Abstract
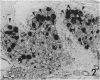
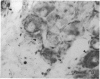
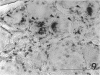
Retrograde transport of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) was used to study the localisation of neurons that innervate the lacrimal gland of the cynomolgous monkey. WGA-HRP-labelled neurons were localised in the ipsilateral trigeminal, superior cervical and ciliary ganglia and in the ipsilateral and contralateral pterygopalatine ganglia. In the trigeminal ganglion WGA-HRP-labelled somata were found in the ophthalmic part (18%) and the maxillary part (5%). Identification of labelled neurons in the ciliary and pterygopalatine ganglia indicates a dual parasympathetic innvervation of the lacrimal gland. There is no known pathway to account for the contralateral location or pterygopalatine neurons. These novel findings are incorporated in a concept of a neural control mechanism for the lacrimal gland.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baljet B., van der Werf F., Otto A. J. Autonomic pathways in the orbit of the human fetus and the rhesus monkey. Doc Ophthalmol. 1989 Aug;72(3-4):247–264. doi: 10.1007/BF00153492. [DOI] [PubMed] [Google Scholar]
- Beckers H. J., Klooster J., Vrensen G. F., Lamers W. P. Ultrastructural identification of trigeminal nerve terminals in the pterygopalatine ganglion of rats: an anterograde tracing and immunohistochemical study. Brain Res. 1991 Aug 23;557(1-2):22–30. doi: 10.1016/0006-8993(91)90111-8. [DOI] [PubMed] [Google Scholar]
- Botelho S. Y., Hisada M., Fuenmayor N. Functional innervation of the lacrimal gland in the cat. Origin of secretomotor fibers in the lacrimal nerve. Arch Ophthalmol. 1966 Oct;76(4):581–588. doi: 10.1001/archopht.1966.03850010583019. [DOI] [PubMed] [Google Scholar]
- Bromberg B. B. Autonomic control of lacrimal protein secretion. Invest Ophthalmol Vis Sci. 1981 Jan;20(1):110–116. [PubMed] [Google Scholar]
- Butler J. M., Ruskell G. L., Cole D. F., Unger W. G., Zhang S. Q., Blank M. A., McGregor G. P., Bloom S. R. Effects of VIIth (facial) nerve degeneration on vasoactive intestinal polypeptide and substance P levels in ocular and orbital tissues of the rabbit. Exp Eye Res. 1984 Oct;39(4):523–532. doi: 10.1016/0014-4835(84)90052-6. [DOI] [PubMed] [Google Scholar]
- Dartt D. A., Shulman M., Gray K. L., Rossi S. R., Matkin C., Gilbard J. P. Stimulation of rabbit lacrimal gland secretion with biologically active peptides. Am J Physiol. 1988 Mar;254(3 Pt 1):G300–G306. doi: 10.1152/ajpgi.1988.254.3.G300. [DOI] [PubMed] [Google Scholar]
- Drummond P. D., Lance J. W. Pathological sweating and flushing accompanying the trigeminal lacrimal reflex in patients with cluster headache and in patients with a confirmed site of cervical sympathetic deficit. Evidence for parasympathetic cross-innervation. Brain. 1992 Oct;115(Pt 5):1429–1445. doi: 10.1093/brain/115.5.1429. [DOI] [PubMed] [Google Scholar]
- Flett D. L., Bell C. Topography of functional subpopulations of neurons in the superior cervical ganglion of the rat. J Anat. 1991 Aug;177:55–66. [PMC free article] [PubMed] [Google Scholar]
- Flügel-Koch C., Kaufman P., Lütjen-Drecoll E. Association of a choroidal ganglion cell plexus with the fovea centralis. Invest Ophthalmol Vis Sci. 1994 Dec;35(13):4268–4272. [PubMed] [Google Scholar]
- Flügel C., Tamm E. R., Mayer B., Lütjen-Drecoll E. Species differences in choroidal vasodilative innervation: evidence for specific intrinsic nitrergic and VIP-positive neurons in the human eye. Invest Ophthalmol Vis Sci. 1994 Feb;35(2):592–599. [PubMed] [Google Scholar]
- Jones L. T. Anatomy of the tear system. Int Ophthalmol Clin. 1973 Spring;13(1):3–22. doi: 10.1097/00004397-197301310-00003. [DOI] [PubMed] [Google Scholar]
- Leblanc G. G., Landis S. C. Target specificity of neuropeptide Y-immunoreactive cranial parasympathetic neurons. J Neurosci. 1988 Jan;8(1):146–155. doi: 10.1523/JNEUROSCI.08-01-00146.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Takami K., Kawai Y., Girgis S., Hillyard C. J., MacIntyre I., Emson P. C., Tohyama M. Distribution of calcitonin gene-related peptide in the rat peripheral nervous system with reference to its coexistence with substance P. Neuroscience. 1985 Aug;15(4):1227–1237. doi: 10.1016/0306-4522(85)90265-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Tanabe T., Ueda S., Kawata M. Immunohistochemical and enzymehistochemical studies of peptidergic, aminergic and cholinergic innervation of the lacrimal gland of the monkey (Macaca fuscata). J Auton Nerv Syst. 1992 Mar;37(3):207–214. doi: 10.1016/0165-1838(92)90042-f. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem. 1978 Feb;26(2):106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Nikkinen A., Lehtosalo J. I., Uusitalo H., Palkama A., Panula P. The lacrimal glands of the rat and the guinea pig are innervated by nerve fibers containing immunoreactivities for substance P and vasoactive intestinal polypeptide. Histochemistry. 1984;81(1):23–27. doi: 10.1007/BF00495396. [DOI] [PubMed] [Google Scholar]
- Nikkinen A., Uusitalo H., Lehtosalo J. I., Palkama A. Distribution of adrenergic nerves in the lacrimal glands of guinea-pig and rat. Exp Eye Res. 1985 May;40(5):751–756. doi: 10.1016/0014-4835(85)90144-7. [DOI] [PubMed] [Google Scholar]
- Prins M., van der Werf F., Baljet B., Otto J. A. Calcitonin gene-related peptide and substance P immunoreactivity in the monkey trigeminal ganglion, an electron microscopic study. Brain Res. 1993 Dec 3;629(2):315–318. doi: 10.1016/0006-8993(93)91337-r. [DOI] [PubMed] [Google Scholar]
- Rossoni R. B., Machado A. B., Machado C. R. Autonomic innervation of the salivary glands in cebid monkeys: a histochemical study. Acta Anat (Basel) 1992;143(3):211–218. doi: 10.1159/000147250. [DOI] [PubMed] [Google Scholar]
- Ruskell G. L. Changes in nerve terminals and acini of the lacrimal gland and changes in secretion induced by autonomic denervation. Z Zellforsch Mikrosk Anat. 1969;94(2):261–281. doi: 10.1007/BF00339361. [DOI] [PubMed] [Google Scholar]
- Ruskell G. L. Nerve terminals and epithelial cell variety in the human lacrimal gland. Cell Tissue Res. 1975;158(1):121–136. doi: 10.1007/BF00219955. [DOI] [PubMed] [Google Scholar]
- Ruskell G. L. The distribution of autonomic post-ganglionic nerve fibres to the lacrimal gland in monkeys. J Anat. 1971 Jul;109(Pt 2):229–242. [PMC free article] [PubMed] [Google Scholar]
- Ruskell G. L. Vasomotor axons of the lacrimal glands monkeys and the ultrastructural identification of sympathetic terminals. Z Zellforsch Mikrosk Anat. 1967;83(3):321–333. doi: 10.1007/BF00336861. [DOI] [PubMed] [Google Scholar]
- Spencer S. E., Sawyer W. B., Wada H., Platt K. B., Loewy A. D. CNS projections to the pterygopalatine parasympathetic preganglionic neurons in the rat: a retrograde transneuronal viral cell body labeling study. Brain Res. 1990 Nov 26;534(1-2):149–169. doi: 10.1016/0006-8993(90)90125-u. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Hardebo J. E., Owman C. Trigeminal fibre collaterals storing substance P and calcitonin gene-related peptide associate with ganglion cells containing choline acetyltransferase and vasoactive intestinal polypeptide in the sphenopalatine ganglion of the rat. An axon reflex modulating parasympathetic ganglionic activity? Neuroscience. 1989;30(3):595–604. doi: 10.1016/0306-4522(89)90154-1. [DOI] [PubMed] [Google Scholar]
- Tan C. K., Zhang Y. L., Wong W. C. A light- and electron microscopic study of tyrosine hydroxylase-like immunoreactivity in the ciliary ganglia of monkey (Macaca fascicularis) and cat. Histol Histopathol. 1995 Jan;10(1):27–34. [PubMed] [Google Scholar]
- Ten Tusscher M. P., Klooster J., Baljet B., Van der Werf F., Vrensen G. F. Pre- and post-ganglionic nerve fibres of the pterygopalatine ganglion and their allocation to the eyeball of rats. Brain Res. 1990 May 28;517(1-2):315–323. doi: 10.1016/0006-8993(90)91043-g. [DOI] [PubMed] [Google Scholar]
- Uddman R., Alumets J., Ehinger B., Håkanson R., Lorén I., Sundler F. Vasoactive intestinal peptide nerves in ocular and orbital structures of the cat. Invest Ophthalmol Vis Sci. 1980 Aug;19(8):878–885. [PubMed] [Google Scholar]
- Whitwell J. ROLE OF THE SYMPATHETIC IN LACRIMAL SECRETION. Br J Ophthalmol. 1961 Jun;45(6):439–445. doi: 10.1136/bjo.45.6.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. E. Pterygopalatine ganglion cytology in monkeys. J Anat. 1984 Sep;139(Pt 2):307–317. [PMC free article] [PubMed] [Google Scholar]
- ten Tusscher M. P., Klooster J., Vrensen G. F. The innervation of the rabbit's anterior eye segment: a retrograde tracing study. Exp Eye Res. 1988 May;46(5):717–730. doi: 10.1016/s0014-4835(88)80058-7. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N., Gorcs T. Synaptic relationships between neurons containing vasopressin, gastrin-releasing peptide, vasoactive intestinal polypeptide, and glutamate decarboxylase immunoreactivity in the suprachiasmatic nucleus: dual ultrastructural immunocytochemistry with gold-substituted silver peroxidase. J Comp Neurol. 1986 Oct 22;252(4):507–521. doi: 10.1002/cne.902520407. [DOI] [PubMed] [Google Scholar]
- van der Werf F., Baljet B., Prins M., Timmerman A., Otto J. A. Innervation of the superior tarsal (Müller's) muscle in the cynomolgus monkey: a retrograde tracing study. Invest Ophthalmol Vis Sci. 1993 Jun;34(7):2333–2340. [PubMed] [Google Scholar]