Abstract
The extensive mining of bastnasite (CeFCO3) has caused pollution of lanthanum (La), cerium (Ce), and fuorine (F) in the surrounding farmland soil, severely threatening the safety of the soil ecosystem. However, the interaction effects of various chemical fractions of La, Ce, and F on the composition of microbial communities are unclear. In our study, high-throughput sequencing was performed based on the pot experiments of four types of combined pollution soils, i.e., La + Ce (LC), Ce + F (CF), La + F (LF), and La + Ce + F (LCF), and the pollution concentration ranges of these three elements of 20–240, 40–450, and 150–900 mg kg–1, respectively. The improved Tessier method was used to investigate the interaction effects of chemical fractions of these elements on the variations in the soil microbial compositions. The result showed the residual form of La (La_RES) displayed restraint on Abditibacteriota, leading to its undetected level in the highest concentration of LC-polluted soils, whereas promoted relative abundance of microbes (Planctomycetota, Elusimicrobiota, Gemmatimonadota, and Rozellomycota) by more than 80%; the exchangeable and organic-bound forms of Ce and F as well as the iron-manganese-bound and residual forms of F were identified as the stress factors for the sensitive bacteria (e.g., WS4, Elusimicrobiota, RCP2-54, and Monoblepharomycota) in CF-polluted soils; in LF-polluted soils, the water-soluble form of La showed the most toxic effect on RCP2-54, Nitrospirota, and FCPU426, leading to decreased relative abundance by more than 80%; while La_RES and iron-manganese-bound form of F were identified as the stress factors for the relative abundance of Nitrospirota, Elusimicrobiota, and GAL15, showing decline of more than 80% in LCF-polluted soils. Our study revealed both inhibition and promotion effects of the element interaction on the growth of microbial communities, providing a certain experimental evidence to support further exploration of the treatment of environmental pollution caused by these elements.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-024-03708-4.
Keywords: Rare earth, Combined pollution soil, Stable chemical fraction, Sensitive microbiome, Tolerant microbiome, Correlation factor
Introduction
Bastnasite (CeFCO3) is one of the rare earth minerals with the widest distribution worldwide [1]. Both lanthanum (La) and cerium (Ce) together account for 70–90% of the total rare earth elements [2]. As the rapid growing of demand for rare earth elements in various fields, the mining and smelting of these elements have caused a series of severe ecological issues in the soil environments [3]. In particular, the pollution of the farmland around the mining area caused by these elements could lead to changes in the physical and chemical properties of the soil [4, 5] and decrease in soil fertility [6], ultimately affecting the growth and development of crops [7, 8] and threatening human health via food chains [9]. Studies have shown that the high La and Ce contents in bastnasite mine areas have sustained inhibitory effects on soil microbial abundance and function [6, 10]. Fluorine (F) in low concentrations would be essential trace nutrient for soil microbes [11], while the concentration of F over 1000 mg kg–1 could cause significant changes in microbial structure [5]. To date, studies have investigated the effects of single element of rare earth ionic mine (e.g., La and Ce) on microbial community [5, 12], whereas the effects of the combined contamination of F with either La or Ce in bastnasite on soil microbial community have not been explored. Therefore, it is urgent to study the response of soil bacterial and fungal communities to the interaction of La, Ce, and F in combined pollution soils. This is important for further development of effective strategies to treat the farmland contaminated by these elements.
Microorganisms have shown various responses to the stress of different combined pollution elements [13, 14]. The significant variations in relative abundance of microbial communities, including the complete disappearance and newly appeared microbes, are considered the appropriate biological indicators for soil pollution evaluation [15]. To evaluate the response of microbiome to the pollution caused by La, Ce, or F, individually, only a few studies have reported the sensitive or tolerant microbial communities to the total contents of these three elements [16–18]. For example, the relative abundance of Cyanobacteria was reduced under the pollution of either La or Ce [19, 20], while the resistant ability was revealed in Actinobacteria under long-term exposure to excessive La or Ce [12, 20]. Excess F can cause significant reduction of bacteria and fungi in the microbial communities [18]. Furthermore, studies have explored the effect of co-contamination of either La, Ce, or F, individually, with another pollutant, especially a heavy metal, on the structure and diversity of soil microorganisms [5, 6, 21]. For example, the synergistic toxicity of high concentrations of La and lead (Pb) significantly increased the relative abundance of Proteobacteria from 19.45 to 29.73%, while the relative abundance of Thaumarchaeota was decreased from 2.76 to 1.53% [21]. However, few studies have assessed the collaborative effects of La and Ce and the synergistic toxic effect of total La and Ce contents on plant metabolism [22]. To date, the effects of combined pollution of two or three elements on microbial communities are still unclear.
Recent studies indicated that the toxicity of La, Ce, or F depends on the different chemical fractions or bioavailability of these elements [3, 23]. For example, the different forms of La exhibit distinct toxic effects on the growth of broad beans under hydroponic conditions [24]. Similarly, the toxic effect of ionic La on Daphnia similis was enhanced compared with the chelated form of La [25]. Therefore, it is necessary to explore the effects of various forms of these elements on the soil bacterial and fungal communities.
Up to now, studies have mainly focused on the variations of the relatively dominant microbial taxa in the soil community co-contaminated by either La, Ce, or F, individually, with another pollutant [5, 6, 21]. Few studies have focused on the disappearance or new appearance of microbes with low relative abundance in polluted soils. Although the dominant microbes are important in shaping the community composition and are involved in soil biogeochemical cycles and nutrient transformation [26], the disappearance or appearance of microbes with low abundances also play important roles in the soil ecological processes, e.g., the soil biogeochemical cycle [27, 28]. Therefore, the disappearance or appearance of microbes as well as the significant changes in their relative abundances should also be taken into consideration during the exploration of the roles of microbiota in shaping the microbial community composition. To date, there is still a lack of research on the response of microbes with different relative abundances to the interaction of different chemical fractions of La, Ce, and F. In particular, there is no study on the responses of sensitive or tolerant microbial communities to the interaction of different chemical fractions of the rare earth elements as well as the ecological processes or functions affected. This is probably due to the technical challenges posed by the complex combined treatments of multiple elements, such as heavy workload, control of the experimental conditions, and the accurate determination of various chemical fractions of the elements [29]. At present, the high-throughput sequencing technology [30] is considered one of the efficient methods to study the different responses of microorganisms to contaminated soils, showing enhanced detection of disappearance, appearance, sensitivity, or tolerance of microorganisms under the interaction of various chemical fractions of the rare earth elements.
In our study, we hypothesize that the various interactions of different chemical fractions of La, Ce, and F in the combined pollution soils have significant effects on the sensitive and tolerant of bacterial and fungal communities. Based on the pot experiments of four different combined pollution soils of two or three elements, i.e., La + Ce (LC), Ce + F (CF), La + F (LF), and La + Ce + F (LCF), high-throughput sequencing was performed on the soil samples with the goals to: (1) investigate the interaction effects of various chemical fractions of these three elements on microbial community composition; (2) identify the sensitive and tolerant microbes under these interaction effects; and (3) detect the key correlation factors (i.e., the different chemical fractions of these elements) responsible for the sensitive and tolerant microbes.
Materials and methods
Soil sample collection and pot experiment
We investigated the pollution level within 5 km of the farmland soil in the mining area, Mianning County (Sichuan, China) through pre-experiment. A total of 15 sampling sites were set up by using the mesh sampling method, with 3 farmland soil samples collected at each site (collection depth 0–20 cm below the surface of the ground) and brought back to the laboratory in aseptic bags to test the contents of La, Ce, and F. The concentrations of La, Ce, and F ranged from 100 to 400, 200 to 800, and 600 to 2000 mg kg –1, respectively. Based on the pollution levels of these elements obtained in our pre-experiments, we designed the low (samples labelled with 1 and 2 in Table 1), middle (samples labelled with 3 in Table 1), and high (samples labelled with 4 and 5 in Table 1) concentrations of soil pot experiments. Therefore, the farmland soil was collected about 15 km away from the bastnaesite mine area in Mianning County (Sichuan, China) and used as control (CK), containing the total contents of La, Ce, and F at 55.89, 177.24, and 540.73 mg kg–1, respectively. It was noted that these concentrations were less than those detected in the areas close to the mine zones.
Table 1.
Pollutant levels of lanthanum (La), cerium (Ce), and fluorine (F) added in the combined pollution soil pot experiments. CK, control; LC, La + Ce contaminated soil; CF, Ce + F contaminated soil; LF, La + F contaminated soil; LCF, La + Ce + F contaminated soil
| Group | La (mg kg–1) | Ce (mg kg –1) | F (mg kg –1) |
|---|---|---|---|
| CK | 0 | 0 | 0 |
| LC1 | 20 | 40 | 0 |
| LC2 | 40 | 80 | 0 |
| LC3 | 80 | 150 | 0 |
| LC4 | 160 | 300 | 0 |
| LC5 | 240 | 450 | 0 |
| CF1 | 0 | 40 | 150 |
| CF2 | 0 | 80 | 300 |
| CF3 | 0 | 150 | 450 |
| CF4 | 0 | 300 | 700 |
| CF5 | 0 | 450 | 900 |
| LF1 | 20 | 0 | 150 |
| LF2 | 40 | 0 | 300 |
| LF3 | 80 | 0 | 450 |
| LF4 | 160 | 0 | 700 |
| LF5 | 240 | 0 | 900 |
| LCF1 | 20 | 40 | 150 |
| LCF2 | 40 | 80 | 300 |
| LCF3 | 80 | 150 | 450 |
| LCF4 | 160 | 300 | 700 |
| LCF5 | 240 | 450 | 900 |
Based on the actual polluted concentration of three elements, four different combined pollution treatments were performed, i.e., La + Ce (LC), Ce + F (CF), La + F (LF), and La + Ce + F (LCF), as well as CK. Each pollution treatment pot was filled with 10 kg control soil and treated with La, Ce, and F (in the above four combinations) provided by dissolved LaCl3·7H2O, CeCl3·7H2O, and NaF, all of analytical grades, respectively (Table 1). The soils were mixed with rare earth elements homogenized before filling the pots, then all pots of control and experimental groups were cultivated at room temperature with 70% field water holding capacity in soil, with three biological replicates performed for each control and experimental treatment. In 6 month, 10 g aged soil samples were collected and stored at – 80 °C for high-throughput sequencing analysis of soil microorganisms. Then, 100 g aged soil samples were collected and air-dried for the determination of soil chemical properties.
High-throughput sequencing analysis of soil microorganisms
The high-throughput sequencing technology, also known as "Next-generation" sequencing technology, is considered one of the efficient methods to study the different responses of microorganisms to contaminated soils. We applied this technology to analyze the response of soil microbial communities to different composite contaminants (i.e., La, Ce, and F). Soil microbial DNA was extracted from all experimental and control groups using a soil DNA kit (Omega Bio-tek Inc., USA). The quality of the acquired DNA was examined on 1% agarose gels [28]. The primer pair 338F/806R was used to amplify the V3-V4 hypervariable regions of bacterial 16S rRNA genes using thermocycler PCR system. The internal transcribed spacer region primers ITS1F and ITS2R were used to amplify fungal DNA. The PCR reactions were conducted using the following program: 3 min of denaturation at 95 °C, 27 and 37 cycles of 30 s at 95 °C for bacteria and fungi, respectively, then 30 s for annealing at 55 °C,followed by 45 s for elongation at 72 °C, and a final extension at 72 °C for 10 min. These PCR products were examined and extracted using a 2% agarose gel, further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, USA), and quantified using QuantiFluor™-ST (Promega, USA) according to the manufacturer’s protocols [31]. PCR-amplified products were purified and sent to Shanghai Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China) for sequencing using Illumina MiSeq platform. The raw sequences have been deposited in the Sequence Read Archive of NCBI database (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1119661).
Bioinformatics analysis of the high-throughput sequencing data was performed using QIIME1.9.1 [32]. All raw sequences were demultiplexed and filtered by QIIME. After filtering the low-quality reads, based on the overlap between PE reads, pairs of reads were merged into a sequence with a minimum overlap length of 10 bp. The maximum mismatch ratio allowed by the overlap area of the merging sequence was 0.2, and the non-conforming sequence was screened. The samples were distinguished based on the barcode and primer at both ends of the first sequence, and the sequence direction was adjusted. The mismatch number of barcode allowed was 0 and the maximum mismatch number of primer was set to 2. These steps were completed using software fastp and FLASH [33].Then a total of 1,923,174 bacterial and 2,155,119 fungal sequences were acquired. respectively, and further grouped into operational taxonomic units (OTUs) using the USEARCH 7-uparse algorithm based on the similarity of 97% [32]. Then, these sequences were clustered into different operational taxonomic units (OTUs) using UPARSE. The OTUs were assigned to different taxonomic ranks of bacteria and fungi at a 70% confidence threshold using ribosomal database project (RDP) classifier algorithm based on 16S rRNA database (SILVA v.138) and ITS fungi database (Unite8.0). Under the combined pollution of different chemical fractions of La, Ce, and F, compared with control samples, microbial taxa at phyla level with the relative abundance reduced over 80%, including the undetected, were defined as sensitive microbes, while the taxa with relative abundance increased over 80%, including the newly detected taxa, were recognized as tolerant microbes.
Determination of soil chemical properties and content of pollutant elements
Standard laboratory methods were used to analyze the soil chemical properties, including pH level and contents of organic matter (OM) [34], total nitrogen (TN) [35], total phosphorus (TP) [36], total potassium (TK) [37], hydrolyzable nitrogen (AN) [38], available phosphorus (AP) [39], and available potassium (AK) [40].
The total La and Ce contents in the soil were determined using a three-acid digestion method (HNO3:HCl:HClO4 = 1:2:2) [12]. All six forms of La and Ce were extracted using an improved Tessier method [41, 42]. Specifically, (a) water-soluble (WS) form, normal temperature water extraction; (b) exchangeable (EX) form, 1 mol L–1 MgCl2 extraction; (c) carbonate-bound (CAR) form, 1 mol L–1 CH3COONa extraction; (d) iron-manganese-bound (FeMn) form, 0.25 mol L–1 NH2OH:HCl extraction; (e) organic-bound (ORG) form, first 0.02 mol L–1 HNO3 and 30% H2O2 mixed extraction, and then 3.2 mol L–1 NH4Ac and 20% HNO3 extraction; and (f) residual (RES) form, the three-acid digestion method (HNO3:HCl:HClO4 = 1:2:2). The different forms of La and Ce were measured using inductively coupled plasma optical emission spectrometry (ICP-OES).
The total content of F was determined using the NaOH fusion-fluoride ion selective electrode method (GBT 22104–2008, China). The five forms of F in the soil were extracted using the continuous grading immersion method. Specifically, (a) WS form, hot water at 70 °C for extraction; (b) EX form, 1 mol L–1 MgCl2 extraction, shaken at 25 °C for 1 h; (c) FeMn form, 0.04 mol L–1 NH2OH:HCl extraction, shaken at 60 °C for 1 h; (d) ORG form, first 0.04 mol L–1 HNO3 and 30% H2O2 extraction at 85 °C for 2 h, and then added with H2O2 and continuous heating at 85 °C for 3 h; after cooling, 25 ml NH4Ac was added and shaken at 25 °C for 0.5 h; and (e) RES form, the NaOH fusion-fluoride ion selective electrode method (GBT 22104–2008, China).
Statistical analysis
The variance and mean differences of the data were analyzed by non-parametric ordination-based analysis and the Fisher’s least significant difference test using SPSS 19.0 (SPSS Inc., USA). OriginPro 9.0 (OriginLab, Northampton, MA, USA) was used to plot the contaminant element components, microbial community composition and relative abundance change. Redundancy analysis was performed using R 4.0.1 software of the vagen package to identify the main correlation factors (i.e., the different chemical fractions of La, Ce, and F) for tolerant and sensitive microbes at phylum level in different combined pollution soils, and we used the step model to detect the lowest AIC value. In this step, the model will automatically screen out the optimal environmental factors in RDA for solving the collinearity. An envfit analysis (envfit function used with 999 permutations) used the ‘vegan’ package to clarify significant factors explaining bacterial composition variation, and the RAD statistics were provided in the supplementary materials (Table S1). The response of the microbes to interaction of various chemical fractions of La, Ce, and F was calculated using the equation [42]: relative abundance change = [(Xp – Xc)/Xc] × 100% where Xp and Xc represented the relative abundances of the microbes in polluted and unpolluted soils, respectively.
Results
Chemical properties of contents of La, Ce, and F
Compared with the CK group, the change in the soil OM, TN, TP, and TK contents were < 2% in the pot experiments. Among other soil properties, the most significant variations were detected in soil pH level in treatment groups (Fig. S1a). The pH of the CK group was 5.15, while the LC-polluted soils were acidic, with a pH range of 3.28–3.47. The pH values of CF-, LF-, and LCF-polluted soils were increased as the pollutant concentrations were increased, ranging from 3.86 to 5.61. However, the pH levels in CF- and LF-polluted treatments were lower than those in the CK group, with the highest increase by 12.26% observed in LC- and LCF-polluted soils. In the four treatment groups, the AN contents were increased as the pollutant concentrations were increased (Fig. S1b), while the AP contents were decreased in all four groups of contaminated soils (Fig. S1c). Compared to the CK group, the AK contents were increased in LC- and LF-contaminated soils but decreased in CF- and LCF-contaminated soils (Fig. S1d).
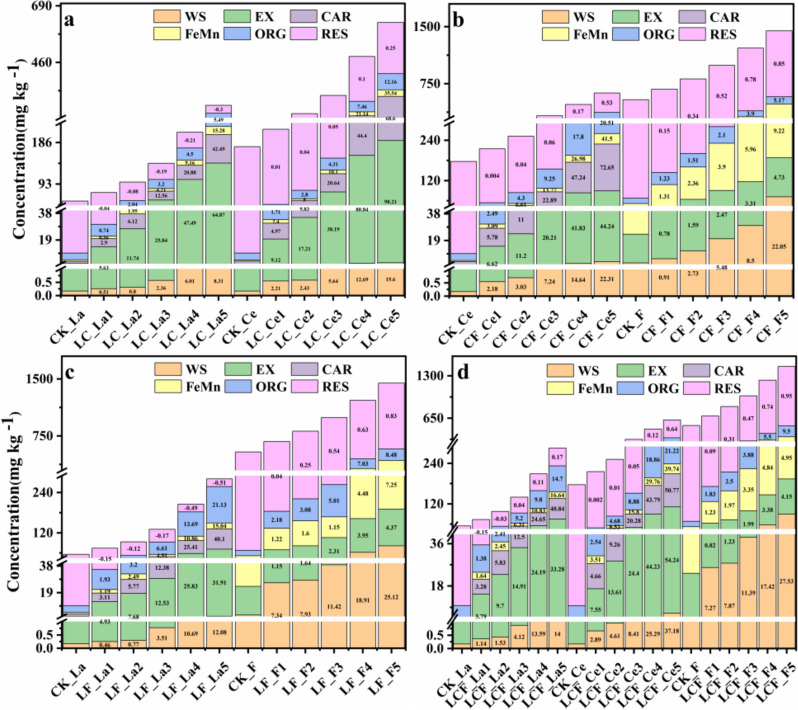
In the four types of combined contamination soils, the contents of most fractions of La, Ce, and F were increased as the pollution concentration was increased except for La_RES of LC- and LF-polluted soils (Fig. 1a, c). In LC-polluted soils, both La_EX and Ce_EX contents showed the highest increases of about 65 and 98 times, respectively (Fig. 1a). In CF-contaminated soils, the highest increases were observed in the contents of Ce_CAR and F_WS by over 72 and 22 times, respectively (Fig. 1b). The highest contents were observed in both La_CAR and F_WS of the LF-polluted soils (Fig. 1c). In the LCF-contaminated soils, the highest increases were detected in the contents of La_CAR, Ce_EX, and F_WS, by over 40, 50, and 27 times, respectively (Fig. 1d). In conclusion, F_WS showed the highest growth rate with the increase of F pollution concentration in all the combined contamination soils containing F (i.e., CF-, LF-, and LCF-polluted soils), while the contents of unstable exchange state and moderately stable carbonate binding state La and Ce showed the highest growth rates in LC-, CF-, and LF-polluted soils, respectively. Notably, the content of La_RES declined with the increase of pollution concentration in both LC and LF treatments.
Fig. 1.
Contents of different chemical fractions of lanthanum (La), cerium (Ce), and fluorine (F) in combined pollution soils of (a) La + Ce (LC), (b) Ce + F (CF), (c) La + F (LF), and (d) La + Ce + F (LCF). WS, water-soluble form; EX, exchangeable form; CAR carbonate-bound form; FeMn, iron-manganese-bound form; ORG, organic-bound form; RES, residual form; CK, control group; LC, interaction treatments of La-Ce; CF, interaction treatments of Ce-F; LF, interaction treatments of La-F; LCF, interaction treatments of La-Ce-F; the numbers within each bar indicated the percentage of change of each chemical fraction of La, Ce or F
Variations in the composition of microbial communities
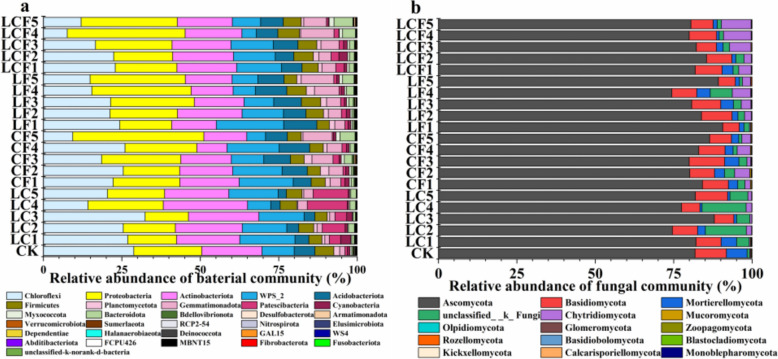
Different contamination soils were revealed with varied effects on the composition of microbial communities. There were a total of 28 bacterial and 15 fungal taxa at the phylum level detected in the unamended CK soil, while in the four groups of polluted soils, a total of 31 bacterial taxa at phylum level, including three newly detected taxa (MBNT15 in LF5-polluted soil, Fibrobacterota in CF5-polluted soil, and Fusobacteriota in LF2- and LF5-polluted soils), were identified (Fig. 2a); no new fungal taxa were detected at the phylum level in the four groups of pollution soils compared to the unamended CK soil (Fig. 2b). Compared with the CK soil, three bacterial taxa at the phylum level, including Abditibacteriota, WS4, and FCPU426, were undetected in the LC-, CF-, and LF-polluted soils (Fig. 2a), while three fungal phyla (i.e., Blastocladiomycota, Kickxellomycota, and Calcarisporiellomycota) were undetected in the LC-contaminated soil, and Blastocladiomycota disappeared in LF- and LCF-polluted soils (Fig. 2b).
Fig. 2.
Soil bacterial (a) and fungal (b) community composition at phylum level. CK, control group; LC, interaction treatments of La-Ce; CF, interaction treatments of Ce-F; LF, interaction treatments of La-F; LCF, interaction treatments of La-Ce-F
Correlation factors of sensitive microbial community
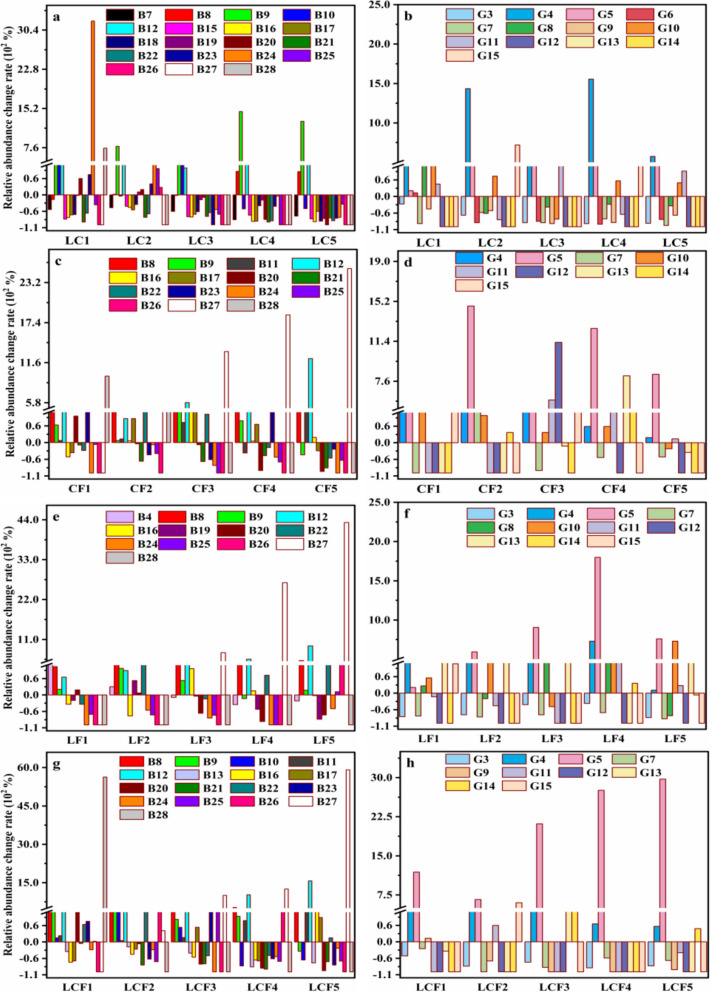
The results showed that the relative abundances of nine bacterial taxa at the phylum level, including Planctomycetota, RCP2-54, Nitrospirota, Elusimicrobiota, Dependentiae, GAL15, Abditibacteriota, WS4 and FCPU426, and five fungal taxa at the phylum level, containing Blastocladiomycota, Kickxellomycota, Calcarisporiellomycota, Mortierellomycota and Zoopagomycota, were all decreased by more than 80% as the contents of the various combinations of La, Ce, and F were increased (Fig. 3).
Fig. 3.
Bacterial (a, c, e, and g) and fungal (b, d, f, and h) taxa at the phylum level with relative abundance either increase or decrease more than 80% in different combined pollution soils of La + Ce (LC), Ce + F (CF), La + F (LF), and La + Ce + F (LCF), respectively. LC, interaction treatments of La-Ce; CF, interaction treatments of Ce-F; LF, interaction treatments of La-F; LCF, interaction treatments of La-Ce-F. Bacterial taxa: B7, Planctomycetota; B8, Gemmatimonadota; B9, Patescibacteria; B10, Cyanobacteria; B11, Myxococcota; B12, Bacteroidota; B13, unclassified-k-norank-d-bacteria; B15, Desulfobacterota; B16, Armatimonadota; B17, Verrucomicrobiota; B18, Sumerlaeota; B19, RCP2-54; B20, Nitrospirota; B21, Elusimicrobiota; B22, Dependentiae; B23, Halanaerobiaeota; B24, Deinococcota; B25, GAL15; B26, WS4; B27, Abditibacteriota; B28, FCPU426. Fungal taxa: G3, Mortierellomycota; G4, unclassified_k fungi; G5, Chytridiomycota; G6, Mucoromycota; G7, Olpidiomycota; G8, Glomeromycota; G9, Zoopagomycota; G10, Rozellomycota; G11, Basidiobolomycota; G12, Blastocladiomycota; G13, Kickxellomycota; G14, Calcarisporiellomycota; G15, Monoblepharomycota
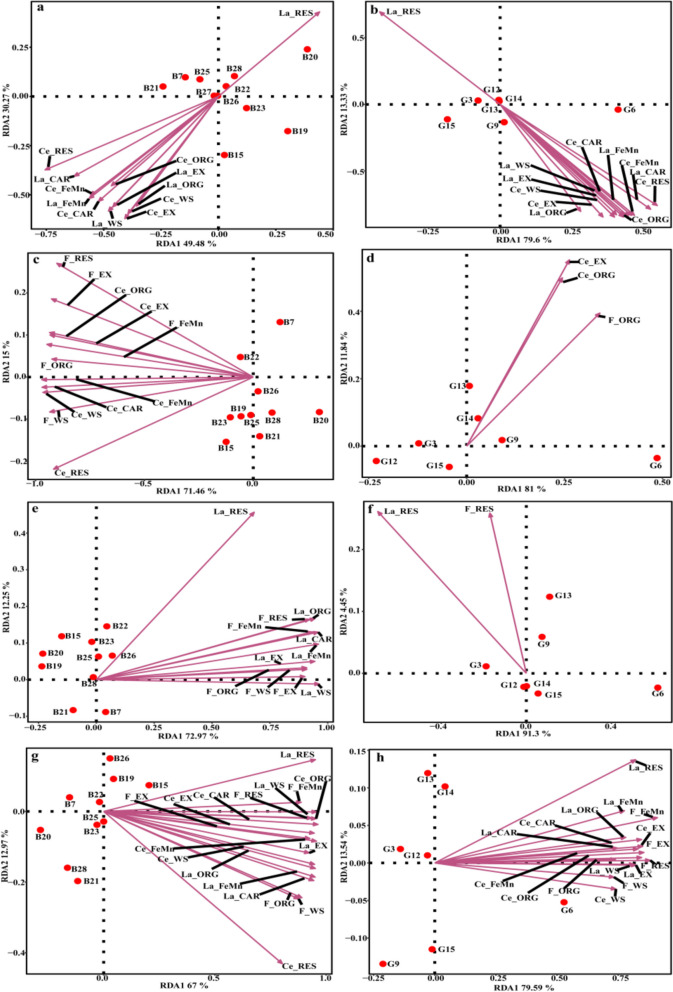
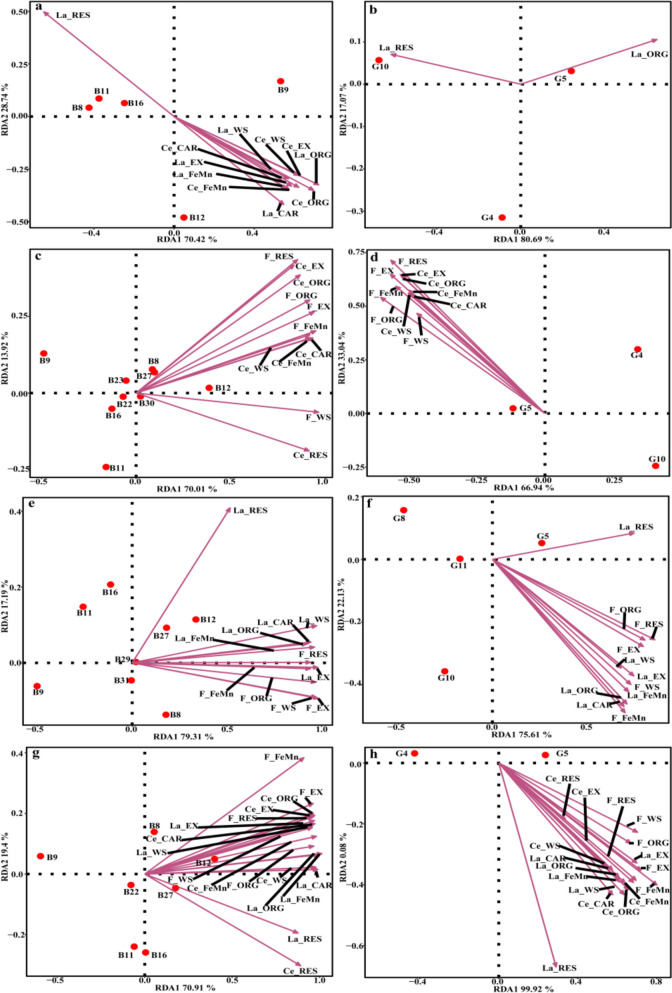
Among these taxa, Abditibacteriota was undetected in LC-polluted soils, while La_RES was negatively correlated with Abditibacteriota (Fig. 4a). WS4 was undetected in CF-polluted soils, Ce_EX, Ce_ORG, F_EX, F_FeMn, F_ORG, and F_RES were negatively correlated with WS4 (p < 0.05; Fig. 4c). La_WS showed negative correlation with the disappearance of FCPU426 in LF-polluted soils (p < 0.05; Fig. 4e). Three fungal phyla, i.e., Blastocladiomycota, Kickxellomycota, and Calcarisporiellomycota, all were undetected in LC-polluted soils, and most forms of La and Ce, except for La_RES, were negatively correlated with these three phyla (p < 0.05; Fig. 4b). In addition, Blastocladiomycota were also undetected in LF- and LCF-polluted soils, with La_RES, and F_RES identified as the correlation factors in LF-polluted soils, and La_WS, La_EX, Ce_WS, and F_WS identified as the correlation factors in LCF-polluted soils (p < 0.05; Fig. 4f, h).
Fig. 4.
Redundancy analysis of the sensitive bacterial and fungal taxa at the phylum level in combined pollution soils of La + Ce (a, b), Ce + F (c, d), La + F (e, f), and La + Ce + F (g, h), respectively. LC, interaction treatments of La-Ce; CF, interaction treatments of Ce-F; LF, interaction treatments of La-F; LCF, interaction treatments of La-Ce-F. Bacterial taxa: B7, Planctomycetota; B15, Desulfobacterota; B19, RCP2-54; B20, Nitrospirota; B21, Elusimicrobiota; B22, Dependentiae; B23, Halanaerobiaeota; B25, GAL15; B26, WS4; B27, Abditibacteriota; B28, FCPU426. Fungal taxa: G3, Mortierellomycota; G6, Mucoromycota; G9, Zoopagomycota; G12, Blastocladiomycota; G13, Kickxellomycota; G14, Calcarisporiellomycota; G15, Monoblepharomycota
Compared with the CK soil, the relative abundance of Nitrospirota were decreased by 100, 95.39, 89.24, and 96.00% in the four groups of combined pollution soils, respectively (Fig. 3a, c, e, and g). Nitrospirota was undetected in LC-polluted soils with high content of pollutants, and the most of the chemical fractions of La and Ce except for La_RES were negatively correlated with this phylum (p < 0.05; Fig. 4a). The relative abundances of Elusimicrobiota was decreased by 91.13, 83.26, and 90.59% in LC-, CF-, and LCF-polluted soils, respectively (Fig. 3a, c, and g), while it had significantly negative correlation with La_RES in LC-polluted soils, and Ce_EX, Ce_ORG, F_EX, F_FeMn, F_ORG, and F_RES in CF-polluted soils, respectively (p < 0.05; Fig. 4a, c). Moreover, La_RES and F_FeMn showed negative correlation with the microbial phyla in LCF-polluted soils (Fig. 4g). The relative abundance of Planctomycetota was decreased by 82.72% (Fig. 3a) and was correlated with the contents of La_ORG, Ce_WS, and Ce_ EX (p < 0.05; Fig. 4a), while the relative abundance of Dependentiae was decreased by 86.98% (Fig. 3a) and was negatively correlation with most chemical fractions of La and Ce except for La_RES in LC-polluted soils (p < 0.05, Fig. 4a). The relative abundance of RCP2-54 was decreased by 81.39% (Fig. 3e) and was negatively correlated with La_WS in LF-polluted soils (p < 0.05; Fig. 4e).
Among the fungal phyla, the relative abundances of Mortierellomycota and Zoopagomycota were decreased by more than 80% in LC- and LCF-polluted soils, while Monoblepharomycota was undetected in the higher concentrations of four groups of combined pollution soils (Fig. 3b, d, f, and h). The relative abundance of Zoopagomycota was negatively correlation with La_RES, while Mortierellomycota was negatively correlation with most forms of La and Ce, except for La_RES, in LC-polluted soils (p < 0.05, Fig. 4b, h). In the LCF-polluted soils, Mortierellomycota was negatively correlation with La_WS, La_EX, Ce_WS, and F_WS, whereas Zoopagomycota was negatively correlation with all of chemical fractions of La, Ce and F, except for La_WS, La_EX, Ce_WS, F_WS, and Ce_RES (p < 0.05, Fig. 4b, h).
Correlation factors of tolerant microbial community
A total of six bacterial phyla, including Gemmatimonadota, Bacteroidota, Patescibacteria, Myxococcota, Armatimonadota, and Abditibacteriota, and four fungal phyla, i.e., Chytridiomycota, Glomeromycota, Rozellomycota, and Basidiobolomycota, were revealed with increased relative abundances exceeding 80% as the pollutant concentrations were increased in the four groups of polluted treatments of soils (Fig. 3).
Specifically, compared with the CK soil, Fibrobacterota were newly detected in CF-polluted soils, and all forms of Ce and F showed positive correlation with the relative abundance of this phylum (p < 0.05; Fig. 5c). Additionally, both MBNT15 and Fusobacteriota were detected in LF-polluted soils, with the former was positively correlation with all chemical fractions of La and F, the latter was only positively correlation with La_EX, F_WS, F_EX, F_FeMn, and F_ORG (p < 0.05; Fig. 5e). Most chemical fractions of La and Ce except for La_RES showed positive correlation with Bacteroidota in LC-polluted soils, while all forms of La, Ce, and F were correlation with this phylum in other three groups of polluted soils (p < 0.05; Fig. 5). The relative abundance of Chytridiomycota was increased by 275–2871% in the four groups of polluted soils (Fig. 3). In addition, the relative abundance of Rozellomycota was increased by 408% and 829% in LC- and LF-polluted soils, respectively, and the relative abundances of Glomeromycota and Basidiobolomycota were increased by 257% and 414%, respectively, in the LF-polluted soils (Fig. 3b and f). These results revealed the tolerance of both Glomeromycota and Basidiobolomycota to the interaction effect of most chemical fractions of La, Ce, and F, except for La_RES (p < 0.05; Fig. 5).
Fig. 5.
Redundancy analysis of the tolerant bacterial and fungal taxa at the phylum level in combined pollution soils of La + Ce (a, b), Ce + F (c, d), La + F (e, f), and La + Ce + F (g, h), respectively. LC, interaction treatments of La-Ce; CF, interaction treatments of Ce-F; LF, interaction treatments of La-F; LCF, interaction treatments of La-Ce-F. Bacterial taxa: B8, Gemmatimonadota; B9, Patescibacteria; B11, Myxococcota; B12, Bacteroidota; B16, Armatimonadota; B22, Dependentiae; B23, Halanaerobiaeota; B27, Abditibacteriota; B28, FCPU426; B29, MBNT15; B30, Fibrobacterota; B31, Fusobacteriota. Fungal taxa: G4, unclassified_k fungi; G5, Chytridiomycota; G8, Glomeromycota; G10, Rozellomycota; G11, Basidiobolomycota
Discussion
The response of microorganisms to pollutant stress is closely reflected in the variations of the taxonomic composition and relative abundance of the microbial communities [13]. Based on the different responsive modes of the microbes to pollutant stress, e.g., the undetected or remarkable decrease in relative abundance as well as newly detected or substantial increase in relative abundance, the microbial taxa are categorized as either sensitive or tolerant groups [27, 43, 44]. In our study, these three groups of microbes were identified in the combined pollution soils to evaluate the effects of the interactions of different chemical fractions of La, Ce, and F on the soil taxonomic compositions of the microbial communities.
Sensitive microbial community
The taxonomic composition change of microbial community can be used as one of the biological indicators of soil pollution [10]. In particular, the undetected or significant decrease in the relative abundances of the microbes are involved in the ecological functions in soils [26].
Undetected microbes
The highest level of stress effects of pollutant on soil microbial communities is represented by the undetected of certain microbes [45]. Our results were consistent with those previously reported, showing the high levels of bioavailability and toxicity in the active forms of WS and EX [46]. It was noteworthy that our results indicated that, not only the active forms of La, Ce, and F, but also the relatively stable chemical fractions of FeMn, ORG, and RES of La, Ce, and F showed high stress effects on the disappearance of the microbial taxa.
A previous study showed that in polluted soils, the disappearance of some microbiome may affect the ecological functions of soil environment [26]. For example, Blastocladiomycota influenced the degradation of organic matters, such as wood fibers, in soil [47]. In our study this phylum were undetected in LC-, LF- and LCF-polluted soils, and may be influence the function of the degradation of organic matter in these polluted soils. Studies have shown that as the dominant genus of phylum Abditibacteriota (Table S1), Abditibacterium could inhabit extreme environments, due to its antibiotic and toxin-resistant properties [48]. Our results showed that Abditibacterium was undetected in LC-polluted soils, indicating that the La_RES toxicity was stronger than those in the other three groups of contaminated soils.
Microbes with significantly reduced relative abundances
The change of microbial relative abundances in contaminated soils is also an important indicator of the level of soil pollution because these alterations are the observable factors in response to the persistent inhibition of pollutants [15, 49]. Although the relative abundance of Nitrospirota declined in all four combined pollution soils, it was undetected in the highest concentration of LC treatment. This was probably due to the synergistic effects of the combined pollution of La and Ce enhanced the toxicity of the different chemical fractions, ultimately inhibiting the growth and metabolism of Nitrospirota [44]. While the toxicity levels of the other three types of polluted soils were lower than that of LC-polluted soil, possibly because F could combine with La and Ce to form relatively stable precipitates [50], thus reducing the toxicity levels of these two rare earth elements. In addition, in the CF-, LF-, and LCF-contaminated soils, the abundance of this bacterial phylum was decreased in the order of LCF > CF > LF; this was probably because that F promoted the formation of relatively stable carbonate binding states of La and Ce in LF- and CF-polluted soils (Fig. 1b, c); in LCF-polluted soil, the highest growth rate was observed in the carbonate binding states of La, while the growth of exchange states of Ce was the highest in LCF (Fig. 1d). Studies have shown that when La and Ce coexist with F, F was more likely to bind with La [51], thus this could be the cause for the higher toxicity level of LCF than that of CF and LF. Nitrospira was the dominant genus in Nitrospirota, and the decline in the relative abundance of Nitrospira (Table S1) could weaken the nitrification function of soils, as previously reported [52], ultimately the nitrification function of LC contaminated soil, among other soil properties, was most affected by this combined contamination.
Previous studies identified GAL15 as the dominant bacterial taxon at the phylum level in the ionic rare earth mining soil [3, 53]. Our study showed that compared with the CK group, the relative abundance of GAL15 was decreased as the increase of the pollutant concentrations of four treatment groups, while the complete disappearance was revealed in treatments of high concentrations of pollutants (Fig. 3). This was probably because that the experimental soils contained the high content of F (540.73 mg kg –1), which caused enhanced stress effect on GAL15 and decreased relative abundance or even complete disappearance of GAL15, suggesting the sensitive property of GAL15 to F. Moreover, studies showed that GAL15 were involved in the inhibition of glycan biosynthesis and metabolism [53], indicating that the decrease in the relative abundance of GAL15 would enhance the glycan biosynthesis and metabolism of the microbial community.
Our studies showed that both active and stable forms of La, Ce, and F played important roles in the variations of the taxonomic composition in the microbial communities. These results were consistent with those previously reported, showing that the active or unstable forms of the elements were the key drivers of variations in soil taxonomic composition of microbial community due to their higher bioavailability [45, 54], while the stable forms of heavy metal elements, such as FeMn, ORG, and RES, are harmful to certain groups of microbes [55]. Interestingly, Elusimicrobiota were sensitive to the RES form of both La and F, probably due to the metabolites of this microbe that could promote dissociation of the stable fractions from soils [55], thus increasing the availability or toxicity of the RES form of both La and F. We note that further studies are needed to clarify the effects of these chemical fractions on the relative abundance of Elusimicrobiota. In the present study, the decrease in the relative abundances of Planctomycetota, Dependentiae, and RCP2-54 were significantly correlated with the chemical fractions, especially the available forms of La or Ce, due to their inhibitory effect on the cell growth [22], as previously reported. Additionally, Gemmataceae and Babeliaceae were the most dominant families of phyla Planctomycetota and Dependentiae, respectively (Table S1), playing the key roles in the metabolism of organic compounds, such as hydrolyzation of various carbohydrates in soils, as previously reported [54, 56]. These results indicated that the available forms of La or Ce significantly reduced the relative abundances of Planctomycetota and Dependentiae, and these two phyla showed the ecological functions, i.e., decomposing organic compounds in soil [56, 57]. Therefore, the reduced relative abundances of these two phyla may further affect their ecological functions of decomposing of organic compounds in soil.
Studies have shown that the fungal communities are sensitive to perturbations or changes in soil ecological system [58]. In our study, the relative abundances of Mortierellomycota and Zoopagomycota were decreased by more than 80% in LC- and LCF-polluted soils. It was noteworthy that the relative abundance of Zoopagomycota was negatively influenced by La_RES, probably because that La_RES was more easily bound to the functional groups of cell membranes in Zoopagomycota to cause disruption of cell membranes [59], resulting in a decrease in the relative abundance of this phylum. Moreover, Mortierella and Syncephalis were the dominant genera of Mortierellomycota and Zoopagomycota, respectively (Table S1). These results were in accordance with those previously reported, showing that the decreased relative abundances of these two genera could affect the mineralization of soil organic carbon, such as the decomposition of humic acid [59, 60].
In general, various microbes show multiple responsive modes to the interaction effects of different forms of elements in the combined pollution soils, including inhibition, insusceptibility, and promotion effects. These interaction effects would change the microbial community composition via stressing the sensitive taxa or promoting the resistant microbial taxa. Additionally, our study showed that the RES forms of the three elements may have restrained the growth of certain microbes.
Tolerant microbial community
Although the pollutants at high concentrations have shown significant inhibitory effect on soil microorganisms, some newly detected or tolerant microbes could be adaptive in the polluted environment via various regulatory mechanisms, such as adsorption of the metal element by cell wall, efflux by metal transporting ATPases, or intracellular bioaccumulation of the metal elements [61, 62]. In our study, the correlation factors responsible for the newly detected microbes or the significant increase in the relative abundance of microbes were investigated to further explore the interaction effects of the different chemical fractions of La, Ce, and F on the microbial community compositions in the combined pollution soils.
Newly detected microbes
Some researchers suggested that the microbes newly detected in contaminated soils may be tolerant to pollutants [12], which was attributed to the changes of soil properties [11]. In our study, Fibrobacterota were newly detected in CF-polluted treatments (Fig. 2a). Previous studies showed that Fibrobacterota produced polysaccharides to adsorb metal ions [63], suggesting the adsorption of all chemical fractions of Ce and F by polysaccharides generated by Fibrobacterota, ultimately preventing the elements from entering cells and making these microbes adapt to CF-polluted treatments and become tolerant bacteria. Our study showed that Fusobacteriota were detected in LF-polluted treatments; these results were consistent with those previously reported, showing the promotion effects of La and F on organisms in a certain concentration range [5, 64]. Therefore, it was speculated that the concentrations of various chemical fractions of La and F were in the tolerance range of the phylum Fusobacteriota, making this phylum the tolerant microbes in LF-polluted soils. It was noted that Fusobacteriota, a group of core intestinal bacteria [65], were newly detected with extremely low relative abundance (ranging from 0.003 to 0.007%) only in LF-polluted soils. Pan et al. [55] showed that the rare microbes play important roles in maintaining the community diversity and correlating multiple ecological functions. Further investigations are necessary to verify the functions of these newly detected microbes in this study.
Microbes with significantly increased relative abundances
Studies have shown that the microbes with significantly increased relative abundances are revealed with strong tolerance to pollutant stress [66]. In our study, the relative abundance of Gemmatimonadota was increased by 79.24, 451.36, 516.06 and 530.89% in four combined pollution soils, respectively (Fig. 3), suggesting the strong tolerance of this phylum in these pollution soils. Previous studies indicated that Gemmatimonadota were the key microbial hosts for heavy metal resistance genes and antibiotic resistance genes [67]. These studies suggested that the phylum Gemmatimonadota contained La, Ce, and F tolerant bacterial taxa. Our results showed that Gemmatimonas was the predominant microorganism of the phylum Gemmatimonadota (Table S1), while studies showed this genus was involved in the mineralization of organic matters [68]. Therefore, the significant increase of the relative abundance of Gemmatimonas could promote the mineralization of organic matters in the four groups of contaminated soils and enhance the soil carbon emissions. Studies have shown that some microbes can produce extracellular polymeric substances to prevent metal ions in contaminated soils from entering the microbial cells [61]. Our results showed that as the dominant genus of Bacteroidota, Mucilaginibacter could produce and secrete extracellular polymeric substances into the surrounding environments to absorb copper (Cu) and zinc (Zn), as previously reported [68]. Therefore, the tolerant property of Bacteroidota was probably due to Mucilaginibacter, which was the dominant genus of this phylum, producing the extracellular polymeric substances to absorb the various chemical fractions of these three elements. These results were consistent with those previously reported, showing that F was an anion that could be absorbed by the extracellular polymeric substance of Mucilaginibacter and the extracellular polymeric substances produced by this genus could exchange anions through electrostatic interactions [53], ultimately promoting the resistance of this bacterial taxon in the four groups of polluted soils.
Despite the weak mobility of the stable forms of La and Ce in the soils [64], our study revealed different effects of these forms on various microbes. For example, La_RES and Ce_RES were the key drivers of the increase in the relative abundances of Myxococcota and Armatimonadota in LCF-polluted soil (Fig. 5g), with Haliangium and Chthonomonas identified as their dominant genera, respectively (Table S1). Studies have shown that Haliangium could enrich phosphorus [69] and Chthonomonas could produce phospholipids [70], suggesting that these two genera could absorb or bind both La_RES and Ce_RES to ultimately promote the bacterial cell proliferation.
The taxonomic composition of fungal community is generally stable or tolerant to pollutants due to the strong fungal adsorption, filtration, and retention of pollutants [71]. In our study, Chytridiomycota, Glomeromycota, Rozellomycota, and Basidiobolomycota were identified as the tolerant fungal phyla in four groups of polluted soils, respectively (Fig. 3). Specifically, the relative abundance of Chytridiomycota was increased by 275–2871% in the four groups of polluted soils (Fig. 3). Our results revealed the tolerance of this phylum to the interaction effect of most chemical fractions of La, Ce, and F, probably due to its capability of synthesizing secondary metabolites, involvement in enzymatic activities, and regulation of metal induced protein synthesis [61], thus producing complexes with the different chemical fractions of La, Ce, and F, ultimately promoting fungal cell proliferation. These results were consistent with those previously reported, showing that these three tolerant fungal phyla could adsorb pollutants through cell walls to protect fungi from pollutant stress and develop resistance to pollutants [72].
In conclusion, Several limitations of this study were noted. We only studied the effects of the chemical fractions of La, Ce, and F pollution elements on microbiomes and the possible effects of microorganisms on the corresponding functions of soil ecology. Although we discussed some relatively dominant bacterial and fungal genera, further explorations are necessary to identify the sensitive microbes at the species level. Moreover, it is still necessary to further evaluate the effects of Cl element introduced by LaCl3·7H2O and CeCl3·7H2O of analytical grades on soil microorganism composition. In summary, the in-depth investigations in these areas would strengthen and verify the findings revealed in our study and provide a strong experimental foundation to support the ecological restoration of La, Ce, and F contaminated soil.
Conclusions
Our study revealed the sensitive and tolerant microbes with multiple responsive modes to the various interactions of different chemical fractions of La, Ce, and F in farmland soils. La_RES was the key correlation factor responsible for the disappearance or the significant increase in the relative abundance of microbes in LC-polluted soils; Ce_EX and four chemical fractions of F (i.e., F_EX, F_FeMn, F_ORG, and F_RES) in CF-polluted soils were identified as the stress factors for the disappearance or the decrease in the relative abundances of microbes. Furthermore, La_WS showed the most toxic effect on bacterial taxa at the phylum level, while all chemical fractions of La and F caused the novel appearance of microbes in LF-polluted soils. Both La_RES and F_FeMn were detected as the stress factors in LCF-polluted soils. Our study further indicated the LC polluted soil had the most toxicity than other three soils on sensitive microbes for La and Ce had the synergistic effects to enhance the toxicity of their chemical fractions. The undetected and newly detected microbes as well as the microbes with significant changes in the relative abundance would affect the mineralization of soil organic matters or the nitrogen transformation. Therefore, interaction groups of various chemical fractions of La, Ce, and F would inhibit or enhance the growth of different microbial communities. In future studies, it is necessary to explore the interactions of various chemical fractions of La, Ce, and F on the ecological functions of sensitive and tolerant microbes at genus level of microbial communities, in order to provide a certain theoretical basis for the ecological restoration of La, Ce, and F contaminated soil.
Supplementary Information
Authors’ contributions
YJ: Conceptualization, Methodology, Data curation, Formal analysis, Investigation, Visualization, Funding acquisition, Writing—Original Draft; DZ: Investigation, Writing—Reviewing and Editing, Data Curation; SZ: Conceptualization, Writing—Reviewing and Editing, Project administration, Funding acquisition, Resource; TL: Supervision, Writing—Reviewing and Editing, Funding acquisition; GW: Supervision, Writing—Reviewing and Editing; XX: Writing—Reviewing and Editing, Funding acquisition; YP: Writing—Reviewing and Editing; LN: Investigation.
Funding
This research was funded by the Sichuan Key Research and Development Projects, China, grant number 2021YFN0018, Sichuan International Science and Technology Innovation Cooperation Project, grant number 2020YFH0159 and Liangshan Prefecture Science and Technology Plan Project, grant number 24YYYJ0030.
Data availability
The datasets generated and/or analysed during the current study are available in the NCBI repository [https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1119661].
Declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gwenzi W, Mangori L, Danha C, Chaukura N, Dunjana N, Sanganyado E. Sources, behaviour, and environmental and human health risks of high-technology rare earth elements as emerging contaminants. Sci Total Environ. 2018;636:299–313. 10.1016/j.scitotenv.2018.04.235. [DOI] [PubMed] [Google Scholar]
- 2.McLemore V. Rare earth elements (REE) deposits associated with great plain margin deposits (Alkaline-Related), southwestern United States and eastern. Resources. 2018;7:8. 10.3390/resources7010008. [Google Scholar]
- 3.Liu JJ, Liu W, Zhang YB, Chen CJ, Wu WX, Zhang TC. Microbial communities in rare earth mining soil after in-situ leaching mining. Sci Total Environ. 2021;755:1–12. 10.1016/j.scitotenv.2020.142521. [DOI] [PubMed] [Google Scholar]
- 4.Wei ZW, Hao ZK, Li XH, Guan ZB, Cai YJ, Liao XR. The effects of phytoremediation on soil bacterial communities in an abandoned mine site of rare earth elements. Sci Total Environ. 2019;670:950–60. 10.1016/j.scitotenv.2019.03.118. [DOI] [PubMed] [Google Scholar]
- 5.Gan CD, Jia YB, Yang JY. Remediation of fluoride contaminated soil with nano-hydroxyapatite amendment: Response of soil fluoride bioavailability and microbial communities. J Hazard Mater. 2021;405:124694. 10.1016/j.jhazmat.2020.124694. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Jiang Q, Li RZ, Zhang B, Zhang JX, Zhang Y. Passivation of lead and cerium in soil facilitated by biochar-supported phosphate-doped ferrihydrite: Mechanisms and microbial community evolution. J Hazard Mater. 2022;436:129090. 10.1016/j.jhazmat.2022.129090. [DOI] [PubMed] [Google Scholar]
- 7.Thomas PJ, Carpenter D, Boutin C, Alliso JE. Rare earth elements (REEs): Effects on germination and growth of selected crop and native plant species. Chemosphere. 2014;96:57–66. 10.1016/j.chemosphere.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Chae Y, Kim D, An YJ. Effects of fluorine on crops, soil exoenzyme activities, and earthworms in terrestrial ecosystems. Ecotox Environ Safe. 2018;151:21–7. 10.1016/j.ecoenv.2017.12.060. [DOI] [PubMed] [Google Scholar]
- 9.Romero-Freire A, Minguez L, Pelletier M, Cayer A, Caillet C, Devin S, Gross EM, Guérold F, Pain-Devin S, Vignati DAL, Giamberini L. Assessment of baseline ecotoxicity of sediments from a prospective mining area enriched in light rare earth elements. Sci Total Environ. 2018;612:831–9. 10.1016/j.scitotenv.2017.08.128. [DOI] [PubMed] [Google Scholar]
- 10.Bergsten-Torralba LR, Magalhães DP, Giese EC, Nascimento CRS, Pinho JVA, Buss DF. Toxicity of three rare earth elements, and their combinations to algae, microcrustaceans, and fungi. Ecotox Environ Safe. 2020;201:110795. 10.1016/j.ecoenv.2020.110795. [DOI] [PubMed] [Google Scholar]
- 11.Yang JY, Wang M, Lu J, Yang K, Wang KP, Liu M, Luo HQ, Pang LN, Wang B. Fluorine in the environment in an endemic fluorosis area in Southwest. China Environ Res. 2020;184:109300. 10.1016/j.envres.2020.109300. [DOI] [PubMed] [Google Scholar]
- 12.Liu JJ, Li C, Ma WD, Wu ZX, Liu W, Wu WX. Exploitation alters microbial community and its co-occurrence patterns in ionic rare earth mining sites. Sci Total Environ. 2023;898:165532. 10.1016/j.scitotenv.2023.165532. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed AM, Tardy V, Bonnineau C, Billard P, Pesce S, Lyautey E. Changes in sediment microbial diversity following chronic copper-exposure induce community copper-tolerance without increasing sensitivity to arsenic. J Hazard Mater. 2020;391:122197. 10.1016/j.jhazmat.2020.122197. [DOI] [PubMed] [Google Scholar]
- 14.Zuo YY, Li Y, Chen H, Ran G, Liu XM. Effects of multi-heavy metal composite pollution on microorganisms around a lead-zinc mine in typical karst areas, southwest China. Ecotox Environ Safe. 2023;262:115190. 10.1016/j.ecoenv.2023.115190. [DOI] [PubMed] [Google Scholar]
- 15.Coban O, DeDeyn GB, Vander PM. Soil microbiota as game-changers in restoration of degraded lands. Sci. 2022;375:990–1000. 10.1126/science.abe0725. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, Xue Y, Xia J, Qu X, Lei B, Yang T, Zhang X, Li N, Zhao H, Wang M, Luo M, Zhang C, Du Y, Yan C. Construction of high quality ultrathin lanthanide oxyiodide nanosheets for enhanced CT imaging and anticancer drug delivery to efficient cancer theranostics. Biomaterials. 2020;230:119670. 10.1016/j.biomaterials.2019.119670. [DOI] [PubMed] [Google Scholar]
- 17.Siciliano A, Guida M, Serafini S, Micillo M, Galdiero E, Carfagna S, Salbitani G, Tommasi F, Lofrano G, Suarez EGP, Gjata I, Brouziotis AA, Trifuoggi M, Liguori R, Race M, Fabbricino M, Libralato G. Long term multi-endpoint exposure of the microalga Raphidocelis subcapitata to lanthanum and cerium. Sci Total Environ. 2021;790:148229. 10.1016/j.scitotenv.2021.148229. [DOI] [PubMed] [Google Scholar]
- 18.Cui SF, Yang JY. Effects of fluorine on the growth of broad bean (Vicia faba L.) and maize (Zea mays L.) and the response of microbial community in soils. Water Air Soil Pollut. 2021;232:27–41. 10.1007/s11270-021-04983-x. [Google Scholar]
- 19.Liu YQ, Yang Q, Zhu MJ, Wang LH, Zhou Q, Yang ZB, Huang XH. Endocytosis in microcystis aeruginosa accelerates the synthesis of microcystins in the presence of lanthanum(III). Harmful Algae. 2020;93:101791. 10.1016/j.hal.2020.101791. [DOI] [PubMed] [Google Scholar]
- 20.Wu D, Hou YY, Cheng J, Han T, Hao NH, Zhang BJ, Fan X, Jia X, Chen FJ, Gong DH, Wang L, McGinne P, Zhao L, Chen SL. Transcriptome analysis of lipid metabolism in response to cerium stress in the oleaginous microalga Nannochloropsis oculata. Sci Total Environ. 2022;838:156420. 10.1016/j.scitotenv.2022.156420. [DOI] [PubMed] [Google Scholar]
- 21.Tao Y, Shen L, Han YS, Li ZX, Cui YH, Lin YL, Qu JH, Zhang Y. Metagenomic study of carbon metabolism in black soil microbial communities under lead-lanthanum stress. J Hazard Mater. 2023;446:130666. 10.1016/j.jhazmat.2022.130666. [DOI] [PubMed] [Google Scholar]
- 22.He EK, Qiu H. Lanthanum and cerium disrupt similar biological pathways and interact synergistically in Triticum aestivum as revealed by metabolomic profiling and quantitative modeling. J Hazard Mater. 2022;426:127831. 10.1016/j.jhazmat.2021.127831. [DOI] [PubMed] [Google Scholar]
- 23.Gunther A, Wollenberg A, Vogel M, Drobot B, Steudtner R, Freitag L, Hubner R, Stumpf T, Raff J. Speciation and spatial distribution of Eu (III) in fungal mycelium. Sci Total Environ. 2022;851:158160. 10.1016/j.scitotenv.2022.158160. [DOI] [PubMed] [Google Scholar]
- 24.Romero-Freire A, González V, Groenenberg J E, Qiu H, Auffan M, Cotelle S, Giamberini L. Cytotoxicity and genotoxicity of lanthanides for Vicia faba L. are mediated by their chemical speciation in different exposure media. Sci Total Environ. 2021; 790, 148223. 10.1016/j.scitotenv.2021.148223. [DOI] [PubMed]
- 25.Egler SG, Roldao TM, Santos GO, Heidelmann GP, Giese EC, Correia FV, Saggioro EM. Acute toxicity of single and combined rare earth element exposures towards Daphnia similis. Ecotox Environ Safe. 2023;251:114538. 10.1016/j.ecoenv.2023.114538. [DOI] [PubMed] [Google Scholar]
- 26.Crowther TW, Vanden-Hoogen J, Wan J, Mayes MA, Keiser AD, Mo L, Averill C, Maynard DS. The global soil community and its influence on biogeochemistry. Sci. 2019;365:v0550. 10.1126/science.aav0550. [DOI] [PubMed] [Google Scholar]
- 27.Kasemodel MC, Sakamoto IK, Varesche MBA, Rodrigues VGS. Potentially toxic metal contamination and microbial community analysis in an abandoned Pb and Zn mining waste deposit. Sci Total Environ. 2019;675:367–79. 10.1016/j.scitotenv.2019.04.223. [DOI] [PubMed] [Google Scholar]
- 28.Rocca JD, Simonin M, Bernhardt ES, Washburne AD, Wright JP. Rare microbial taxa emerge when communities collide: freshwater and marine microbiome responses to experimental mixing. Ecology. 2020;101:e02956. 10.1002/ecy.2956. [DOI] [PubMed] [Google Scholar]
- 29.Zhou M, Zhu S, Wei X, Xie Y. Electrodialytic remediation of fluorine contaminated soil using 2-electrolysis compartment. Arch Agron Soil Sci. 2019;65:886–96. 10.1080/03650340.2018.1537484. [Google Scholar]
- 30.Nottingham AT, Fierer N, Turner BL, Whitaker J, Ostle NJ, McNamara NP, Bardgett RD, Leff JW, Salinas N, Silman MR, Kruuk L, Meir P. Microbes follow Humboldt: temperature drives plant and soil microbial diversity patterns from the Amazon to the Andes. Ecology. 2018;99:2455–66. 10.1002/ecy.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan X, Zhang S, Zhong Q, Gong G, Wang G, Guo X, Xu X. Effects of soil chemical properties and fractions of Pb, Cd, and Zn on bacterial and fungal communities. Sci Total Environ. 2020;715:136904. 10.1016/j.scitotenv.2020.136904. [DOI] [PubMed] [Google Scholar]
- 32.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:141–5. 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun B, Gu L, Bao L, Zhang S, Wei Y, Bai Z, Zhuang G, Zhuang X. Application of biofertilizer containing Bacillus subtilis reduced the nitrogen loss in agricultural soil. Soil Biol Biochem. 2020;148:107911. 10.1016/j.soilbio.2020.107911. [Google Scholar]
- 35.Bremner JM. Nitrogen Total. In: Sparus DL, editor. Methods of Soil Analysis Part 3. Chemical Methods; 1996. 10.2136/sssabookser5.3.c37. [DOI]
- 36.Kara D, Ozsavasci C, Alkan M. Investigation of suitable digestion methods for the determination of total phosphorus in soils. Talanta. 1997;44:2027–32. 10.1016/S0039-9140(97)00014-3. [DOI] [PubMed] [Google Scholar]
- 37.Helmke P A, Sparks D L. Lithium, sodium, potassium, rubidium, and cesium. Methods of Soil Analysis: Part 3 chemical Methods, 5.3. 1996. 10.2136/sssabookser5.3.c19.
- 38.Sharma KL, Lal M, Reddy KS, Indoria AK, Srinivas K, Singh VK, Prabhakar M, Chandrika DS, Vasavi M, Haindavi P, Gayatri DLA. Long term effect of different levels of surface crop residue application on hydrolyzable and non-hydrolyzable nitrogen fractions in sorghum (Sorghum bicolor (L.) Moench) - cowpea (Vigna unguiculata) system in rainfed alfisols. Commun. Soil Sci Plan, 2021;53:337–345.
- 39.Sinegani AAS, Hosseinpur A. Evaluation of effect of different sterilization methods on soil biomass phosphorus extracted with NaHCO3. Plant Soil Environ. 2010;56:156–162. 10.17221/86/2009-PSE.
- 40.Demiss M, Beyene S, Kidanu S. Comparison of soil extractants and spectral reflectance measurement for estimation of available soil potassium in some Ethiopian soils. Eurasian Soil Sci. 2020;53:1100–9. 10.1134/S1064229320080049. [Google Scholar]
- 41.Tessier A, Campbell PGC, Bisson M. Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem. 1979;51:844–51. 10.1021/ac50043a017. [Google Scholar]
- 42.Sut-Lohmann M, Ramezany S, Kastner F, Raab T, Heinrich M, Grimm M. Using modified Tessier sequential extraction to specify potentially toxic metals at a former sewage farm. J Environ Manage. 2021;304:114229. 10.1016/j.jenvman.2021.114229. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Wang S, Prete D, Xue S, Nan Z, Zang F, Zhang Q. Accumulation and interaction of fluoride and cadmium in the soil-wheat plant system from the wastewater irrigated soil of an oasis region in northwest China. Sci Total Environ. 2017;595:344–51. 10.1016/j.scitotenv.2017.03.288. [DOI] [PubMed] [Google Scholar]
- 44.Chen XM, Zhao Y, Zhao XY, Wu JQ, Zhu LJ, Zhang X, Wei ZM, Liu Y, He PP. Selective pressures of heavy metals on microbial community determine microbial functional roles during composting: Sensitive, resistant and actor. J Hazard Mater. 2020;398:122858. 10.1016/j.jhazmat.2020.122858. [DOI] [PubMed] [Google Scholar]
- 45.Ji M, Kong W, Stegen J, Yue L, Wang F, Dong X, Cowan DA, Ferrari BC. Distinct assembly mechanisms underlie similar biogeographical patterns of rare and abundant bacteria in Tibetan Plateau grassland soils. Environ Microbiol. 2020;22:2261–72. 10.1111/1462-2920.14993. [DOI] [PubMed] [Google Scholar]
- 46.Feng W, Zhang S, Zhong Q, Wang G, Pan X, Xu X, Zhou W, Li T, Luo L, Zhang Y. Soil washing remediation of heavy metal from contaminated soil with EDTMP and PAA: properties, optimization, and risk assessment. J Hazard Mater. 2020;381:120997. 10.1016/j.jhazmat.2019.120997. [DOI] [PubMed] [Google Scholar]
- 47.Powell MJ, Letcher PM, James TY. Ultrastructural characterization of the host–parasite interface between Allomyces anomalus (Blastocladiomycota) and Rozella allomycis (Cryptomycota). Fungal Biol. 2017;121:561–72. 10.1016/j.funbio.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Yue ZF, Zhang J, Ding CF, Wang YR, Zhou ZG, Yu XL, Zhang TL, Wang XX. Transfer and distribution of antibiotic resistance genes in the soil-peanut system receiving manure for years. Sci Total Environ. 2023;869:161742. 10.1016/j.scitotenv.2023.161742. [DOI] [PubMed] [Google Scholar]
- 49.Rachelle EB, Wyatt H, Maria FC, Terry CH, Rex McAlileya L, James HC. Variation in microbial community structure correlates with heavy-metal contamination in soils decades after mining ceased. Soil Biol Biochem. 2018;126:57–63. 10.1016/j.soilbio.2018.08.011. [Google Scholar]
- 50.Zhang HC, Xie DR, Zhang H, Jiang M, Huang H, Wan Y, Liao Y, Zhao SL. Reaction mechanism of fluoride conversion into BF4 during sulphuric acid leaching of roasted bastnaesite. J Rare Earth. 2021;39:186–93. 10.1016/j.jre.2020.03.005. [Google Scholar]
- 51.Xie T, Shi Y. Effects of LaF3/CeF3 on the friction transfer of PTFE-based composites. Tribol Int. 2021;161:107069. 10.1016/j.triboint.2021.107069. [Google Scholar]
- 52.Zhao JY, Qin ST, Pan P, Chen DK, Tang SD, Chen LH, Wang XL, Gu MH, Tang FY, He JH, Wen RH, He B. Microbial driving mechanism of soil conditioner on reducing cadmium uptake by rice and improving soil environment. Agr Ecosyst Environ. 2023;349:108452. 10.1016/j.agee.2023.108452. [Google Scholar]
- 53.Li JB, Meng DL, Wang XZ, Lara BJLA, Song SX, Xia L. Sources and succession of microorganisms in industrial coal flotation system. Fuel. 2023;342:127917. 10.1016/j.fuel.2023.127917. [Google Scholar]
- 54.Wang Z, Wang H, Wang H, Li Q, Li Y. Effect of soil washing on heavy metal removal and soil quality: a two-sided coin. Ecotox Environ Safe. 2020;203:110981. 10.1016/j.ecoenv.2020.110981. [DOI] [PubMed] [Google Scholar]
- 55.Pan XM, Zhang SR, Li T, Ouyang JY, Gong GS, Wang GY, Xu XX, Pu YL, Long LL, Jia YX. Response of microbiomes with different abundances to removal of metal fractions by soil washing. Ecotox Environ Safe. 2022;242:113862. 10.1016/j.ecoenv.2022.113862. [DOI] [PubMed] [Google Scholar]
- 56.Dedysh SN, Ivanova AA. Planctomycetes in boreal and subarctic wetlands: diversity patterns and potential ecological functions. Microbiol Ecol. 2019;95:2008415. 10.1080/00103624.2021.2008415. [DOI] [PubMed] [Google Scholar]
- 57.Li ZY, Li WH, Wang JL, Zhang JZ, Wang ZH. Drip irrigation shapes the soil bacterial communities and enhances jujube yield by regulating the soil moisture content and nutrient levels. Agr Water Manage. 2023;289:108563. 10.1016/j.agwat.2023.108563. [Google Scholar]
- 58.Bai LF, Zhang XQ, Li BZ, Sun FC, Zhao XQ, Wang YF, Lu ZY, Zhang DJ, Fang J. Fungal communities are more sensitive to nitrogen fertilization than bacteria in different spatial structures of silage maize under short-term nitrogen fertilization. Appl Soil Ecol. 2022;170:104275. 10.1016/j.apsoil.2021.104275. [Google Scholar]
- 59.Wang YZ, Zhang HF, Zhang YP, Fei JC, Rong XM, Peng JW, Luo GW. Crop rotation-driven changes in rhizosphere metabolite profiles regulate soil microbial diversity and functional capacity. Agr Ecosyst Environ. 2023;358:108716. 10.1016/j.agee.2023.108716. [Google Scholar]
- 60.Liu XJ, Zhang Y, Li P, Xiao L. Siltation of check dams alters microbial communities and thus limits organic carbon mineralization. Soil Till Res. 2024;236:105949. 10.1016/j.still.2023.105949. [Google Scholar]
- 61.Ansari A, Pena-Bahamonde J, Fanourakis SK, Hu Y, Rodrigues DF. Microbially-induced mineral scaling in desalination conditions: Mechanisms and effects of commercial antiscalants. Water Res. 2020;179:115863. 10.1016/j.watres.2020.115863. [DOI] [PubMed] [Google Scholar]
- 62.Ibrahim IM, Konnova SA, Elena NS, Sigida EN, Lyubun EV, Muratova AY, Fedonenko YP, Elbanna K. Bioremediation potential of a halophilic Halobacillus sp. strain, EG1HP4QL: exopolysaccharide production, crude oil degradation, and heavy metal tolerance. Extremophiles. 2020;24:157–166. 10.1007/s00792-019-01143-2. [DOI] [PubMed]
- 63.Ransom-Jones E, Jones DL, McCarthy AJ, McDonald JE. The Fibrobacteres: an important phylum of cellulose-degrading bacteria. Microb Ecol. 2012;63:267–81. 10.1007/s00248-011-9998-1. [DOI] [PubMed] [Google Scholar]
- 64.Kotelnikova A, Fastovets I, Rogova O, Volkov DS, Stolbova V. Toxicity assay of lanthanum and cerium in solutions and soil. Ecotox Environ Safe. 2019;167:20–8. 10.1016/j.ecoenv.2018.09.117. [DOI] [PubMed] [Google Scholar]
- 65.Xie Z, Li YT, Xiong K, Tu ZH, Waiho K, Yang CY, Deng YW, Li SS, Fang JKH, Hu MH, Dupont S, Wang YJ. Combined effect of salinity and hypoxia on digestive enzymes and intestinal microbiota in the oyster Crassostrea hongkongensis. Environ Pollut. 2023;331:121921. 10.1016/j.envpol.2023.121921. [DOI] [PubMed] [Google Scholar]
- 66.Yang TY, Tang GT, Li L, Ma LC, Zhao YY, Guo ZC. Interactions between bacteria and eukaryotic microorganisms and their response to soil properties and heavy metal exchangeability nearby a coal-fired power plant. Chemosphere. 2022;302:134829. 10.1016/j.chemosphere.2022.134829. [DOI] [PubMed] [Google Scholar]
- 67.Li ZQ, Wang XQ, Zhang BB, Li BY, Du HH, Wu ZB, Rashid A, Mensah CO, Lei M. Transmission mechanisms of antibiotic resistance genes in arsenic-contaminated soil under sulfamethoxazole stress. Environ Pollut. 2023;326:121488. 10.1016/j.envpol.2023.121488. [DOI] [PubMed] [Google Scholar]
- 68.Li YP, You LX, Yang XJ, Yu YS, Zhang HT, Yang B, Chorover J, Feng RW, Rensing C. Extrapolymeric substances (EPS) in Mucilaginibacter rubeus P2 displayed efficient metal (loid) bio-adsorption and production was induced by copper and zinc. Chemosphere. 2022;291:132712. 10.1016/j.chemosphere.2021.132712. [DOI] [PubMed] [Google Scholar]
- 69.Zhou X, Shi A, Rensing C, Yang J, Ni W, Xing S, Yang W. Wood vinegar facilitated growth and Cd/Zn phytoextraction of Sedum alfredii Hance by improving rhizosphere chemical properties and regulating bacterial community. Environ Pollut. 2022;305:119266. 10.1016/j.envpol.2022.119266. [DOI] [PubMed] [Google Scholar]
- 70.Chen JF, Liu YY, Yang YW, Tang MZ, Wang RJ, Jiang LT, Tian YP, Hu HW, Zhang X, Wei YS. Bacterial community structure and gene function prediction in response to long-term running of dual graphene modified bioelectrode bioelectrochemical systems. Bioresource Technol. 2020;309:123398. 10.1016/j.biortech.2020.123398. [DOI] [PubMed] [Google Scholar]
- 71.Passarini MRZ, Ottoni JR, Costa PES, Hissa DC, Falcão RM, Melo VMM, Balbino VQ, Mendonça LAR, Lima MGS, Coutinho HDM, Verde LCL. Fungal community diversity of heavy metal contaminated soils revealed by metagenomics. Arch Microbiol. 2022;204:255. 10.1007/s00203-022-02860-7. [DOI] [PubMed] [Google Scholar]
- 72.Shah T, Munsif FD, Amato R, Nie L. Lead toxicity induced phytotoxic impacts on rapeseed and clover can be lowered by biofilm forming lead tolerant bacteria. Chemosphere. 2020;246:125766. 10.1016/j.chemosphere.2019.125766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the NCBI repository [https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1119661].