Abstract
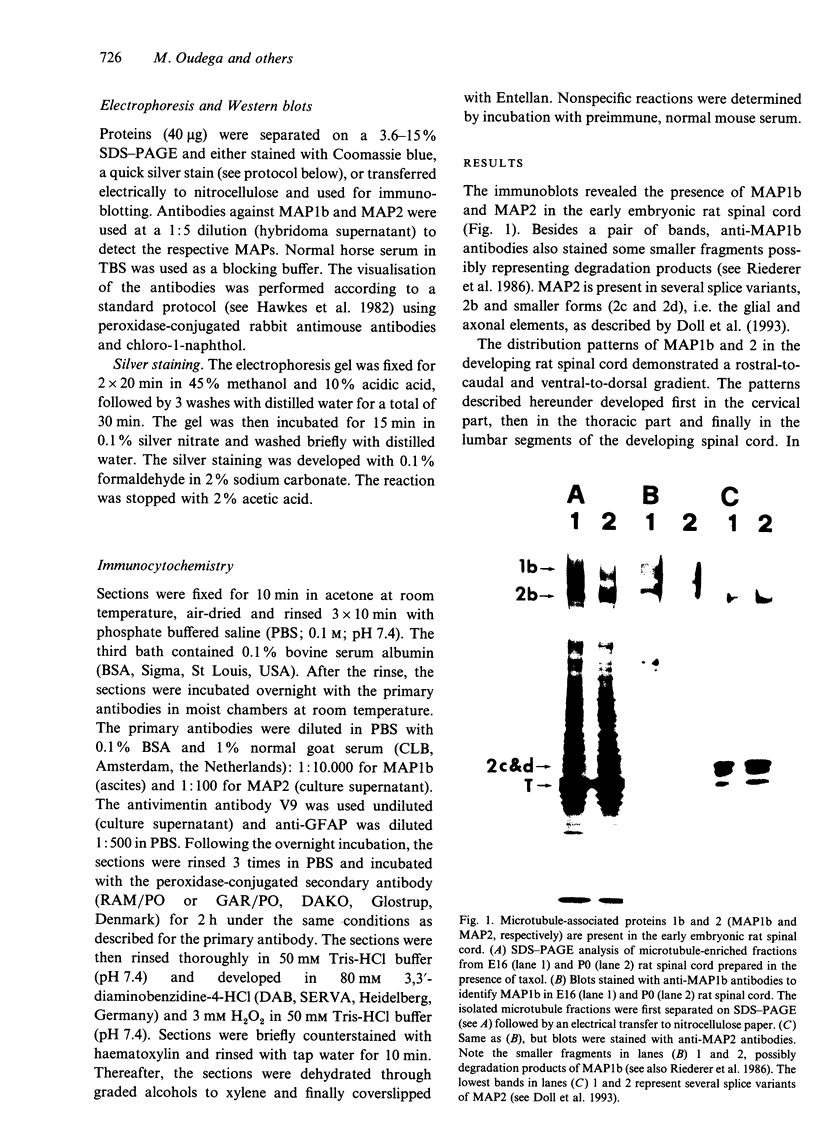
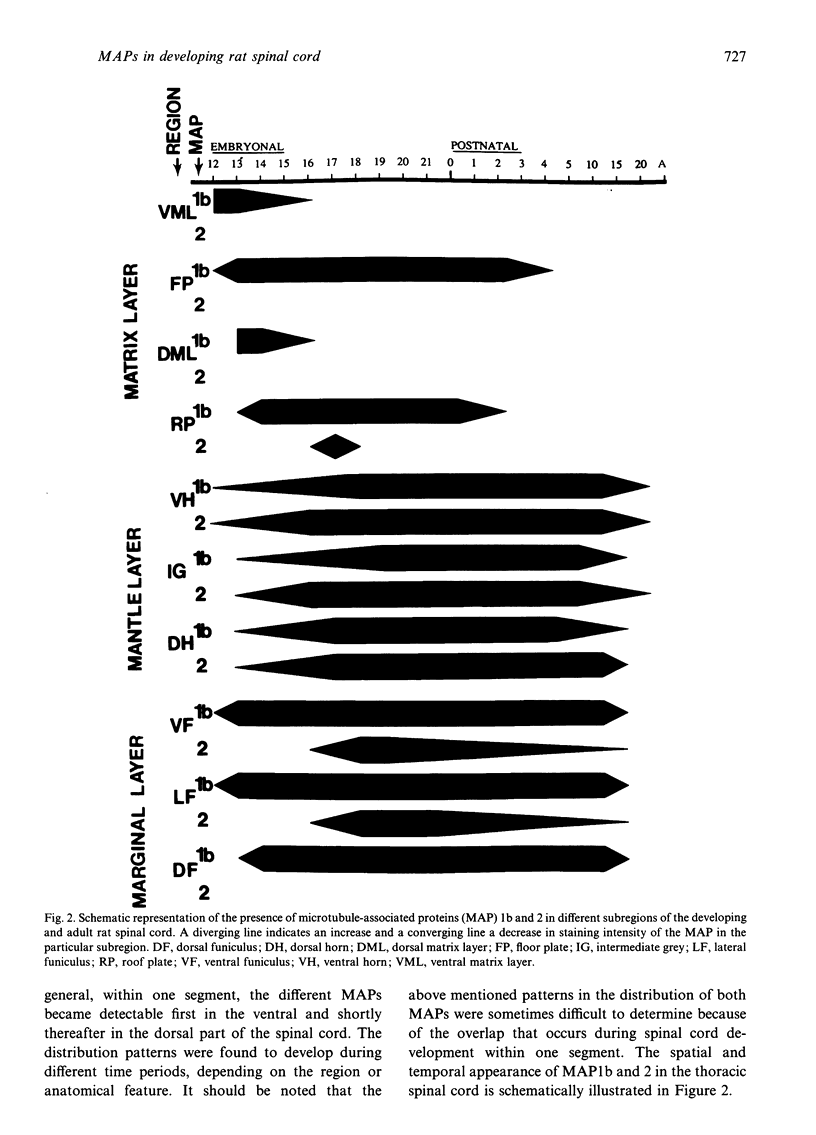
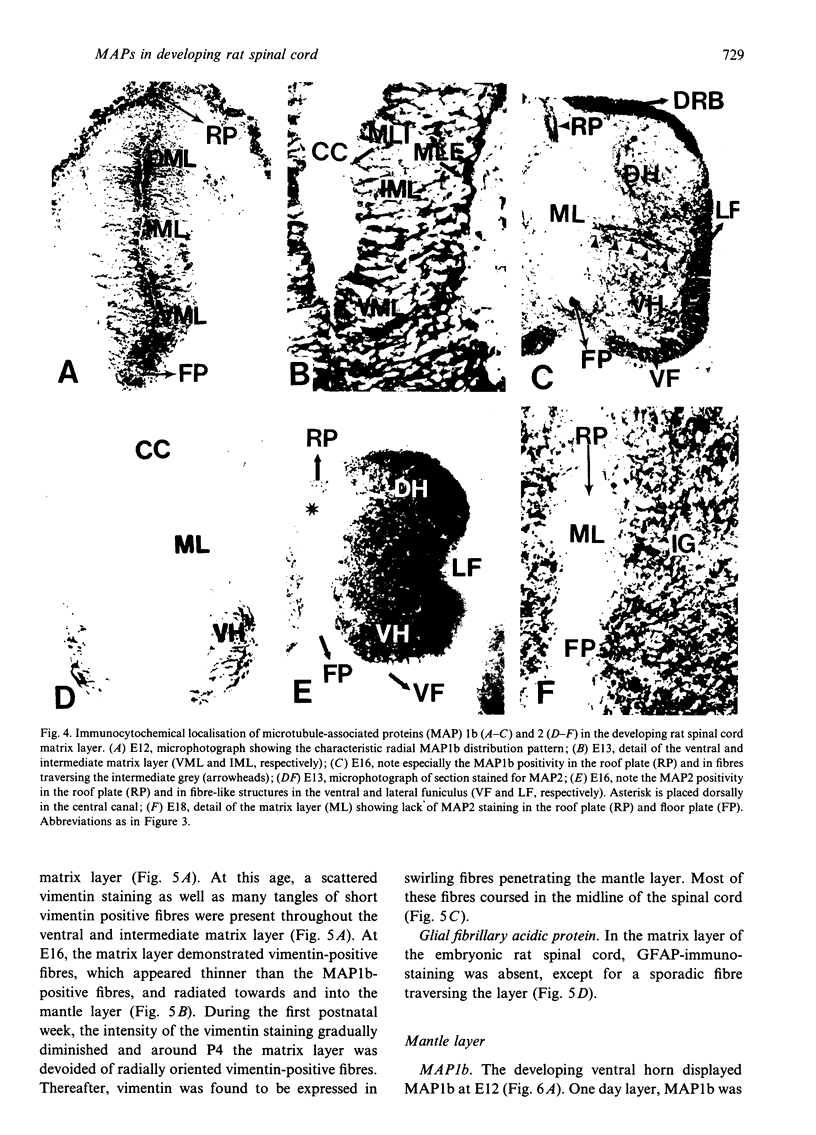
The straightforward anatomical organisation of the developing and mature rat spinal cord was used to determine and interpret the time of appearance and expression patterns of microtubule-associated proteins (MAP) 1b and 2. Immunoblots revealed the presence of MAP1b and 2 in the early embryonic rat spinal cord and confirmed the specificity of the used anti-MAP mouse monoclonal antibodies. The immunocytochemical data demonstrated a rostral-to-caudal and ventral-to-dorsal gradient in the expression of MAP1b/2 within the developing spinal cord. In the matrix layer, MAP1b was found in a distinct radial pattern distributed between the membrana limitans interna and externa between embryonal day (E)12 and E15. Immunostaining for vimentin revealed that this MAP1b pattern was morphologically and topographically different from the radial glial pattern which was present in the matrix layer between E13 and E19. The ventral-to-dorsal developmental gradient of the MAP1b staining in the spinal cord matrix layer indicates a close involvement of MAP1b either in the organisation of the microtubules in the cytoplasmatic extensions of the proliferating neuroblasts or neuroblast mitosis. MAP2 could not be detected in the developing matrix layer. In the mantle and marginal layer, MAP1b was abundantly present between E12 and postnatal day (P)0. After birth, the staining intensity for MAP1b gradually decreased in both layers towards a faint appearance at maturity. The distribution patterns suggest an involvement of MAP1b in the maturation of the motor neurons, the contralaterally and ipsilaterally projecting axons and the ascending and descending long axons of the rat spinal cord. MAP2 was present in the spinal cord grey matter between E12 and maturity, which reflects a role for MAP2 in the development as well as in the maintenance of microtubules. The present description of the expression patterns of MAP1b and 2 in the developing spinal cord suggests important roles of the two proteins in various morphogenetic events. The findings may serve as the basis for future studies on the function of MAP1b and 2 in the development of the central nervous system.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aletta J. M., Lewis S. A., Cowan N. J., Greene L. A. Nerve growth factor regulates both the phosphorylation and steady-state levels of microtubule-associated protein 1.2 (MAP1.2). J Cell Biol. 1988 May;106(5):1573–1581. doi: 10.1083/jcb.106.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumal R., Kahn H. J., Bailey D., Phillips M. J., Hanna W. The value of immunohistochemistry in increasing diagnostic precision of undifferentiated tumours by the surgical pathologist. Histochem J. 1984 Oct;16(10):1061–1078. doi: 10.1007/BF01002895. [DOI] [PubMed] [Google Scholar]
- Bernhardt R., Matus A. Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeletons. J Comp Neurol. 1984 Jun 20;226(2):203–221. doi: 10.1002/cne.902260205. [DOI] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Kim H., Caceres A., Payne M. R., Rebhun L. I. Heterogeneity of microtubule-associated protein 2 during rat brain development. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5613–5617. doi: 10.1073/pnas.81.17.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M. M., Slaughter T., Fischer I. Microtubule-associated protein 1b (MAP1b) is concentrated in the distal region of growing axons. J Neurosci. 1994 Feb;14(2):857–870. doi: 10.1523/JNEUROSCI.14-02-00857.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugg B., Matus A. PC12 cells express juvenile microtubule-associated proteins during nerve growth factor-induced neurite outgrowth. J Cell Biol. 1988 Aug;107(2):643–650. doi: 10.1083/jcb.107.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D., Cumming R. Ontogeny of microtubule-associated protein 2 in rat cerebellum: differential expression of the doublet polypeptides. Neuroscience. 1984 Jan;11(1):156–167. doi: 10.1016/0306-4522(84)90220-3. [DOI] [PubMed] [Google Scholar]
- Caceres A., Binder L. I., Payne M. R., Bender P., Rebhun L., Steward O. Differential subcellular localization of tubulin and the microtubule-associated protein MAP2 in brain tissue as revealed by immunocytochemistry with monoclonal hybridoma antibodies. J Neurosci. 1984 Feb;4(2):394–410. doi: 10.1523/JNEUROSCI.04-02-00394.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert R., Anderton B. H. A microtubule-associated protein (MAP1) which is expressed at elevated levels during development of the rat cerebellum. EMBO J. 1985 May;4(5):1171–1176. doi: 10.1002/j.1460-2075.1985.tb03756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden M. J., Trojanowski J. Q., Schlaepfer W. W., Lee V. M. Two-stage expression of neurofilament polypeptides during rat neurogenesis with early establishment of adult phosphorylation patterns. J Neurosci. 1987 Nov;7(11):3489–3504. doi: 10.1523/JNEUROSCI.07-11-03489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall J. E., Jacobson M., Kosik K. S. Ontogenesis of microtubule-associated protein 2 (MAP2) in embryonic mouse cortex. Brain Res. 1986 Jul;393(1):127–133. doi: 10.1016/0165-3806(86)90072-6. [DOI] [PubMed] [Google Scholar]
- Doll T., Meichsner M., Riederer B. M., Honegger P., Matus A. An isoform of microtubule-associated protein 2 (MAP2) containing four repeats of the tubulin-binding motif. J Cell Sci. 1993 Oct;106(Pt 2):633–639. doi: 10.1242/jcs.106.2.633. [DOI] [PubMed] [Google Scholar]
- Domínguez J. E., Buendia B., López-Otín C., Antony C., Karsenti E., Avila J. A protein related to brain microtubule-associated protein MAP1B is a component of the mammalian centrosome. J Cell Sci. 1994 Feb;107(Pt 2):601–611. [PubMed] [Google Scholar]
- Faundez V., Alvarez J. Microtubules and calibers in developing axons. J Comp Neurol. 1986 Aug 1;250(1):73–80. doi: 10.1002/cne.902500107. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Busciglio J., Cáceres A. Microtubule formation and neurite growth in cerebellar macroneurons which develop in vitro: evidence for the involvement of the microtubule-associated proteins, MAP-1a, HMW-MAP2 and Tau. Brain Res Dev Brain Res. 1989 Oct 1;49(2):215–228. doi: 10.1016/0165-3806(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Gordon-Weeks P. R., Mansfield S. G., Alberto C., Johnstone M., Moya F. A phosphorylation epitope on MAP 1B that is transiently expressed in growing axons in the developing rat nervous system. Eur J Neurosci. 1993 Oct 1;5(10):1302–1311. doi: 10.1111/j.1460-9568.1993.tb00916.x. [DOI] [PubMed] [Google Scholar]
- Gordon-Weeks P. R. Organization of microtubules in axonal growth cones: a role for microtubule-associated protein MAP 1B. J Neurocytol. 1993 Sep;22(9):717–725. doi: 10.1007/BF01181317. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Liem R. K., Shelanski M. L. Regulation of a high molecular weight microtubule-associated protein in PC12 cells by nerve growth factor. J Cell Biol. 1983 Jan;96(1):76–83. doi: 10.1083/jcb.96.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Hisanaga S., Shiomura Y. MAP2 is a component of crossbridges between microtubules and neurofilaments in the neuronal cytoskeleton: quick-freeze, deep-etch immunoelectron microscopy and reconstitution studies. J Neurosci. 1988 Aug;8(8):2769–2779. doi: 10.1523/JNEUROSCI.08-08-02769.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber G., Matus A. Differences in the cellular distributions of two microtubule-associated proteins, MAP1 and MAP2, in rat brain. J Neurosci. 1984 Jan;4(1):151–160. doi: 10.1523/JNEUROSCI.04-01-00151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Rodionov V. I., Gelfand V. I., Rosenblat V. A. Microtubule-associated protein MAP1 promotes microtubule assembly in vitro. FEBS Lett. 1981 Dec 7;135(2):241–244. doi: 10.1016/0014-5793(81)80791-0. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Ivanov I. E., Lee G. H., Cowan N. J. Organization of microtubules in dendrites and axons is determined by a short hydrophobic zipper in microtubule-associated proteins MAP2 and tau. Nature. 1989 Nov 30;342(6249):498–505. doi: 10.1038/342498a0. [DOI] [PubMed] [Google Scholar]
- Mansfield S. G., Diaz-Nido J., Gordon-Weeks P. R., Avila J. The distribution and phosphorylation of the microtubule-associated protein MAP 1B in growth cones. J Neurocytol. 1991 Dec;20(12):1007–1022. doi: 10.1007/BF01187918. [DOI] [PubMed] [Google Scholar]
- Marani E. A method for orienting cryostat sections for three-dimensional reconstructions. Stain Technol. 1978 Sep;53(5):265–268. doi: 10.3109/10520297809111943. [DOI] [PubMed] [Google Scholar]
- Mareck A., Fellous A., Francon J., Nunez J. Changes in composition and activity of microtubule-associated proteins during brain development. Nature. 1980 Mar 27;284(5754):353–355. doi: 10.1038/284353a0. [DOI] [PubMed] [Google Scholar]
- Matus A., Huber G., Bernhardt R. Neuronal microdifferentiation. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):775–782. doi: 10.1101/sqb.1983.048.01.079. [DOI] [PubMed] [Google Scholar]
- Matus A. Microtubule-associated proteins and neuronal morphogenesis. J Cell Sci Suppl. 1991;15:61–67. doi: 10.1242/jcs.1991.supplement_15.9. [DOI] [PubMed] [Google Scholar]
- Matus A. Microtubule-associated proteins: their potential role in determining neuronal morphology. Annu Rev Neurosci. 1988;11:29–44. doi: 10.1146/annurev.ne.11.030188.000333. [DOI] [PubMed] [Google Scholar]
- Molander C., Xu Q., Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol. 1984 Nov 20;230(1):133–141. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- Nornes H. O., Das G. D. Temporal pattern of neurogenesis in spinal cord of rat. I. An autoradiographic study--time and sites of origin and migration and settling patterns of neuroblasts. Brain Res. 1974 Jun 14;73(1):121–138. doi: 10.1016/0006-8993(74)91011-7. [DOI] [PubMed] [Google Scholar]
- Nunez J. Differential expression of microtubule components during brain development. Dev Neurosci. 1986;8(3):125–141. doi: 10.1159/000112248. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Non-motor microtubule-associated proteins. Curr Opin Cell Biol. 1991 Feb;3(1):52–58. doi: 10.1016/0955-0674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- Oudega M., Marani E. Acetylcholinesterase in the developing rat spinal cord: an enzyme histochemical study. Eur J Morphol. 1990;28(2-4):379–393. [PubMed] [Google Scholar]
- Oudega M., Marani E. Expression of vimentin and glial fibrillary acidic protein in the developing rat spinal cord: an immunocytochemical study of the spinal cord glial system. J Anat. 1991 Dec;179:97–114. [PMC free article] [PubMed] [Google Scholar]
- Peng I., Binder L. I., Black M. M. Biochemical and immunological analyses of cytoskeletal domains of neurons. J Cell Biol. 1986 Jan;102(1):252–262. doi: 10.1083/jcb.102.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer B. M., Guadano-Ferraz A., Innocenti G. M. Difference in distribution of microtubule-associated proteins 5a and 5b during the development of cerebral cortex and corpus callosum in cats: dependence on phosphorylation. Brain Res Dev Brain Res. 1990 Nov 1;56(2):235–243. doi: 10.1016/0165-3806(90)90088-g. [DOI] [PubMed] [Google Scholar]
- Riederer B. M., Moya F., Calvert R. Phosphorylated MAP1b, alias MAP5 and MAP1x, is involved in axonal growth and neuronal mitosis. Neuroreport. 1993 Jun;4(6):771–774. doi: 10.1097/00001756-199306000-00044. [DOI] [PubMed] [Google Scholar]
- Riederer B. M., Porchet R., Marugg R. A., Binder L. I. Solubility of cytoskeletal proteins in immunohistochemistry and the influence of fixation. J Histochem Cytochem. 1993 Apr;41(4):609–616. doi: 10.1177/41.4.8450200. [DOI] [PubMed] [Google Scholar]
- Riederer B. M. Production of an affinity-purified antibody against an aldehyde-treated neurofilament protein for use in immunocytochemistry. Brain Res Bull. 1993;30(5-6):623–627. doi: 10.1016/0361-9230(93)90092-p. [DOI] [PubMed] [Google Scholar]
- Riederer B. M. Some aspects of the neuronal cytoskeleton in development. Eur J Morphol. 1990;28(2-4):347–378. [PubMed] [Google Scholar]
- Riederer B., Cohen R., Matus A. MAP5: a novel brain microtubule-associated protein under strong developmental regulation. J Neurocytol. 1986 Dec;15(6):763–775. doi: 10.1007/BF01625193. [DOI] [PubMed] [Google Scholar]
- Riederer B., Matus A. Differential expression of distinct microtubule-associated proteins during brain development. Proc Natl Acad Sci U S A. 1985 Sep;82(17):6006–6009. doi: 10.1073/pnas.82.17.6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer Beat M., Innocenti Giorgio M. MAP2 Isoforms in Developing Cat Cerebral Cortex and Corpus Callosum. Eur J Neurosci. 1992;4(12):1376–1386. doi: 10.1111/j.1460-9568.1992.tb00163.x. [DOI] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloboda R. D., Rosenbaum J. L. Decoration and stabilization of intact, smooth-walled microtubules with microtubule-associated proteins. Biochemistry. 1979 Jan 9;18(1):48–55. doi: 10.1021/bi00568a008. [DOI] [PubMed] [Google Scholar]
- Takemura R., Okabe S., Umeyama T., Kanai Y., Cowan N. J., Hirokawa N. Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. J Cell Sci. 1992 Dec;103(Pt 4):953–964. doi: 10.1242/jcs.103.4.953. [DOI] [PubMed] [Google Scholar]
- Tanaka E. M., Kirschner M. W. Microtubule behavior in the growth cones of living neurons during axon elongation. J Cell Biol. 1991 Oct;115(2):345–363. doi: 10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. P., Binder L. I., Matus A. I. Neuronal microtubule-associated proteins in the embryonic avian spinal cord. J Comp Neurol. 1988 May 1;271(1):44–55. doi: 10.1002/cne.902710106. [DOI] [PubMed] [Google Scholar]
- Tucker R. P. The roles of microtubule-associated proteins in brain morphogenesis: a review. Brain Res Brain Res Rev. 1990 May-Aug;15(2):101–120. doi: 10.1016/0165-0173(90)90013-e. [DOI] [PubMed] [Google Scholar]
- Ulloa L., Avila J., Díaz-Nido J. Heterogeneity in the phosphorylation of microtubule-associated protein MAP1B during rat brain development. J Neurochem. 1993 Sep;61(3):961–972. doi: 10.1111/j.1471-4159.1993.tb03609.x. [DOI] [PubMed] [Google Scholar]
- Vallee R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J Cell Biol. 1982 Feb;92(2):435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viereck C., Tucker R. P., Matus A. The adult rat olfactory system expresses microtubule-associated proteins found in the developing brain. J Neurosci. 1989 Oct;9(10):3547–3557. doi: 10.1523/JNEUROSCI.09-10-03547.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar B., Matus A. Microtubule-associated protein 2 and the organization of cellular microtubules. J Neurocytol. 1993 Sep;22(9):727–734. doi: 10.1007/BF01181318. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Spooner B. S., Wessells N. K. Axon growth: roles of microfilaments and microtubules. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1206–1212. doi: 10.1073/pnas.66.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauner W., Kratz J., Staunton J., Feick P., Wiche G. Identification of two distinct microtubule binding domains on recombinant rat MAP 1B. Eur J Cell Biol. 1992 Feb;57(1):66–74. [PubMed] [Google Scholar]
- van Muijen G. N., Ruiter D. J., van Leeuwen C., Prins F. A., Rietsema K., Warnaar S. O. Cytokeratin and neurofilament in lung carcinomas. Am J Pathol. 1984 Sep;116(3):363–369. [PMC free article] [PubMed] [Google Scholar]