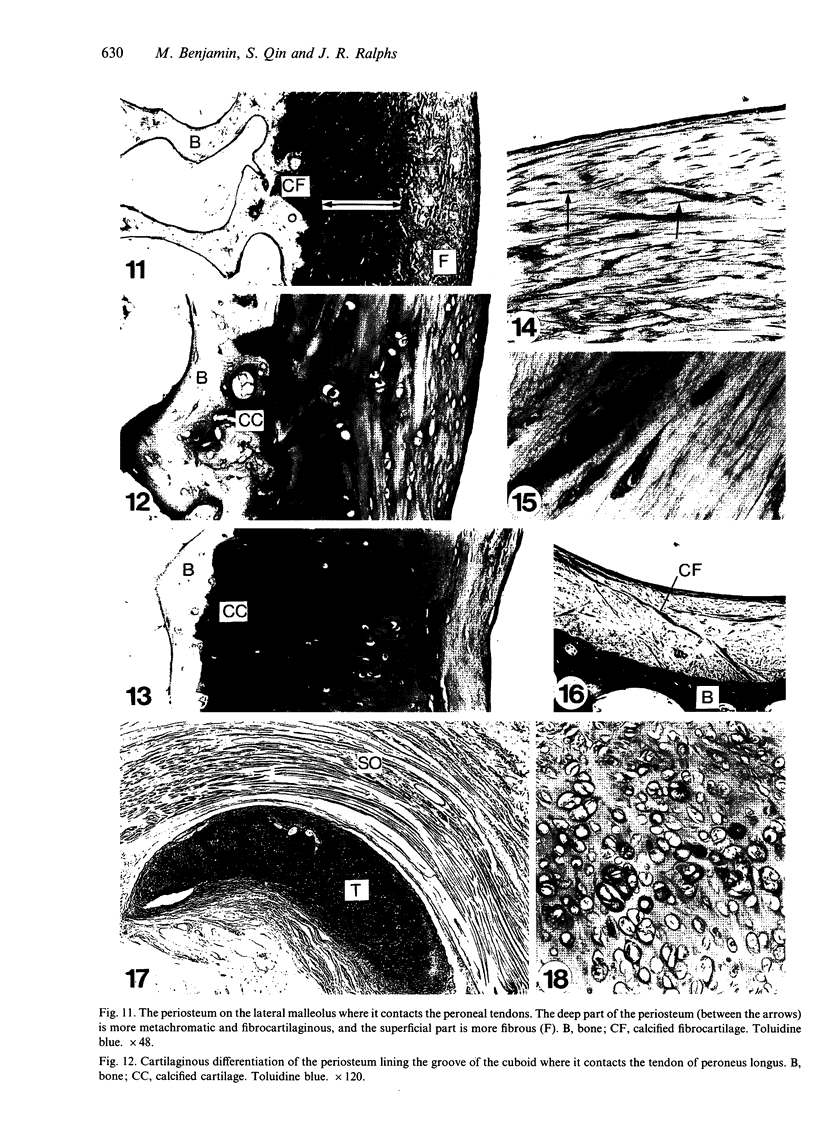
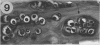Abstract
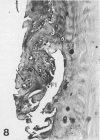
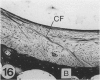
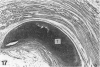
The presence of fibrocartilage in tendons that wrap around bony or fibrous pulleys is well known. It is an adaptation to resisting compression or shear, but the extent to which the structure of most human tendons is modified where they contact pulleys is less clear, for there has been no single comprehensive survey of a large number of sites. Less is known of the structure of the corresponding pulleys. In the present study, 38 regions of tendons that wrap around bony pulleys or pass beneath fibrous retinacula have been studied in routine histology sections taken from each of 2 or 3 elderly dissecting room cadavers. Most of the corresponding pulleys have also been examined. Fibrocartilage was present in 22 of the 38 tendon sites and it was most conspicuous where the tendons pressed predominantly against bone rather than retinacula and where they showed a large change in direction. Fibrocartilage was more characteristic of tendons at the ankle than the wrist, probably because the long axis of the foot is at right angles to that of the leg. There was considerable variation in the structure of tendon fibrocartilage. The most fibrocartilaginous tendons had oval or round cells embedded in a highly metachromatic matrix with interwoven or spiralling collagen fibres. At other sites, fibrocartilage cells were arranged in rows between parallel collagen fibres. The differences probably relate to differences in development. A single tendon could be modified at successive points along its length and fibrocartilage could be present in the endotenon and epitenon as well as in the tendon itself. Pathological changes seen in 'wrap around' tendons were fragmentation and partial delamination of the compressed surface, chondrocyte clustering, fatty infiltration and bone formation. Three types of pulleys were described for tendons--bony prominences and grooves, fibrous retinacula and synovial joints. The extent of cartilaginous differentiation on the periosteum of bony pulleys frequently mirrored that in the corresponding tendon. The cartilage or fibrocartilage prevents the tendon from 'sawing' through the bone. Some of the best known retinacula were largely fibrous, though the inferior peroneal retinaculum and the trochlea for the superior oblique were cartilaginous. The results underline the considerable regional heterogeneity in different tendons and their pulleys. They show that one tendon is not like another and that tendons may need to be carefully selected for particular surgical transfers or joint reconstructions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
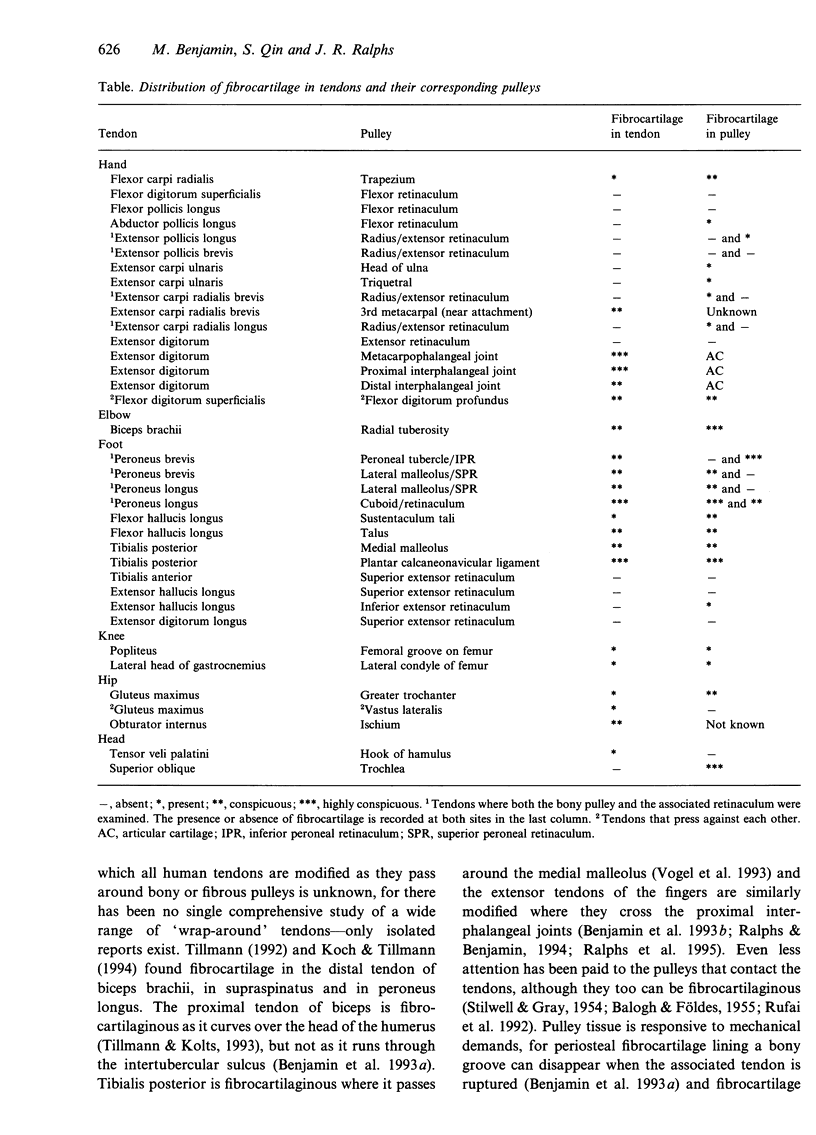
- Amiel D., Frank C., Harwood F., Fronek J., Akeson W. Tendons and ligaments: a morphological and biochemical comparison. J Orthop Res. 1984;1(3):257–265. doi: 10.1002/jor.1100010305. [DOI] [PubMed] [Google Scholar]
- BALOGH G., FOLDES I. Die funktionelle Gewebestrukture der Sehnenfurchen. Acta Morphol Acad Sci Hung. 1955;5(3-4):355–368. [PubMed] [Google Scholar]
- Benjamin M., Ralphs J. R., Newell R. L., Evans E. J. Loss of the fibrocartilaginous lining of the intertubercular sulcus associated with rupture of the tendon of the long head of biceps brachii. J Anat. 1993 Apr;182(Pt 2):281–285. [PMC free article] [PubMed] [Google Scholar]
- Evanko S. P., Vogel K. G. Ultrastructure and proteoglycan composition in the developing fibrocartilaginous region of bovine tendon. Matrix. 1990 Dec;10(6):420–436. doi: 10.1016/s0934-8832(11)80150-2. [DOI] [PubMed] [Google Scholar]
- Gillard G. C., Reilly H. C., Bell-Booth P. G., Flint M. H. The influence of mechanical forces on the glycosaminoglycan content of the rabbit flexor digitorum profundus tendon. Connect Tissue Res. 1979;7(1):37–46. doi: 10.3109/03008207909152351. [DOI] [PubMed] [Google Scholar]
- Greenlee T. K., Jr, Beckham C., Pike D. A fine structural study of the development of the chick flexor digital tendon: a model for synovial sheathed tendon healing. Am J Anat. 1975 Jul;143(3):303–313. doi: 10.1002/aja.1001430304. [DOI] [PubMed] [Google Scholar]
- Harwood F. L., Amiel D. Differential metabolic responses of periarticular ligaments and tendon to joint immobilization. J Appl Physiol (1985) 1992 May;72(5):1687–1691. doi: 10.1152/jappl.1992.72.5.1687. [DOI] [PubMed] [Google Scholar]
- Kannus P., Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991 Dec;73(10):1507–1525. [PubMed] [Google Scholar]
- Koob T. J., Clark P. E., Hernandez D. J., Thurmond F. A., Vogel K. G. Compression loading in vitro regulates proteoglycan synthesis by tendon fibrocartilage. Arch Biochem Biophys. 1992 Oct;298(1):303–312. doi: 10.1016/0003-9861(92)90127-i. [DOI] [PubMed] [Google Scholar]
- Merrilees M. J., Flint M. H. Ultrastructural study of tension and pressure zones in a rabbit flexor tendon. Am J Anat. 1980 Jan;157(1):87–106. doi: 10.1002/aja.1001570109. [DOI] [PubMed] [Google Scholar]
- Ralphs J. R., Benjamin M. The joint capsule: structure, composition, ageing and disease. J Anat. 1994 Jun;184(Pt 3):503–509. [PMC free article] [PubMed] [Google Scholar]
- Ralphs J. R., Tyers R. N., Benjamin M. Development of functionally distinct fibrocartilages at two sites in the quadriceps tendon of the rat: the suprapatella and the attachment to the patella. Anat Embryol (Berl) 1992;185(2):181–187. doi: 10.1007/BF00185920. [DOI] [PubMed] [Google Scholar]
- Robbins J. R., Vogel K. G. Regional expression of mRNA for proteoglycans and collagen in tendon. Eur J Cell Biol. 1994 Aug;64(2):264–270. [PubMed] [Google Scholar]
- Rufai A., Benjamin M., Ralphs J. R. Development and ageing of phenotypically distinct fibrocartilages associated with the rat Achilles tendon. Anat Embryol (Berl) 1992 Dec;186(6):611–618. doi: 10.1007/BF00186984. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Koob T. J. Structural specialization in tendons under compression. Int Rev Cytol. 1989;115:267–293. doi: 10.1016/s0074-7696(08)60632-4. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Ordög A., Pogány G., Oláh J. Proteoglycans in the compressed region of human tibialis posterior tendon and in ligaments. J Orthop Res. 1993 Jan;11(1):68–77. doi: 10.1002/jor.1100110109. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Sandy J. D., Pogány G., Robbins J. R. Aggrecan in bovine tendon. Matrix Biol. 1994 Mar;14(2):171–179. doi: 10.1016/0945-053x(94)90006-x. [DOI] [PubMed] [Google Scholar]