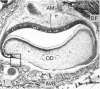
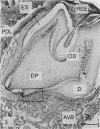
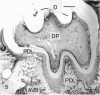
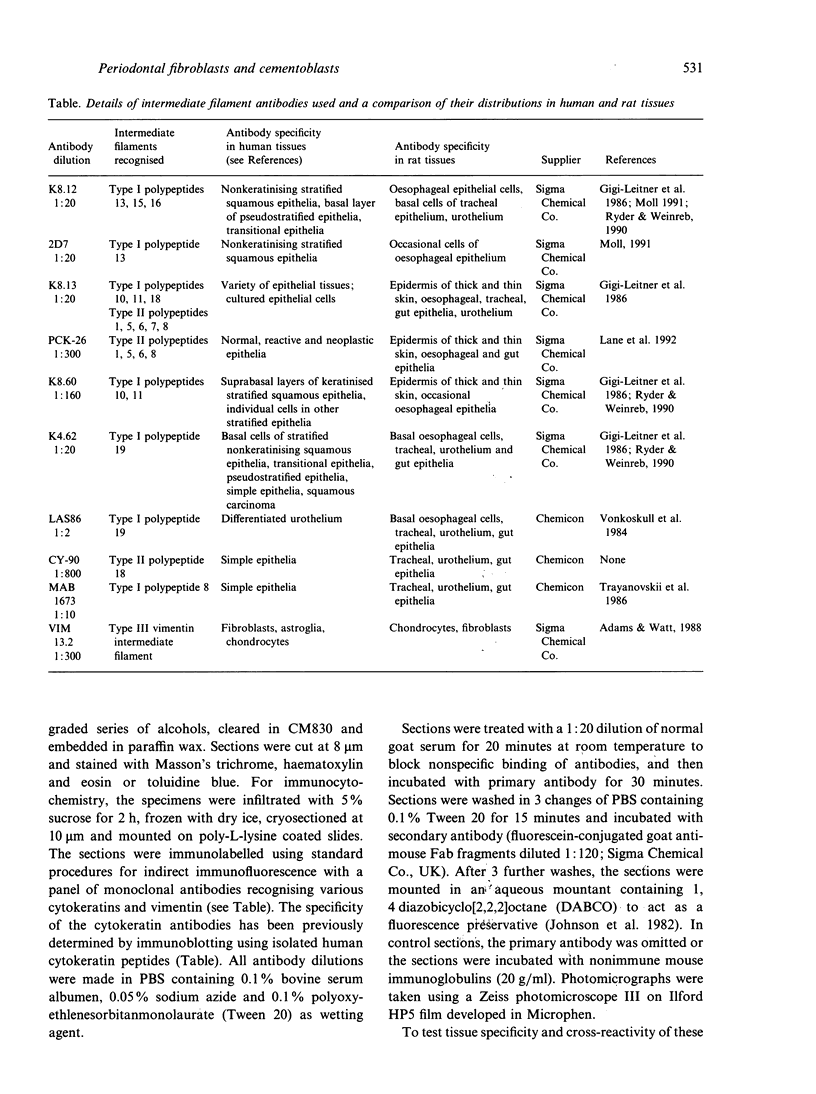
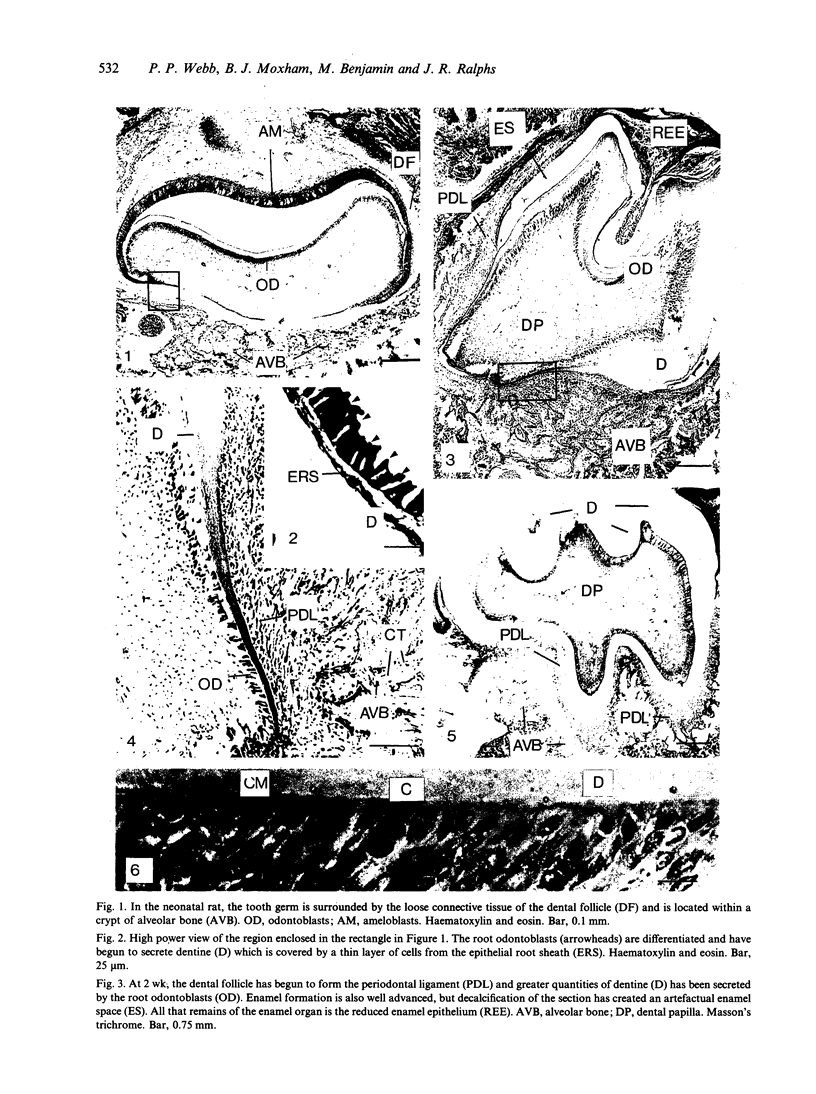
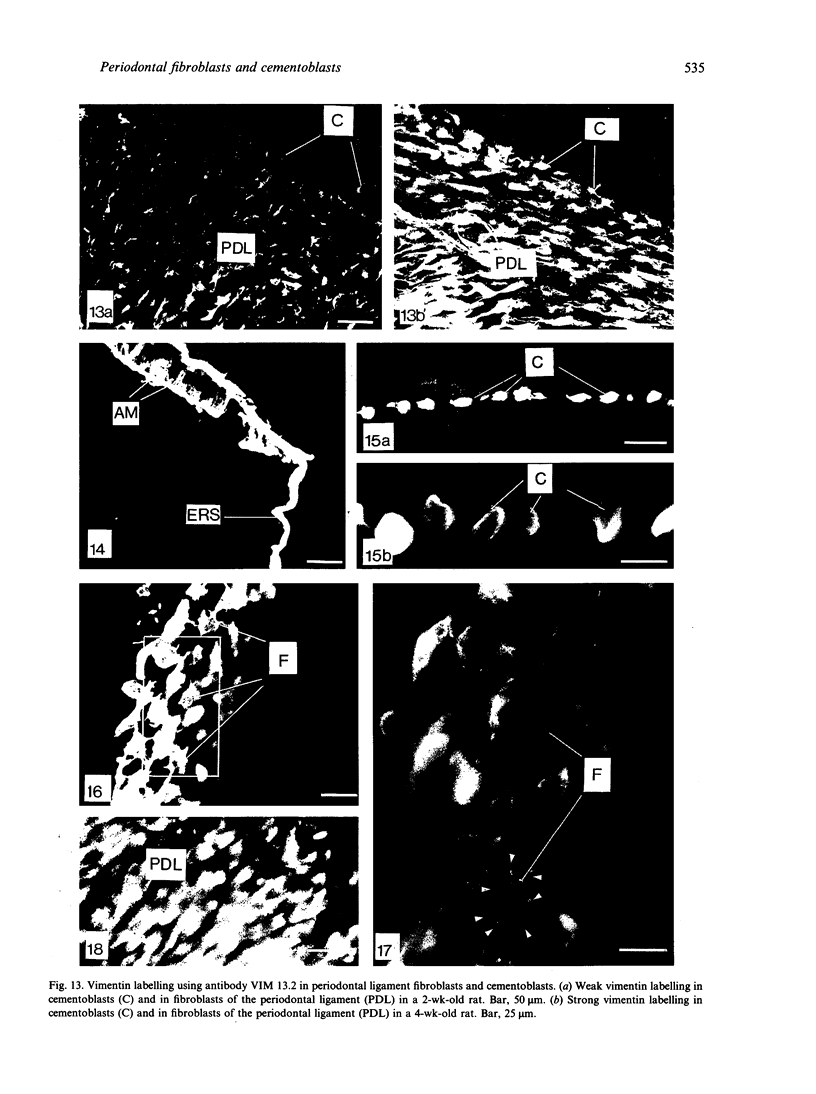
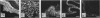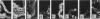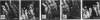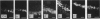Abstract
The distributing of vimentin and cytokeratin intermediate filaments within the cells of the dental follicle and developing periodontal ligament is described during eruption of the rat 1st molar tooth. Alcohol-fixed tissues from animals ranging from neonates to 12 wk old were cryosectioned, immunolabelled with monoclonal antibodies against vimentin and a range of cytokeratins and examined by indirect immunofluorescence. Vimentin was observed in follicular and periodontal ligament fibroblasts in all animals and at all stages of eruption. It was also observed in cementoblasts after disruption of the epithelial root sheath (of Hertwig) which is responsible for determining the shape of the developing root. Prior to eruption, cytokeratins were restricted to epithelial components of the developing tooth, including the root sheath. However, they were seen in cementoblasts on disruption of the root sheath at 2 wk and in periodontal ligament fibroblasts at 3 wk after birth, when the tooth was erupting but had not reached occlusion. On occlusion (at 4 wk), fibroblasts no longer labelled for cytokeratins but cementoblasts associated with acellular cementum formation continued to express them. These results demonstrate temporal and spatial changes within the cells of the developing periodontal connective tissues and suggest that the appearance of cytokeratins in periodontal fibroblasts and cementoblasts may be related to mechanical changes during tooth eruption. Further, the results suggest different origins for cementoblasts associated with cellular and acellular cementum formation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. An unusual strain of human keratinocytes which do not stratify or undergo terminal differentiation in culture. J Cell Biol. 1988 Nov;107(5):1927–1938. doi: 10.1083/jcb.107.5.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M., Archer C. W., Ralphs J. R. Cytoskeleton of cartilage cells. Microsc Res Tech. 1994 Aug 1;28(5):372–377. doi: 10.1002/jemt.1070280503. [DOI] [PubMed] [Google Scholar]
- Breitbart R. E., Andreadis A., Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. doi: 10.1146/annurev.bi.56.070187.002343. [DOI] [PubMed] [Google Scholar]
- Cho M. I., Lin W. L., Garant P. R. Occurrence of epidermal growth factor-binding sites during differentiation of cementoblasts and periodontal ligament fibroblasts of the young rat: a light and electron microscopic radioautographic study. Anat Rec. 1991 Sep;231(1):14–24. doi: 10.1002/ar.1092310104. [DOI] [PubMed] [Google Scholar]
- Darling A. I., Levers B. G. The pattern of eruption of some human teeth. Arch Oral Biol. 1975 Feb;20(2):89–96. doi: 10.1016/0003-9969(75)90159-4. [DOI] [PubMed] [Google Scholar]
- Eggli P. S., Hunziker E. B., Schenk R. K. Quantitation of structural features characterizing weight- and less-weight-bearing regions in articular cartilage: a stereological analysis of medial femoral condyles in young adult rabbits. Anat Rec. 1988 Nov;222(3):217–227. doi: 10.1002/ar.1092220302. [DOI] [PubMed] [Google Scholar]
- Eichner R., Sun T. T., Aebi U. The role of keratin subfamilies and keratin pairs in the formation of human epidermal intermediate filaments. J Cell Biol. 1986 May;102(5):1767–1777. doi: 10.1083/jcb.102.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti P., Fekete D. M., Patterson M., Lane E. B. Transient expression of simple epithelial keratins by mesenchymal cells of regenerating newt limb. Dev Biol. 1989 Jun;133(2):415–424. doi: 10.1016/0012-1606(89)90045-6. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Appelhans B., Schmid E., Freudenstein C., Osborn M., Weber K. Identification and characterization of epithelial cells in mammalian tissues by immunofluorescence microscopy using antibodies to prekeratin. Differentiation. 1979;15(1):7–25. doi: 10.1111/j.1432-0436.1979.tb01030.x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. V., Coppock S. M., Green H., Cleveland D. W. Two distinct classes of keratin genes and their evolutionary significance. Cell. 1981 Nov;27(1 Pt 2):75–84. doi: 10.1016/0092-8674(81)90362-7. [DOI] [PubMed] [Google Scholar]
- Gigi-Leitner O., Geiger B., Levy R., Czernobilsky B. Cytokeratin expression in squamous metaplasia of the human uterine cervix. Differentiation. 1986;31(3):191–205. doi: 10.1111/j.1432-0436.1986.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M., Franke W. W. Pair formation and promiscuity of cytokeratins: formation in vitro of heterotypic complexes and intermediate-sized filaments by homologous and heterologous recombinations of purified polypeptides. J Cell Biol. 1985 Nov;101(5 Pt 1):1826–1841. doi: 10.1083/jcb.101.5.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn L., Franke W. W. High frequency of cytokeratin-producing smooth muscle cells in human atherosclerotic plaques. Differentiation. 1989 Mar;40(1):55–62. doi: 10.1111/j.1432-0436.1989.tb00813.x. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Davidson R. S., McNamee K. C., Russell G., Goodwin D., Holborow E. J. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods. 1982 Dec 17;55(2):231–242. doi: 10.1016/0022-1759(82)90035-7. [DOI] [PubMed] [Google Scholar]
- Kasper M., Karsten U., Stosiek P., Moll R. Distribution of intermediate-filament proteins in the human enamel organ: unusually complex pattern of coexpression of cytokeratin polypeptides and vimentin. Differentiation. 1989 Jun;40(3):207–214. doi: 10.1111/j.1432-0436.1989.tb00600.x. [DOI] [PubMed] [Google Scholar]
- Kasper M., Moll R., Stosiek P., Karsten U. Patterns of cytokeratin and vimentin expression in the human eye. Histochemistry. 1988;89(4):369–377. doi: 10.1007/BF00500639. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W. Intermediate filaments: new proteins, some answers, more questions. Curr Opin Cell Biol. 1995 Feb;7(1):46–54. doi: 10.1016/0955-0674(95)80044-1. [DOI] [PubMed] [Google Scholar]
- Knapp A. C., Franke W. W. Spontaneous losses of control of cytokeratin gene expression in transformed, non-epithelial human cells occurring at different levels of regulation. Cell. 1989 Oct 6;59(1):67–79. doi: 10.1016/0092-8674(89)90870-2. [DOI] [PubMed] [Google Scholar]
- Kuruc N., Franke W. W. Transient coexpression of desmin and cytokeratins 8 and 18 in developing myocardial cells of some vertebrate species. Differentiation. 1988 Sep;38(3):177–193. doi: 10.1111/j.1432-0436.1988.tb00212.x. [DOI] [PubMed] [Google Scholar]
- Lane E. B., Rugg E. L., Navsaria H., Leigh I. M., Heagerty A. H., Ishida-Yamamoto A., Eady R. A. A mutation in the conserved helix termination peptide of keratin 5 in hereditary skin blistering. Nature. 1992 Mar 19;356(6366):244–246. doi: 10.1038/356244a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lesot H., Meyer J. M., Ruch J. V., Weber K., Osborn M. Immunofluorescent localization of vimentin, prekeratin and actin during odontoblast and ameloblast differentiation. Differentiation. 1982;21(2):133–137. doi: 10.1111/j.1432-0436.1982.tb01206.x. [DOI] [PubMed] [Google Scholar]
- Lester K. S. The unusual nature of root formation in molar teeth of the laboratory rat. J Ultrastruct Res. 1969 Sep;28(5):481–506. doi: 10.1016/s0022-5320(69)80035-3. [DOI] [PubMed] [Google Scholar]
- Lombardi T., Samson J., Mühlhauser J., Fiore-Donno G., Maggiano N., Castellucci M. Expression of intermediate filaments and actins in human dental pulp and embryonic dental papilla. Anat Rec. 1992 Dec;234(4):587–592. doi: 10.1002/ar.1092340414. [DOI] [PubMed] [Google Scholar]
- Lu X., Quinlan R. A., Steel J. B., Lane E. B. Network incorporation of intermediate filament molecules differs between preexisting and newly assembling filaments. Exp Cell Res. 1993 Sep;208(1):218–225. doi: 10.1006/excr.1993.1240. [DOI] [PubMed] [Google Scholar]
- Luo W., Slavkin H. C., Snead M. L. Cells from Hertwig's epithelial root sheath do not transcribe amelogenin. J Periodontal Res. 1991 Jan;26(1):42–47. doi: 10.1111/j.1600-0765.1991.tb01624.x. [DOI] [PubMed] [Google Scholar]
- Mackenzie I. C., Gao Z. Patterns of cytokeratin expression in the epithelia of inflamed human gingiva and periodontal pockets. J Periodontal Res. 1993 Jan;28(1):49–59. doi: 10.1111/j.1600-0765.1993.tb01050.x. [DOI] [PubMed] [Google Scholar]
- Markl J., Franke W. W. Localization of cytokeratins in tissues of the rainbow trout: fundamental differences in expression pattern between fish and higher vertebrates. Differentiation. 1988 Dec;39(2):97–122. doi: 10.1111/j.1432-0436.1988.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Melcher A. H., Correia M. A. Remodelling of periodontal ligament in erupting molars of mature rats. J Periodontal Res. 1971;6(2):118–125. doi: 10.1111/j.1600-0765.1971.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Mochan E., Armor L., Sporer R. Interleukin 1 stimulation of plasminogen activator production in cultured gingival fibroblasts. J Periodontal Res. 1988 Jan;23(1):28–32. doi: 10.1111/j.1600-0765.1988.tb01023.x. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moll R., Krepler R., Franke W. W. Complex cytokeratin polypeptide patterns observed in certain human carcinomas. Differentiation. 1983;23(3):256–269. doi: 10.1111/j.1432-0436.1982.tb01291.x. [DOI] [PubMed] [Google Scholar]
- Moll R. Molecular diversity of cytokeratins: significance for cell and tumor differentiation. Acta Histochem Suppl. 1991;41:117–127. [PubMed] [Google Scholar]
- Moxham B. J., Berkovitz B. K. Continuous monitoring of the movements of erupting and newly erupted teeth of limited growth (ferret mandibular canines) and their responses to hexamethonium. Arch Oral Biol. 1988;33(12):919–923. doi: 10.1016/0003-9969(88)90023-4. [DOI] [PubMed] [Google Scholar]
- Owens P. D. The root surface in human teeth: a microradiographic study. J Anat. 1976 Nov;122(Pt 2):389–401. [PMC free article] [PubMed] [Google Scholar]
- Owens P. D. Ultrastructure of Hertwig's epithelial root sheath during early root development in premolar teeth in dogs. Arch Oral Biol. 1978;23(2):91–104. doi: 10.1016/0003-9969(78)90145-0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Lumsden A. G. Development of periodontal ligament and alveolar bone in homografted recombinations of enamel organs and papillary, pulpal and follicular mesenchyme in the mouse. Arch Oral Biol. 1987;32(4):281–289. doi: 10.1016/0003-9969(87)90022-7. [DOI] [PubMed] [Google Scholar]
- Pelissier A., Ouhayoun J. P., Sawaf M. H., Forest N. Evolution of cytokeratin expression in developing human tooth germ. J Biol Buccale. 1990 Jun;18(2):99–108. [PubMed] [Google Scholar]
- Perera K. A., Tonge C. H. Fibroblast cell population kinetics in the mouse molar periodontal ligament and tooth eruption. J Anat. 1981 Sep;133(Pt 2):281–300. [PMC free article] [PubMed] [Google Scholar]
- Perera K. A., Tonge C. H. Fibroblast cell proliferation in the mouse molar periodontal ligament. J Anat. 1981 Aug;133(Pt 1):77–90. [PMC free article] [PubMed] [Google Scholar]
- Pitaru S., Aubin J. E., Bhargava U., Melcher A. H. Immunoelectron microscopic studies on the distributions of fibronectin and actin in a cellular dense connective tissue: the periodontal ligament of the rat. J Periodontal Res. 1987 Jan;22(1):64–74. doi: 10.1111/j.1600-0765.1987.tb01541.x. [DOI] [PubMed] [Google Scholar]
- Ralphs J. R., Tyers R. N., Benjamin M. Development of functionally distinct fibrocartilages at two sites in the quadriceps tendon of the rat: the suprapatella and the attachment to the patella. Anat Embryol (Berl) 1992;185(2):181–187. doi: 10.1007/BF00185920. [DOI] [PubMed] [Google Scholar]
- Ryder M. I., Weinreb R. N. Cytokeratin patterns in corneal, limbal, and conjunctival epithelium. An immunofluorescence study with PKK-1, 8.12, 8.60, and 4.62 anticytokeratin antibodies. Invest Ophthalmol Vis Sci. 1990 Nov;31(11):2230–2234. [PubMed] [Google Scholar]
- Scott J. The development, structure, and function of alveolar bone. Dent Pract Dent Rec. 1968 Sep;19(1):19–22. [PubMed] [Google Scholar]
- Skalli O., Goldman R. D. Recent insights into the assembly, dynamics, and function of intermediate filament networks. Cell Motil Cytoskeleton. 1991;19(2):67–79. doi: 10.1002/cm.970190202. [DOI] [PubMed] [Google Scholar]
- Slavkin H. C., Bessem C., Fincham A. G., Bringas P., Jr, Santos V., Snead M. L., Zeichner-David M. Human and mouse cementum proteins immunologically related to enamel proteins. Biochim Biophys Acta. 1989 Apr 25;991(1):12–18. doi: 10.1016/0304-4165(89)90021-4. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Wilson C., Matthews J. B. An immunocytochemical study of keratin reactivity during rat odontogenesis. Histochemistry. 1990;94(3):329–335. doi: 10.1007/BF00266636. [DOI] [PubMed] [Google Scholar]
- TROTT J. R. The development of the periodontal attachment in the rat. Acta Anat (Basel) 1962;51:313–328. doi: 10.1159/000142326. [DOI] [PubMed] [Google Scholar]
- Ten Cate A. R., Mills C., Solomon G. The development of the periodontium. A transplantation and autoradiographic study. Anat Rec. 1971 Jul;170(3):365–379. doi: 10.1002/ar.1091700312. [DOI] [PubMed] [Google Scholar]
- Tenorio D., Cruchley A., Hughes F. J. Immunocytochemical investigation of the rat cementoblast phenotype. J Periodontal Res. 1993 Nov;28(6 Pt 1):411–419. [PubMed] [Google Scholar]
- von Koskull H., Aula P., Trejdosiewicz L. K., Virtanen I. Identification of cells from fetal bladder epithelium in human amniotic fluid. Hum Genet. 1984;65(3):262–267. doi: 10.1007/BF00286514. [DOI] [PubMed] [Google Scholar]