Abstract
Malignant proliferating trichilemmal tumors (MPTTs), arising from the external root sheath of hair follicles, are exceptionally rare, with limited documentation of their genetic alterations. We present a case of a 64-year-old African American woman who initially presented with a gradually enlarging nodule on her posterior scalp. An initial biopsy at an outside hospital suggested metastatic adenocarcinoma or squamous cell carcinoma (SCC) of an uncertain origin. A subsequent wide local excision revealed a 2.0 cm tumor demonstrating characteristic trichilemmal keratinization, characterized by an abrupt transition from the nucleated epithelium to a laminated keratinized layer, confirming MPTT. Immunohistochemistry demonstrated diffuse p53 expression, patchy CD 34 expression, focal HER2 membranous expression, and patchy p16 staining (negative HPV ISH). A molecular analysis identified TP53 mutation and amplifications in the ERBB2 (HER2), BRD4, and TYMS. Additional gene mutations of uncertain significance included HSPH1, ATM, PDCD1 (PD-1), BARD1, MSH3, LRP1B, KMT2C (MLL3), GNA11, and RUNX1. Assessments for the homologous recombination deficiency, PD-L1 expression, gene rearrangement, altered splicing, and DNA mismatch repair gene expression were negative. The confirmation of ERBB2 (HER2) amplification in the MPTT through a molecular analysis suggests potential therapeutic avenues involving anti-HER2 monoclonal antibodies. The presence of the TP53 mutation, without the concurrent gene mutations typically observed in SCC, significantly aided in this differential diagnosis.
Keywords: malignant proliferating trichilemmal tumors (MPTT), CD34, p53, HER2
1. Introduction
Malignant proliferating trichilemmal tumors (MPTTs) are uncommon malignant dermal neoplasms that arise from the external root sheath of hair follicles [1]. They typically manifest as solitary, painless masses on the scalps of elderly women, often presenting with ulceration, necrosis, or hemorrhage [1,2].
Under microscopic examination, the tumor displays a lobulated growth pattern with clusters of proliferating atypical epithelium showing trichilemmal differentiation, characterized by extensive trichilemmal keratinization and extension into adjacent tissues. MPTTs have the potential to exhibit aggressive behavior, including local invasion, recurrence, and metastasis. The tumor can be mistakenly diagnosed as squamous cell carcinoma (SCC). Distinguishing features, such as a history of slow tumor growth, the presence of trichilemmal keratinization, and findings from immunohistochemistry, are often used in the differential diagnosis.
The genetic alterations associated with MPTTs are poorly documented in the literature, which hinders the comprehensive characterization of their behavior, the prediction of clinical outcomes, and the development of targeted therapies. In this study, we present a case of MPTT with molecular analysis and provide a literature review to enhance the understanding of this rare condition.
2. Case Report
2.1. Clinical Presentation
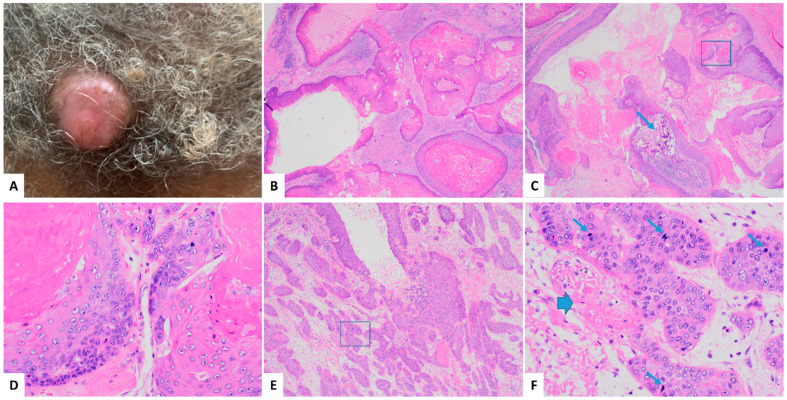
The patient, a 64-year-old African American woman, noticed a slowly growing nodule on her posterior scalp over one year (Figure 1A). A biopsy conducted at another hospital initially suggested metastatic adenocarcinoma or squamous cell carcinoma (SCC) of an uncertain origin. She subsequently presented to our institution for further evaluation. A computed tomography (CT) scan revealed a 2.2 cm exophytic, partially calcified, heterogeneous soft tissue mass on the left occipital scalp, adjacent to the local left suboccipital musculature without bone invasion. The patient underwent wide local excision with 2 cm margins and limited lymph node dissection. Pathological examination confirmed MPTT with clear margins. Two months later, she completed a 5-week course of radiation therapy. At the 10-month follow-up, the patient remained disease-free.
Figure 1.
Clinical presentation and histologic examination of malignant proliferating trichilemmal tumors (MPTT) on a hematoxylin and eosin stain (H and E). The tumor presented as a 2.0 cm mass on the left occipital scalp (A). Microscopically, a histological examination revealed a solid and cystic dermal neoplasm, with smaller cystic spaces (B) 20X and a larger cyst exhibiting infolding bands of tumor cells with calcification indicated by an arrow (C) 20X. The tumor displayed an abrupt transition from the nucleated epithelium to a densely laminated keratinized layer without an intermediate granular layer (D) higher magnification of the squared area in (C) 200X. There were areas with invasive irregular tumor nests in the desmoplastic stroma, composed of nonkeratinizing tumor cells (E) 40X, showing moderate nuclear pleomorphism, frequent mitoses (indicated by narrow arrows), and occasional necrosis (indicated by wide arrow) (F) higher magnification of the squared area in (E) 200X.
2.2. Histopathologic Findings
The wide local excision specimen consisted of a skin ellipse measuring 6.5 × 6 × 1.5 cm, containing a central exophytic mass measuring 2 × 2 × 1.5 cm. The cut surface revealed a solid and cystic mass located 2.0 cm from the closest peripheral margin and 0.3 cm from the nearest deep margin. A histological examination revealed a solid and cystic dermal neoplasm (Figure 1B,C) showing characteristic trichilemmal keratinization, characterized by an abrupt transition from a nucleated epithelium to a densely laminated keratinized layer without an intermediate granular layer (Figure 1D). Areas with invasive tumor nests composed of nonkeratinizing tumor cells were observed (Figure 1E), displaying moderate nuclear pleomorphism, frequent mitoses, and occasional necrosis (Figure 1F). The findings of an invasive nonkeratinizing component in the tumor, characterized by nuclear pleomorphism, frequent mitoses, and necrosis, suggest a diagnosis of malignancy.
Immunohistochemistry (IHC) was performed using prediluted antibodies provided by the vendors. A standard antigen retrieval method was employed for the antibodies on the BenchMark Ultra automated IHC system. Specifically, Ventana’s Cell Conditioning Solution 1 (CC1), a pre-diluted Tris-based buffer provided by Ventana, was used as the antigen retrieval solution. This solution was applied at a high temperature (approximately 100 °C) on the automated slide stainer to effectively unmask the target antigens within the tissue prior to staining. Additional details about the antibodies are provided in Table 1.
Table 1.
Monoclonal antibodies utilized in immunohistochemical studies in the current MPTT case.
| Antibody (Monoclonal) | CK17 | P53 | CD34 | Ki-67 | HER2 | P16 |
|---|---|---|---|---|---|---|
| Clone | SP95 | Bp53-11 | QBEnd/10 | 30-9 | 4B5 | E6H4 |
| Vender | Ventana /Roche |
Ventana /Roche |
Cell Marque | Ventana /Roche |
Ventana /Roche |
Ventana /Roche |
| Catalog # | 790-4560 | 760-2542 | 134M-18 | 790-4286 | 790-7167 | 805-4713 |
| Species | Rabbit | Mouse | Mouse | Rabbit | Rabbit | Mouse |
#: Number.
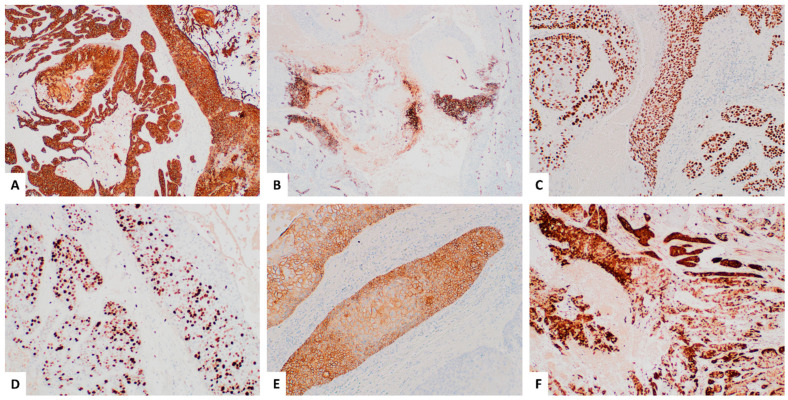
The tumor demonstrated a diffuse membranous and cytoplasmic expression of CK17 (Figure 2A) and a diffuse nuclear expression of p53 (Figure 2C), along with patchy membranous positivity for CD34 (Figure 2B). The Ki-67 proliferative index was approximately 30% in the most active areas (Figure 2D). Focal HER2 overexpression, characterized by a complete membranous staining pattern, was assessed using breast cancer criteria (Figure 2E). HER2 FISH was positive. Additionally, focal/patchy p16 staining was observed (Figure 2F), while high risk HPV RNA in situ hybridization was negative. The diagnosis of MPTT was confirmed, with no evidence of lymph node involvement.
Figure 2.
Immunohistochemistry (IHC) of the malignant proliferating trichilemmal tumor. IHC revealed diffuse expression of CK17 (A) 40X and p53 (C) 100X in the tumor, along with patchy positivity for CD34 (B) 40X. The Ki-67 proliferative index was approximately 30% in the hottest spots (D) 100X. Focal HER2 overexpression with a complete membranous staining pattern was observed (E) 100X. Additionally, patchy p16 staining was noted (F) 40X, whereas high risk HPV RNA in situ hybridization was negative.
2.3. Molecular Study
Molecular analysis was conducted as send-out tests, performed and interpreted by TEMPUS. The assay description from the report is as follows: “The Tempus xT (version 4) assay is a custom oncology testing panel consisting of 648 genes, with single nucleotide variants (SNVs), insertions and deletions (indels), copy number variants (CNVs), and chromosomal rearrangements (translocations) detected by hybrid capture next-generation sequencing (NGS) using custom-designed IDT probes.” The main genomic variants detected in the current case are summarized in Table 2.
Table 2.
The main genomic variants detected in the current case of MPTT.
| Clinical Summary | Gene | Alteration/Mutation Effect |
|---|---|---|
| Potentially Actionable | ERBB2 (HER2) | Copy number gain |
| Biologically Relevant | TP53 | c.527G > T p.C176F NM_000546 Missense variant—loss of funcgtion (LOF) |
| BRD4 | Copy number gain | |
| TYMS | Copy number gain | |
| Unknown Significance | HSPH1 | c.2348G > T p.R783L Missense variant NM_001286504 |
| ATM | c.8938C > G p.L2980V Missense variant NM_000051 | |
| PDCD1 (PD-1) | c.715G > A p.V239M Missense variant NM_005018 | |
| BARD1 | c.1339C > A p.L447I Missense variant NM_000465 | |
| MSH3 | c.177_178ins(18) p.A59_A60insPPAPPA Inframe insertion NM_002439 | |
| LRP1B | c.10414G > A p.D3472N Splice region variant NM_018557 | |
| KMT2C (MLL3) c | c.7443-2dup Splice region variant NM_170606 | |
| GNA11 | c.735+1_736-1del Splice region variant NM_002067 | |
| RUNX1 | c.1265A > C p.E422A Missense variant NM_001754 |
NGS molecular analysis unveiled the genetic profile of the tumor, revealing a TP53 mutation (p.C176F missense variant) and amplifications in ERBB2 (HER2), BRD4, and TYMS. Additional gene mutations of uncertain significance included HSPH1, ATM, PDCD1 (PD-1), BARD1, MSH3, LRP1B, KMT2C (MLL3), GNA11, and RUNX1. Further evaluations for homologous recombination deficiency (HRD), PD-L1 expression, gene rearrangement, and altered splicing via RNA sequencing yielded negative results. Notably, there was no loss of expression detected in the DNA mismatch repair genes (MLH1, PMS2, MSH2, and MSH6).
3. Literature Review
A PubMed literature review spanning 24 years (from 2000, with expectations for a comparison of molecular results) summarized in Table 3, documented 41 studies on MPTTs of the scalp, involving 60 patients, including our case, aged 19–87 years (average of 57), with the majority being female (71.2%, 42/59; gender data missing in one study). The duration of MPTTs ranged widely from one month to 40 years, with the tumor sizes varying from 1.0 to 30.0 cm. A clinical follow-up was available for 78.3% (47/60) of the patients. The local recurrence rate was 21.7% (13/60). Metastases occurred in 23.3% (14/60) of the cases, involving lymph nodes and/or other sites, with the lymph nodes being the most common metastatic site (64.3%, 9/14 cases). The metastatic sites also included the brain, the base of the skull, the cistern sinus, the lung, the pleura, and the pancreas. The mortality rate was 11.7% (7/60). Notably, none of the previous reports included a molecular study.
Table 3.
Literature review of 41 studies on malignant proliferating trichilemmal tumor of the scalp covering 60 cases, including the current study, over the past 24 years (2000–2024) in PubMed.
| Year [Ref #] |
Age | Case # /Sex (F/M) |
Duration (mo, y) | Size (cm) |
Local Recur (#) | Metastasis /Site (#) |
F-U (mo, y) (#) |
|---|---|---|---|---|---|---|---|
| 2000 [3] | 61 | 1/F | 20 y | 16.0 | NA | Brain | 12 mo/died |
| 2001 [4,5] | 51 | 1/F | 6 y | 1.2 | NA | None | NA |
| 32 | 1/M | 1 y | 8.0 | None | LN, Brain | 6 mo | |
| 2002 [6] | 60 | 1/F | 4 y | 5.0 | NA | None | L to F-U |
| 2003 [7] | 69 | 1/F | 2 y | 2.0 | Recur | LN | 8 mo |
| 2004 [8] | 41–87 | 11/F, 3/M | 1 mo–20 y | 1.0–9.0 | Recur (3) | LN (1) | 3 mo–8.5 y Died (2) Ϯ |
| 2005 [9] | 33 | 1/M | 1 y | 10.0 | None | None | ~1.5 y |
| 2006 [10] | 54 | 1/F | 3 y | 3.0 | None | None | 2 y |
| 2007 [11,12] | 32 | 1/F | NA | 12 | Recur | LN | 4 mo |
| 19 | 1/M | NA | 5.0 | Recur | BOS, CS, Lung | 5 y/died | |
| 50 | 1F | 3 mo | 6.7 | NA | LN | NA | |
| 2008 [13,14,15] | 72 | 1F | 10 y | 2.0 (recur) | Recur | None | 32 mo |
| 32 | 1M | 1 y | 2.5 | None | LN | 9 mo/died Ϯ | |
| 76 | 1F | Recent | 2.0 | None | None | 11 mo | |
| 2009 [16,17] | 85 | 1F | 1 y | 15.0 | None | None | 14 mo |
| 58 | 1F | 2 mo | 5.5 | NA | None | L to F-U | |
| 2010 [18,19] | 58 | 1F | 1 y | 3.2 | NA | None | NA |
| 41 | 1F | 1 y | NA | NA | None | NA | |
| 51 | 1F | NA | 16 | Recur | None | 54 mo | |
| 53 | 1M | Rapid | 20 | Recur | Brain | 9 mo/died | |
| 2011 [20,21] | 65 | 1F | 9 y | 2.0 | None | None | 6 mo |
| 57 | 1F | Many y | 7.0 | NA | None | L to F-U | |
| 2012 [22,23] | 73 | 1F | 3 y | 6.0 | NA | None | L to F-U |
| 25 | 1F | 1 y | 4.0 | None | None | 1 y | |
| 2013 [24] | 62 | 1F | NA | NA | None | Left pleura | 3 y |
| 2014 [25,26,27] | 65 | 1F | 4 mo | 3.0 | NA | None | NA |
| 32 | 1M | 8 mo | 15.0 | NA | None | F-U | |
| 26 | 1F | 1 y | 6.0 | Recur | LN | 1 mo | |
| 2015 [28,29,30] | 67 | 1M | 3 mo | 3.0 | None | None | 6 mo |
| 48 | 1F | NA | 4.0 (recur) | Recur | None | ~2 y | |
| 65 | 1M | 40 y | NA | None | LN, Pancreas | 15 mo | |
| 2016 [31,32,33] | 61 | 1M | 15 y | 15.0 | NA | None | L to F-U |
| 29 | 1M | 7 y | 30.0 | NA | Lung | 2 mo/died | |
| 42 | 1M | 11 y | 22.0 | Recur | None | 28 mo | |
| 2017 [34] | 64 | 1F | 18 mo | 12.0 | None | None | 3 mo |
| 2018 [35] | 56 | 1F | 1 y | 1.8 | Recur | None | 6 mo |
| 2019 [36] | 68 | 1F | 20 y | ~15.0 | NA | None | NA |
| 2020 [37] | Old | 2F | NA | NA | None | None | 2 y |
| 2021 [38] | 46 | 1F | Many y | 1.5 | NA | None | F-U |
| 2022 [39,40] | 85 | 1M | 2 y | 6.8 | None | None | 7 mo |
| 69 | 1M | 1 y | 2.0 | Recur | LN, Lung | 26 mo/died | |
| 55 | 1F | 1.5 y | 11.0 | None | None | 10 mo | |
| NA | 1 (NA) | NA | 10.0 | Recur | None | 2 mo/died | |
| 2023 [41] | 86 | 1F | Several y | 4.0 | None | None | 2 y |
| 2024 [42] | 87 | 1M | NA | 6.0 | None | None | 6 mo |
| Current c | 64 | 1F | 1 y | 2.0 | None | None | 10 mo |
Ref: reference; #: number of cases; F: female; M: male; mo: month(s); y: year(s); cm: centimeter(s); NA: not available; LN: lymph node; BOS: base of skull; CS: cistern sinus; L to F-U: lost to follow-up; C: case. Ϯ Total two patients died of diseases other than MPTT.
4. Discussion
A previous literature review on MPTTs of the scalp reported an average patient age of 55 years, ranging from 26 to 85 years [2]. In our study of MPTTs of the scalp, the average age at presentation was 57 years, ranging from 19 to 87 years. The youngest MPTT patient, aged 19, had Keratitis–Ichthyosis–Deafness (KID) syndrome, a rare ectodermal dysplasia [8]. Females constituted the majority of the patients at 71.2%, consistent with previous studies [2,16]. The tumor sizes ranged from 1.0 to 30.0 cm in our study, compared to a range of 1–28 cm reported previously [2]. The duration of the MPTTs varied widely from 1 month to 40 years in our current study, whereas prior findings indicated a range of 1 to 30 years [16].
MPTTs of the scalp present a broad spectrum of differential diagnoses clinically, ranging from benign conditions such as trichilemmal cysts, pilomatricomas, and trichoepitheliomas to malignant entities like squamous cell carcinoma (SCC), basal cell carcinoma, and trichilemmal carcinoma. Clinically, MPTTs are most frequently mistaken for SCC. However, distinguishing MPTTs from SCC is critical, as MPTTs exhibit a significantly higher potential for local destruction and metastasis [2].
According to the WHO Classification of Tumors (5th edition, online version), MPTT falls within the morphological spectrum of PTT, which includes benign, atypical (intermediate), and rare malignant lesions [43]. MPTTs often arise from the transformation of a benign PTT and display aggressive clinical behavior and atypical histological features. Key differentiating characteristics include a rapidly enlarging mass, necrosis or hemorrhage, local invasion, or evidence of bone erosion on imaging. Early imaging studies, such as contrast-enhanced CT or, in some cases, magnetic resonance imaging (MRI), are essential for evaluating the extent of bone involvement and tumor invasion. Additionally, positron emission tomography (PET) can be employed to detect metastatic disease and assess the full extent of the tumor spread [2].
Histologically, MPTTs exhibit histopathologic features akin to a trichilemmal cyst, characterized by epithelial infoldings within cystic spaces [1].Tumor cells demonstrate abrupt differentiation towards large, monomorphic keratinocytes without a granular layer. Distinguishing MPTT from SCC can be challenging in areas with malignant features. The immunostains for CD34, p53, and Ki-67 have been documented to assist in MPTT diagnosis [16,18,20,33,44]. Four articles referenced the use of Ki-67 [9,20,28,39], with two reporting that MPTT demonstrated a 20% proliferative index labeled by Ki-67, supporting the diagnosis of malignancy [20,39]. We observed a proliferative index of 30% in the hottest spots. In our case, MPTT showed CK17 positivity, an epithelial marker. The tumor displayed focal or patchy CD34 immunoreactivity, suggesting trichilemmal differentiation from the outer root sheath, with most of the cells negative for CD34. Herrero et al. proposed that CD34 negativity could indicate tumor cell undifferentiation [43]. Therefore, CD34 expression aids in distinguishing MPTT from SCC. P53 overexpression and a high proliferative index labeled by Ki-67 confirm tumor malignancy, excluding diagnoses such as PTT and trichilemmal cysts. In this case, focal HER2 expression with complete membranous staining was noted. HER2 FISH was positive. Patchy but very focal strong p16 expression poses a diagnostic challenge, potentially leading to HPV-mediated SCC misdiagnosis in small biopsies.
Our molecular study identified amplifications in ERBB2 (HER2), BRD4 (Bromodomain protein 4), and TYMS (Thymidylate synthase). The ERBB2 amplification corroborates the IHC findings of HER2 overexpression, suggesting potential benefits of targeted HER2 therapies in MPTT using anti-HER2 monoclonal antibodies. BRD4, known for its frequent overexpression and role in drug resistance, has been proposed as a therapeutic target [45]. The amplification of TYMS, indicative of increased thymidylate synthase activity, may contribute to resistance against TYMS inhibitors, providing crucial insights for treatment planning [46].
In this case, a molecular analysis also detected a TP53 mutation, specifically the p.C176F missense variant located in exon 4 of TP53, characterized by a G to T substitution at nucleotide position 527. This mutation is distinct from previously reported mutations, such as the point mutation (CGT > CAT) at codon 273 in exon 8 of the TP53 gene observed in scalp MPTTs [44]. The presence of this TP53 mutation in MPTT, alongside the absence of the mutations commonly found in SCC such as CDKN2A, PIK3CA, KMT2D, and NOTCH1 [47], significantly contributed to the differential diagnosis.
Additional gene mutations of uncertain significance included HSPH1, ATM, PDCD1 (PD-1), BARD1, MSH3, LRP1B, KMT2C (MLL3), GNA11, and RUNX1. Although their specific roles in this tumor type are not fully understood, these mutations have the potential to influence the tumor’s pathogenesis and may affect its response to treatment.
Surgical management remains the cornerstone of treatment for MPTTs, complemented by radiotherapy and chemotherapy as adjuvant therapies in cases of aggressiveness or recurrence. Our study observed a local recurrence rate of 21.7%, consistent with the 24% reported in prior analyses [2]. Metastasis occurred in 23.3% of cases, predominantly affecting the lymph nodes, with additional sites including the brain, the base of the skull, the cistern sinus, the lung, pleura, and the pancreas, indicating both the lymphatic and hematogenous spread of MPTTs. The mortality rate was 11.7%, predominantly associated with cases showing recurrence and/or metastasis. Ye et al. established histologic criteria categorizing PPTs into three subgroups, facilitating the assessment of tumor behavior [8]. Benign PPTs often respond well to simple excision, while low-grade and high-grade MPTTs may necessitate more extensive surgical intervention and additional therapies [8]. Several chemotherapeutic regimens were used with limited success, with the CAV protocol (cisplatin, adriamycin, and vindesine) being the most commonly administered [2]. However, there is a potential risk of developing squamous cell carcinoma (SCC) due to malignant transformation induced by a high-dose chemotherapy regimen [2].
In summary, our study presents a case of MPTT of the scalp in an elderly woman, accompanied by a comprehensive literature review, detailed immunostain analysis, and genetic profiling. The identification of HER2 overexpression via IHC, confirmed by ERBB2 (HER2) amplification using FISH and NGS, suggests the potential therapeutic benefits of anti-HER2 monoclonal antibodies in treating aggressive MPTTs. The presence of TP53 mutation, distinct from the gene mutations typical of SCC, significantly aids in differential diagnosis. The amplification of BRD4 and TYMS, alongside mutations in other genes of uncertain significance, may contribute to understanding MPTT pathogenesis and guide future treatment strategies. Importantly, our molecular analysis of MPTT marks the first documented instance in the literature.
Although we present molecular analysis findings on MPTT, our data were derived from a single case, which limits the generalizability of the conclusions. Further molecular studies involving larger case series are essential to deepen our understanding of the molecular pathogenesis of MPTT. The mortality rate of this tumor, as previously mentioned, is 11.7%, emphasizing the clinical significance of addressing its aggressive behavior. Reducing the rates of recurrence and metastasis is crucial to improving patient outcomes and lowering the mortality associated with this tumor. A comprehensive molecular analysis may not only provide insights into the underlying mechanisms driving tumor progression but also identify potential therapeutic targets, paving the way for the development of targeted treatments.
Author Contributions
M.H.A. and Y.X.: conception of the work and design of the work. M.H.A., H.S., A.H.D., C.J.F., A.D.H., D.J.H. and Y.X.: acquisition of data, analysis of data, and interpretation of data. M.H.A., H.S., A.H.D., C.J.F., A.D.H., D.J.H. and Y.X.: drafting the work, and revising the work critically for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Due to the inability to meet in person, the author received verbal consent from the patient for the article. Written consent was obtained after the article was explained to the patient, who agreed to allow one of the authors to act as her representative and sign the consent on her behalf.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This case report received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jones E.W. Proliferating epidermoid cysts. Arch. Dermatol. 1966;94:11–19. doi: 10.1001/archderm.1966.01600250017002. [DOI] [PubMed] [Google Scholar]
- 2.Osto M.B., Parry N.B., Rehman R.B., Ahmed U.B., Mehregan D. Malignant Proliferating Trichilemmal Tumor of the Scalp: A Systematic Review. Am. J. Dermatopathol. 2021;43:851–866. doi: 10.1097/DAD.0000000000001991. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y.C., Vandersteen D.P., Park H.J., Cinn Y.W. A case of giant proliferating trichilemmal tumor with malignant transformation. J. Dermatol. 2000;27:687–688. doi: 10.1111/j.1346-8138.2000.tb02257.x. [DOI] [PubMed] [Google Scholar]
- 4.Mathis E.D., Honningford J.B., Rodriguez H.E., Wind K.P., Connolly M.M., Podbielski F.J. Malignant proliferating trichilemmal tumor. Am. J. Clin. Oncol. 2001;24:351–353. doi: 10.1097/00000421-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Bae S.B., Lee K.K., Kim J.S., Lee J.H., Lee N.S., Lee G.T., Park S.K., Won J.H., Baick S.H., Hong D.S., et al. A case of malignant proliferating trichilemmoma of the scalp with multiple metastases. Korean J. Intern. Med. 2001;16:40–43. doi: 10.3904/kjim.2001.16.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sethi S., Singh U.R. Proliferating trichilemmal cyst: Report of two cases, one benign and the other malignant. J. Dermatol. 2002;29:214–220. doi: 10.1111/j.1346-8138.2002.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 7.Jung J., Bin Cho S., Yun M., Lee K.H., Chung K.Y. Metastatic malignant proliferating trichilemmal tumor detected by positron emission tomography. Dermatol. Surg. 2003;29:872–874. doi: 10.1046/j.1524-4725.2003.29237.x. [DOI] [PubMed] [Google Scholar]
- 8.Ye J., Nappi O., Swanson P.E., Patterson J.W., Wick M.R. Proliferating pilar tumors: A clinicopathologic study of 76 cases with a proposal for definition of benign and malignant variants. Am. J. Clin. Pathol. 2004;122:566–574. doi: 10.1309/0XLEGFQ64XYJU4G6. [DOI] [PubMed] [Google Scholar]
- 9.Tierney E., Ochoa M.-T., Rudkin G., Soriano T.T. Mohs’ micrographic surgery of a proliferating trichilemmal tumor in a young black man. Dermatol. Surg. 2005;31:359–363. doi: 10.1097/00042728-200503000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Filippou D.K., Filippou G., Trigka A., Condilis N., Kiparidou E., Skandalakis P., Rizos S. Malignant proliferating trichilemmal tumour of the scalp. Report of a case and a short review of the literature. Ann. Ital. Chir. 2006;77:179–181. [PubMed] [Google Scholar]
- 11.Nyquist G.G., Mumm C., Grau R., Crowson A.N., Shurman D.L., Benedetto P., Allen P., Lovelace K., Smith D.W., Frieden I., et al. Malignant proliferating pilar tumors arising in KID syndrome: A report of two patients. Am. J. Med. Genet. Part A. 2007;143A:734–741. doi: 10.1002/ajmg.a.31635. [DOI] [PubMed] [Google Scholar]
- 12.Budrukkar A., Siddha M., Shet T., Deshpande M., Basu A., Patil N., Bhalavat R. Malignant pilar tumor of the scalp: A case report and review of literature. J. Cancer Res. Ther. 2007;3:240–243. doi: 10.4103/0973-1482.39001. [DOI] [PubMed] [Google Scholar]
- 13.Cecchi R., Rapicano V., De Gaudio C. Malignant proliferating trichilemmal tumour of the scalp managed with micrographic surgery and sentinel lymph node biopsy. J. Eur. Acad. Dermatol. Venereol. 2008;22:1258–1259. doi: 10.1111/j.1468-3083.2008.02611.x. [DOI] [PubMed] [Google Scholar]
- 14.Nakai N., Takenaka H., Hamada S., Kishimoto S. Identical p53 gene mutation in malignant proliferating trichilemmal tumour of the scalp and small cell carcinoma of the common bile duct: The necessity for therapeutic caution? Br. J. Dermatol. 2008;159:482–485. doi: 10.1111/j.1365-2133.2008.08631.x. [DOI] [PubMed] [Google Scholar]
- 15.Trabelsi A., Stita W., Gharbi O., Kanani N., Sriha B., Korbi S. Malignant proliferating trichilemmal tumor of the scalp: A case report. Dermatol. Online J. 2008;14:11. doi: 10.5070/D343F7R58V. [DOI] [PubMed] [Google Scholar]
- 16.Garg P.K., Dangi A., Khurana N., Hadke N.S. Malignant proliferating trichilemmal cyst: A case report with review of literature. Malays. J. Pathol. 2009;31:71–76. [PubMed] [Google Scholar]
- 17.Kini J.R., Kini H. Fine-needle aspiration cytology in the diagnosis of malignant proliferating trichilemmal tumor: Report of a case and review of the literature. Diagn. Cytopathol. 2009;37:744–747. doi: 10.1002/dc.21100. [DOI] [PubMed] [Google Scholar]
- 18.Chaichamnan K., Satayasoontorn K., Puttanupaab S., Attainsee A. Malignant proliferating trichilemmal tumors with CD34 expression. J. Med. Assoc. Thai. 2010;93((Suppl. S6)):S28–S34. [PubMed] [Google Scholar]
- 19.Makiese O., Chibbaro S., Hamdi S., Mirone G., George B. Huge proliferating trichilemmal tumors of the scalp: Report of six cases. Plast. Reconstr. Surg. 2010;126:18e–19e. doi: 10.1097/PRS.0b013e3181dbc48e. [DOI] [PubMed] [Google Scholar]
- 20.Gulati H.K., Deshmukh S., Anand M., Morale V., Pande D.P., Jadhav S.E. Low-grade malignant proliferating pilar tumor simulating a squamous-cell carcinoma in an elderly female: A case report and immunohistochemical study. Int. J. Trichol. 2011;3:98–101. doi: 10.4103/0974-7753.90818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khaled A., Kourda M., Fazaa B., Kourda J., Ben Jilani S., Kamoun M.R., Zermani R. Malignant proliferating trichilemmal cyst of the scalp: Histological aspects and nosology. Pathologica. 2011;103:73–76. [PubMed] [Google Scholar]
- 22.Goyal S., Jain B.B., Jana S., Bhattacharya S.K. Malignant proliferating trichilemmal tumor. Indian J. Dermatol. 2012;57:50–52. doi: 10.4103/0019-5154.92679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma R., Verma P., Yadav P., Sharma S. Proliferating trichilemmal tumor of scalp: Benign or malignant, A dilemma. J. Cutan. Aesthetic Surg. 2012;5:213–215. doi: 10.4103/0974-2077.101394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katrancioglu O., Akkas Y., Akkas E.A., Cevit R., Kilickap S. Metastasis to pleura of malignant trichilemmal tumor. Respir. Case Rep. 2013;2:150–153. doi: 10.5505/respircase.2013.81300. [DOI] [Google Scholar]
- 25.Deshmukh B., Kulkarni M., Momin Y., Sulhyan K. Malignant proliferating trichilemmal tumor: A case report and review of literature. J. Cancer Res. Ther. 2014;10:767–769. doi: 10.4103/0973-1482.136036. [DOI] [PubMed] [Google Scholar]
- 26.Shetty P.K., Jagirdar S., Balaiah K., Shetty D. Malignant proliferating trichilemmal tumor in young male. Indian J. Surg. Oncol. 2014;5:43–45. doi: 10.1007/s13193-013-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubhashi S., Jadhav S., Parasnis A., Patil C. Recurrent malignant proliferating trichilemmal tumor with lymph node metastasis in a young woman. J. Postgrad. Med. 2014;60:400–402. doi: 10.4103/0022-3859.143973. [DOI] [PubMed] [Google Scholar]
- 28.Fieleke D.R., Goldstein G.D. Malignant proliferating trichilemmal tumor treated with Mohs surgery: Proposed protocol for diagnostic work-up and treatment. Dermatol. Surg. 2015;41:292–294. doi: 10.1097/DSS.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 29.Reddy L.G., Jayakumar V., Rajan S., Bilimagga R.S., Priyadarshini V., Anbumani S. Recurrent malignant proliferating trichilemmal tumour of the scalp: A case report. J. Radiother. Pract. 2015;14:307–310. doi: 10.1017/S1460396915000229. [DOI] [Google Scholar]
- 30.Trikudanathan G., Shaukat A., Bakman Y. Proliferating Pilar Tumor of Scalp Metastasizing to Pancreas: Diagnosis with Endoscopic Ultrasound-guided Fine-needle Aspiration. Clin. Gastroenterol. Hepatol. 2015;13:e164–e165. doi: 10.1016/j.cgh.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Morgado B., Agostini P., Rivero A., Silva N. Extensive and ulcerated malignant proliferating trichilemmal (pilar) tumour, arising from multiple, large, degenerated trichilemmal (pilar) cysts. BMJ Case Rep. 2016;2016:bcr2015209785. doi: 10.1136/bcr-2015-209785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lobo L., Amonkar A.D., Dontamsetty V.V. Malignant Proliferating Trichilemmal Tumour of the Scalp with Intra-Cranial Extension and Lung Metastasis-a Case Report. Indian J. Surg. 2016;78:493–495. doi: 10.1007/s12262-015-1427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X., Li W., Yuan H., Gu W., Chen D. Surgical treatment of rare giant malignant tumors of the scalp: A report of 3 cases with different tumor types. Oncol. Lett. 2016;12:3411–3416. doi: 10.3892/ol.2016.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naciri I., Hassam B. Une tumeur exophytique du cuir chevelu [Exophytic tumor of the scalp] Pan Afr. Med. J. 2017;28:45. doi: 10.11604/pamj.2017.28.45.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh P., Usman A., Motta L., Khan I. Malignant proliferating trichilemmal tumour. BMJ Case Rep. 2018:2018. doi: 10.1136/bcr-2018-224460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demirdag H., Serel S., Akay B., Kirmizi A., Heper A.O. Malignant proliferating trichilemmal tumour arising on a trichilemmal cyst with dermoscopic and histopathologic findings. J. Eur. Acad. Dermatol. Venereol. 2019;33:e322–e324. doi: 10.1111/jdv.15621. [DOI] [PubMed] [Google Scholar]
- 37.Rajbhar R., Anvikar A., Sulhyan K. Clinicopathological correlation of malignant skin tumors: A retrospective study of 5 years. Int. J. Health Sci. 2020;14:18–25. [PMC free article] [PubMed] [Google Scholar]
- 38.Joshi T.P., Marchand S., Tschen J. Malignant Proliferating Trichilemmal Tumor: A Subtle Presentation in an African American Woman and Review of Immunohistochemical Markers for This Rare Condition. Cureus. 2021;13:e17289. doi: 10.7759/cureus.17289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kemaloğlu C.A., Öztürk M., Aydın B., Canöz Ö., Eğilmez O. Malignant proliferating trichilemmal tumor of the scalp: Report of 4 cases and a short review of the literature. Case Rep. Plast. Surg. Hand Surg. 2022;9:158–164. doi: 10.1080/23320885.2022.2077208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamal I.B., Hali F., Marnissi F., Chiheb S. The Proliferating and Malignant Proliferating Trichilemmal Cyst: An Anatomo-Clinical Study of Three Cases. Ski. Appendage Disord. 2022;8:161–164. doi: 10.1159/000518354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu M., Liao C., Zhao Z., Zhou Z., Liu Y., Wang X., Zhang G. Local narrow margin excision sequential with modified ALA-PDT for successful treatment of an 86-year-old patient with malignant proliferating trichilemmal tumor. Photodiagn. Photodyn. Ther. 2023;42:103524. doi: 10.1016/j.pdpdt.2023.103524. [DOI] [PubMed] [Google Scholar]
- 42.Jia K., Feng L., Qian L.-L. Malignant proliferating trichilemmal tumor successfully treated with integrated traditional Chinese and Western medicine: A case report. Asian J. Surg. 2024;47:2399–2400. doi: 10.1016/j.asjsur.2024.01.053. [DOI] [PubMed] [Google Scholar]
- 43.WHO Classification of Tumor online, Skin Tumors (5th Edition, Online Version) [(accessed on 5 November 2024)]. Available online: https://tumourclassification.iarc.who.int/chaptercontent/64/143.
- 44.Herrero J., Monteagudo C., Ruiz A., Bosch L. Malignant proliferating trichilemmal tumours: An histopathological and immunohistochemical study of three cases with DNA ploidy and morphometric evaluation. Histopathology. 1998;33:542–546. doi: 10.1046/j.1365-2559.1998.00549.x. [DOI] [PubMed] [Google Scholar]
- 45.Yongprayoon V., Wattanakul N., Khomate W., Apithanangsiri N., Kasitipradit T., Nantajit D., Tavassoli M. Targeting BRD4: Potential therapeutic strategy for head and neck squamous cell carcinoma (Review) Oncol. Rep. 2024;51:74. doi: 10.3892/or.2024.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lima A., Azevedo R., Sousa H., Seabra V., Medeiros R. Current Approaches for TYMS Polymorphisms and their Importance in Molecular Epidemiology and Pharmacogenetics. Pharmacogenomics. 2013;14:1337–1351. doi: 10.2217/pgs.13.118. [DOI] [PubMed] [Google Scholar]
- 47.Chang D., Shain A.H. The landscape of driver mutations in cutaneous squamous cell carcinoma. NPJ Genom. Med. 2021;6:61. doi: 10.1038/s41525-021-00226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding authors.