. All research involving human subjects conducted at Vitalant conform to the principles contained in the Belmont Report and are subject to the Common Rule and subparts B, C, and D of the US Department of Health and Human Services regulations at 45 CFR part 46. We reported study methodology based on the Strengthening the Reporting of Observational Studies in Epidemiology guidelines [63].
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41366-024-01603-6.
DATA AVAILABILITY
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
REFERENCES
- 1.World Health Organization. WHO Coronavirus (COVID-19) dashboard. Geneva, Switzerland: World Health Organization; 2023. https://covid19.who.int/. [Google Scholar]
- 2.Toussi SS, Hammond JL, Gerstenberger BS, Anderson AS. Therapeutics for COVID-19. Nat Microbiol. 2023;8:771–86. 10.1038/s41564-023-01356-4. [DOI] [PubMed] [Google Scholar]
- 3.El-Sadr WM, Vasan A, El-Mohandes A. Facing the new Covid-19 reality. N Engl J Med. 2023;388:385–7. 10.1056/NEJMp2213920. [DOI] [PubMed] [Google Scholar]
- 4.Nalbandian A, Desai AD, Wan EY. Post-COVID-19 condition. Annu Rev Med. 2023;74:55–64. 10.1146/annurev-med-043021-030635. [DOI] [PubMed] [Google Scholar]
- 5.Mueller MR, Ganesh R, Hurt RT, Beckman TJ. Post-COVID conditions. Mayo Clin Proc. 2023;98:1071–8. 10.1016/j.mayocp.2023.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. New ICD-10-CM code for post-COVID conditions, following the 2019 novel coronavirus (COVID-19). 2021. https://www.cdc.gov/nchs/data/icd/announcement-new-icd-code-for-post-covid-condition-april-2022-final.pdf.
- 7.Morello R, Mariani F, Mastrantoni L, De Rose C, Zampino G, Munblit D, et al. Risk factors for post-COVID-19 condition (Long Covid) in children: a prospective cohort study. eClinicalMedicine. 2023;59. 10.1016/j.eclinm.2023.101961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steenblock C, Schwarz PEH, Ludwig B, Linkermann A, Zimmet P, Kulebyakin K, et al. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9:786–98. 10.1016/s2213-8587(21)00244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stefan N Metabolic disorders, COVID-19 and vaccine-breakthrough infections. Nat Rev Endocrinol. 2022;18:75–6. 10.1038/s41574-021-00608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected—obesity, impaired metabolic health and COVID-19. Nat Rev Endocrinol. 2021;17:135–49. 10.1038/s41574-020-00462-1. [DOI] [PubMed] [Google Scholar]
- 11.Almuwaqqat Z, Hui Q, Liu C, Zhou JJ, Voight BF, Ho Y-L, et al. Long-term body mass index variability and adverse cardiovascular outcomes. JAMA Netw Open. 2024;7:e243062. 10.1001/jamanetworkopen.2024.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiterer M, Rajan M, Gómez-Banoy N, Lau JD, Gomez-Escobar LG, Ma L, et al. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab. 2021;33:2174–88.e5. 10.1016/j.cmet.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez-Colón GJ, Ratnasiri K, Chen H, Jiang S, Zanley E, Rustagi A, et al. SARS-CoV-2 infection drives an inflammatory response in human adipose tissue through infection of adipocytes and macrophages. Sci Transl Med. 2022;14:eabm9151. 10.1126/scitranslmed.abm9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo S, Liang Y, Wong THT, Schooling CM, Au Yeung SL. Identifying factors contributing to increased susceptibility to COVID-19 risk: a systematic review of Mendelian randomization studies. Int J Epidemiol. 2022;51:1088–105. 10.1093/ije/dyac076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsampasian V, Elghazaly H, Chattopadhyay R, Debski M, Naing TKP, Garg P, et al. Risk factors associated with post−COVID-19 condition: a systematic review and meta-analysis. JAMA Intern Med. 2023;183:566–80. 10.1001/jamainternmed.2023.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peluso MJ, Deeks SG. Early clues regarding the pathogenesis of long-COVID. Trends Immunol. 2022;43:268–70. 10.1016/j.it.2022.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell CD, Lone NI, Baillie JK. Comorbidities, multimorbidity and COVID-19. Nat Med. 2023;29:334–43. 10.1038/s41591-022-02156-9. [DOI] [PubMed] [Google Scholar]
- 18.Lissner L, Odell PM, D’Agostino RB, Stokes J, Kreger BE, Belanger AJ, et al. Variability of body weight and health outcomes in the Framingham population. N Engl J Med. 1991;324:1839–44. 10.1056/nejm199106273242602. [DOI] [PubMed] [Google Scholar]
- 19.Blair SN, Shaten J, Brownell K, Collins G, Lissner L. Body weight change, all-cause mortality, and cause-specific mortality in the multiple risk factor intervention trial. Ann Intern Med. 1993;119:749–57. 10.7326/0003-4819-119-7_part_2-199310011-00024. [DOI] [PubMed] [Google Scholar]
- 20.Hussain SM, Newman AB, Beilin LJ, Tonkin AM, Woods RL, Neumann JT, et al. Associations of change in body size with all-cause and cause-specific mortality among healthy older adults. JAMA Netw Open. 2023;6:e237482. 10.1001/jamanetworkopen.2023.7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dasa O, Smith SM, Howard G, Cooper-DeHoff RM, Gong Y, Handberg E, et al. Association of 1-year blood pressure variability with long-term mortality among adults with coronary artery disease: a post hoc analysis of a randomized clinical trial. JAMA Netw Open. 2021;4:e218418. 10.1001/jamanetworkopen.2021.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Jin R, Jin X, Wu Z, Zhang H, Han Z, et al. Separate and joint associations of remnant cholesterol accumulation and variability with carotid atherosclerosis: a prospective cohort study. J Am Heart Assoc. 2023;12:e029352. 10.1161/JAHA.122.029352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098. 10.1136/bmj.i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schutte AE, Kollias A, Stergiou GS. Blood pressure and its variability: classic and novel measurement techniques. Nat Rev Cardiol. 2022;19:643–54. 10.1038/s41569-022-00690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheikh AB, Sobotka PA, Garg I, Dunn JP, Minhas AMK, Shandhi MMH, et al. Blood pressure variability in clinical practice: past, present and the future. J Am Heart Assoc. 2023;12:e029297. 10.1161/JAHA.122.029297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark D III, Nicholls SJ, St John J, Elshazly MB, Ahmed HM, Khraishah H, et al. Visit-to-visit blood pressure variability, coronary atheroma progression, and clinical outcomes. JAMA Cardiol. 2019;4:437–43. 10.1001/jamacardio.2019.0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Sun X, Von Cannon JL, Kon ND, Ferrario CM, Groban L. Estrogen receptors are linked to angiotensin-converting enzyme 2 (ACE2), ADAM metal-lopeptidase domain 17 (ADAM-17), and transmembrane protease serine 2 (TMPRSS2) expression in the human atrium: insights into COVID-19. Hypertens Res. 2021;44:882–4. 10.1038/s41440-021-00626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messerli FH, Rimoldi SF, Bangalore S. Blood pressure variability and arterial stiffness—chicken or egg? JAMA Cardiol. 2019;4:1050. 10.1001/jamacardio.2019.2730. [DOI] [PubMed] [Google Scholar]
- 29.Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103–18. 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chavakis T, Alexaki VI, Ferrante AW. Macrophage function in adipose tissue homeostasis and metabolic inflammation. Nat Immunol. 2023;24:757–66. 10.1038/s41590-023-01479-0. [DOI] [PubMed] [Google Scholar]
- 31.Chaib S, Tchkonia T, Kirkland JL. Cellular senescence and senolytics: the path to the clinic. Nat Med. 2022;28:1556–68. 10.1038/s41591-022-01923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–74. 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt CA, Tchkonia T, Niedernhofer LJ, Robbins PD, Kirkland JL, Lee S. COVID-19 and cellular senescence. Nat Rev Immunol. 2023;23:251–63. 10.1038/s41577-022-00785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones JM, Opsomer JD, Stone M, Benoit T, Ferg RA, Stramer SL, et al. Updated US infection- and vaccine-induced SARS-CoV-2 seroprevalence estimates based on blood donations, July 2020-December 2021. JAMA. 2022;328:298–301. 10.1001/jama.2022.9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones JM, Stone M, Sulaeman H, Fink RV, Dave H, Levy ME, et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020-May 2021. JAMA. 2021;326:1400–9. 10.1001/jama.2021.15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Department of Health and Human Services, Office of the Assistant Secretary for Health. National research action plan on long COVID. Washington, DC; 2022. [Google Scholar]
- 37.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. WHO Technical Report Series 894. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 38.Müller SA, Isaaka L, Mumm R, Scheidt-Nave C, Heldt K, Schuster A, et al. Pre-valence and risk factors for long COVID and post-COVID-19 condition in Africa: a systematic review. Lancet Glob Health. 2023;11:e1713–24. 10.1016/S2214-109X(23)00384-4. [DOI] [PubMed] [Google Scholar]
- 39.VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37:17–32. 10.1146/annurev-publhealth-032315-021402. [DOI] [PubMed] [Google Scholar]
- 40.Geserick M, Vogel M, Gausche R, Lipek T, Spielau U, Keller E, et al. Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med. 2018;379:1303–12. 10.1056/NEJMoa1803527. [DOI] [PubMed] [Google Scholar]
- 41.Sponholtz TR, van den Heuvel ER, Xanthakis V, Vasan RS. Association of variability in body mass index and metabolic health with cardiometabolic disease risk. J Am Heart Assoc. 2019;8:e010793. 10.1161/jaha.118.010793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chidambaram V, Shanmugavel Geetha H, Kumar A, Majella MG, Sivakumar RK, Voruganti D, et al. Association of lipid levels with COVID-19 infection, disease severity and mortality: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:862999. 10.3389/fcvm.2022.862999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahat RK, Rathore V, Singh N, Singh N, Singh SK, Shah RK, et al. Lipid profile as an indicator of COVID-19 severity: a systematic review and meta-analysis. Clin Nutr ESPEN. 2021;45:91–101. 10.1016/j.clnesp.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ketter E, Randall G. Virus impact on lipids and membranes. Annu Rev Virol. 2019;6:319–40. 10.1146/annurev-virology-092818-015748. [DOI] [PubMed] [Google Scholar]
- 45.Sheppard JP, Nicholson BD, Lee J, McGagh D, Sherlock J, Koshiaris C, et al. Association between blood pressure control and coronavirus disease 2019 outcomes in 45 418 symptomatic patients with hypertension. Hypertension. 2021;77:846–55. 10.1161/HYPERTENSIONAHA.120.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li FK, An DW, Guo QH, Zhang YQ, Qian JY, Hu WG, et al. Day-by-day blood pressure variability in hospitalized patients with COVID-19. J Clin Hypertens. 2021;23:1675–80. 10.1111/jch.14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jagannatha GNP, Yasmin AAADA, Pradnyana IWAS, Kamardi S, Pradnyaandara IGBMA, Pangkahila EE, et al. Therapeutic target and clinical impact of day-to-day blood pressure variability in hypertensive patients with covid-19. Hypertens Res. 2023;46:165–74. 10.1038/s41440-022-01077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He C, Liu C, Yang J, Tan H, Ding X, Gao X, et al. Prognostic significance of day-by-day in-hospital blood pressure variability in COVID-19 patients with hypertension. J Clin Hypertens. 2022;24:224–33. 10.1111/jch.14437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirten RP, Danieletto M, Tomalin L, Choi KH, Zweig M, Golden E, et al. Use of physiological data from a wearable device to identify SARS-CoV-2 infection and symptoms and predict COVID-19 diagnosis: observational study. J Med Internet Res. 2021;23:e26107. 10.2196/26107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Natarajan A, Su H-W, Heneghan C. Assessment of physiological signs associated with COVID-19 measured using wearable devices. NPG. Digit Med 2020;3:156. 10.1038/s41746-020-00363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20:442–7. 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buttia C, Llanaj E, Raeisi-Dehkordi H, Kastrati L, Amiri M, Meçani R, et al. Prognostic models in COVID-19 infection that predict severity: a systematic review. Eur J Epidemiol. 2023;38:355–72. 10.1007/s10654-023-00973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thillainadesan S, Madsen S, James DE, Hocking SL. The impact of weight cycling on health outcomes in animal models: a systematic review and meta-analysis. Obes Rev. 2022;23:e13416. 10.1111/obr.13416. [DOI] [PubMed] [Google Scholar]
- 54.Tschöp MH, Friedman JM. Seeking satiety: from signals to solutions. Sci Transl Med. 2023;15:eadh4453. 10.1126/scitranslmed.adh4453. [DOI] [PubMed] [Google Scholar]
- 55.Sakers A, De Siqueira MK, Seale P, Villanueva CJ. Adipose-tissue plasticity in health and disease. Cell. 2022;185:419–46. 10.1016/j.cell.2021.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carmody RN, Bisanz JE. Roles of the gut microbiome in weight management. Nat Rev Microbiol. 2023;21:535–50. 10.1038/s41579-023-00888-0. [DOI] [PubMed] [Google Scholar]
- 57.Thaweethai T, Jolley SE, Karlson EW, Levitan EB, Levy B, McComsey GA, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329:1934–46. 10.1001/jama.2023.8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaichana U, Man KKC, Chen A, Wong ICK, George J, Wilson P, et al. Definition of post–COVID-19 condition among published research studies. JAMA Netw Open. 2023;6:e235856. 10.1001/jamanetworkopen.2023.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–7. 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.National Institute for Health and Care Excellence. COVID-19 rapid guideline: managing the long-term effects of COVID-19. 2021. https://www.nice.org.uk/guidance/ng188. [PubMed]
- 61.Altmann DM, Whettlock EM, Liu S, Arachchillage DJ, Boyton RJ. The immunology of long COVID. Nat Rev Immunol. 2023. 10.1038/s41577-023-00904-7. [DOI] [PubMed] [Google Scholar]
- 62.Imai M, Ito M, Kiso M, Yamayoshi S, Uraki R, Fukushi S, et al. Efficacy of antiviral agents against omicron subvariants BQ.1.1 and XBB. N Engl J Med. 2022;388:89–91. 10.1056/NEJMc2214302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–24. 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.


