Abstract
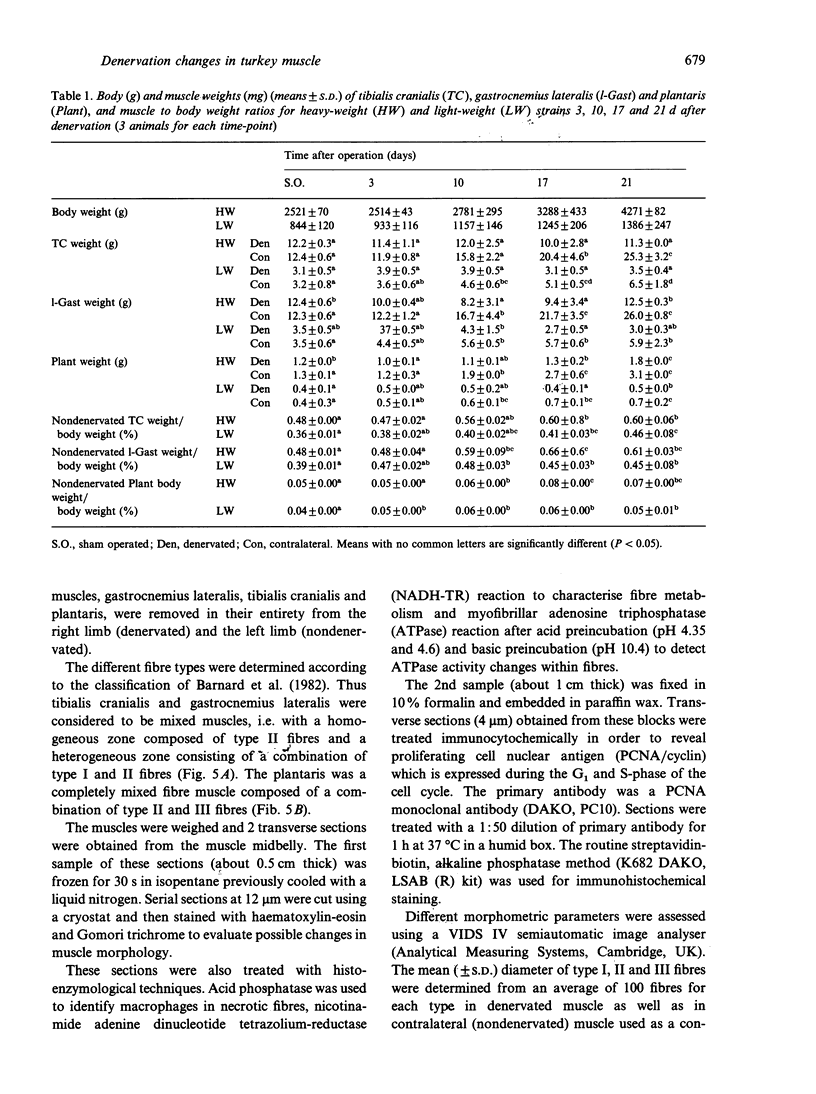
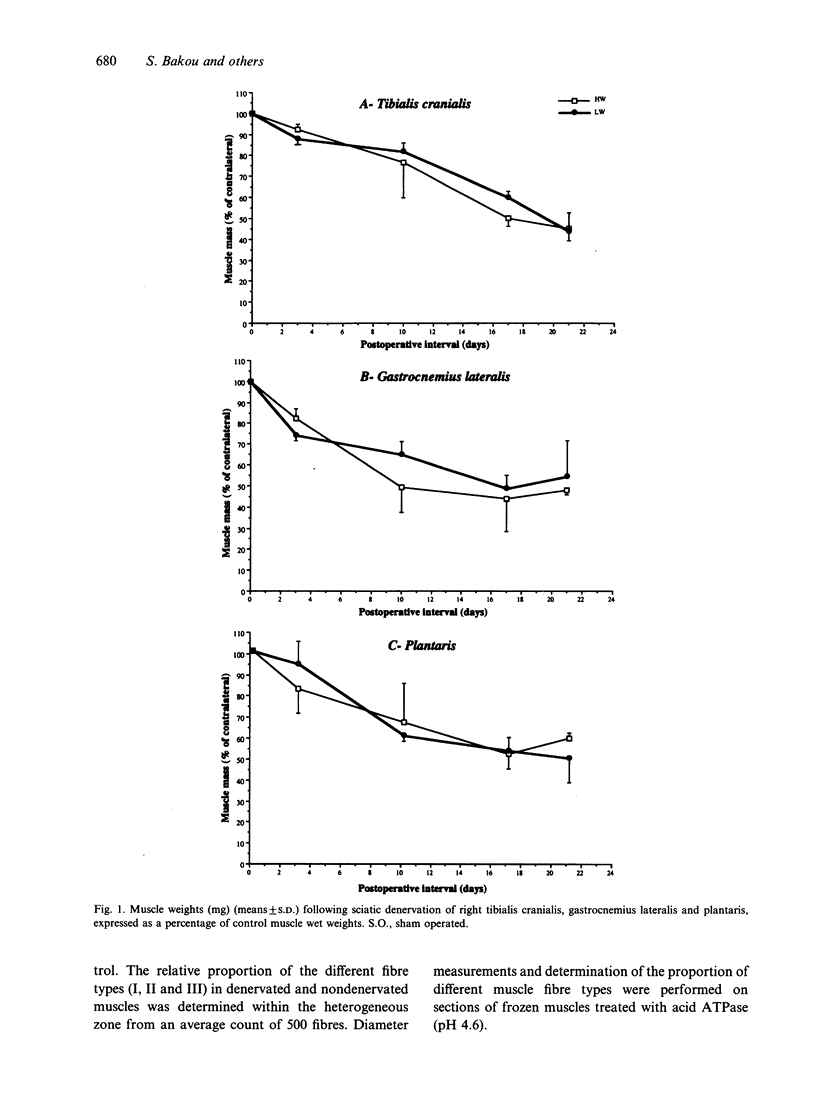
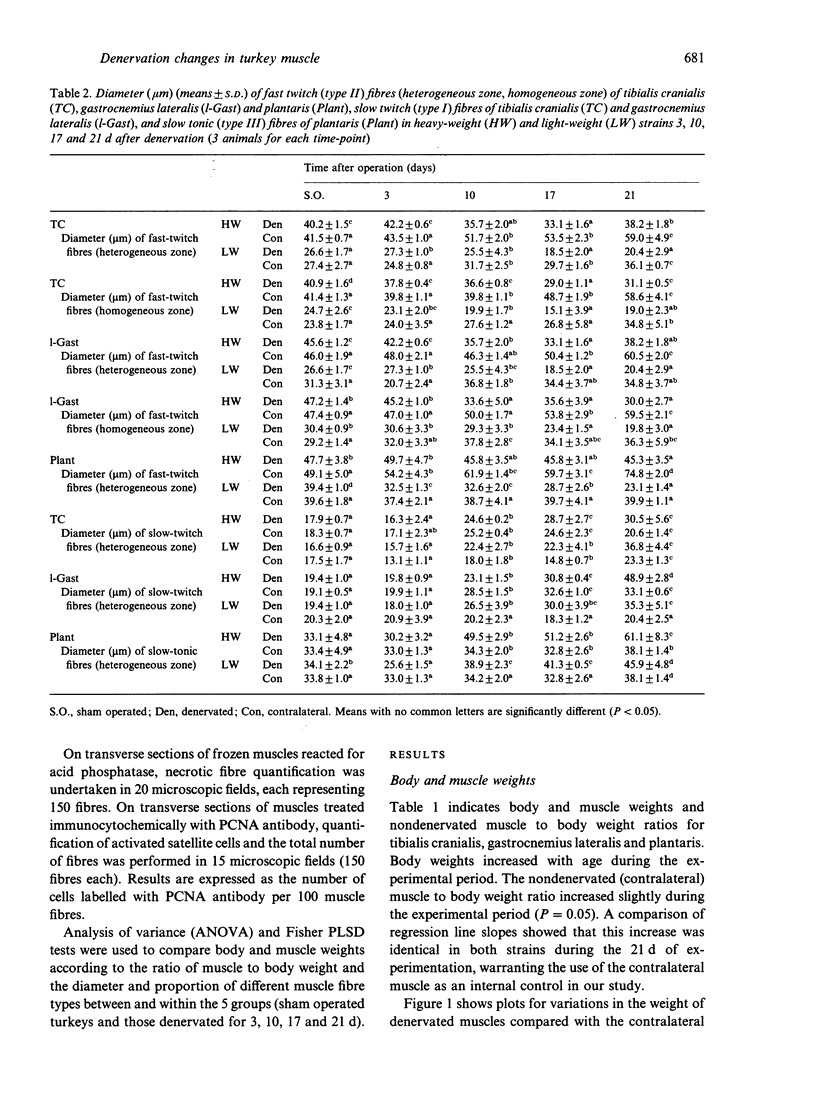
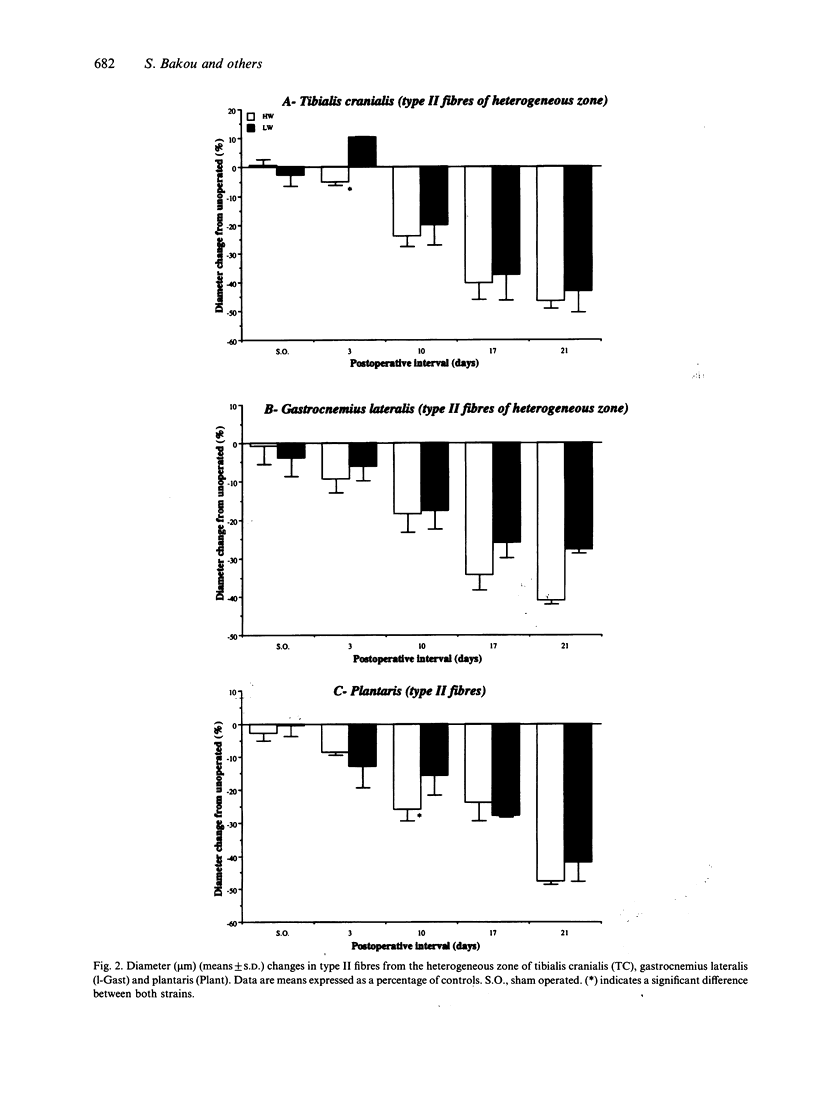
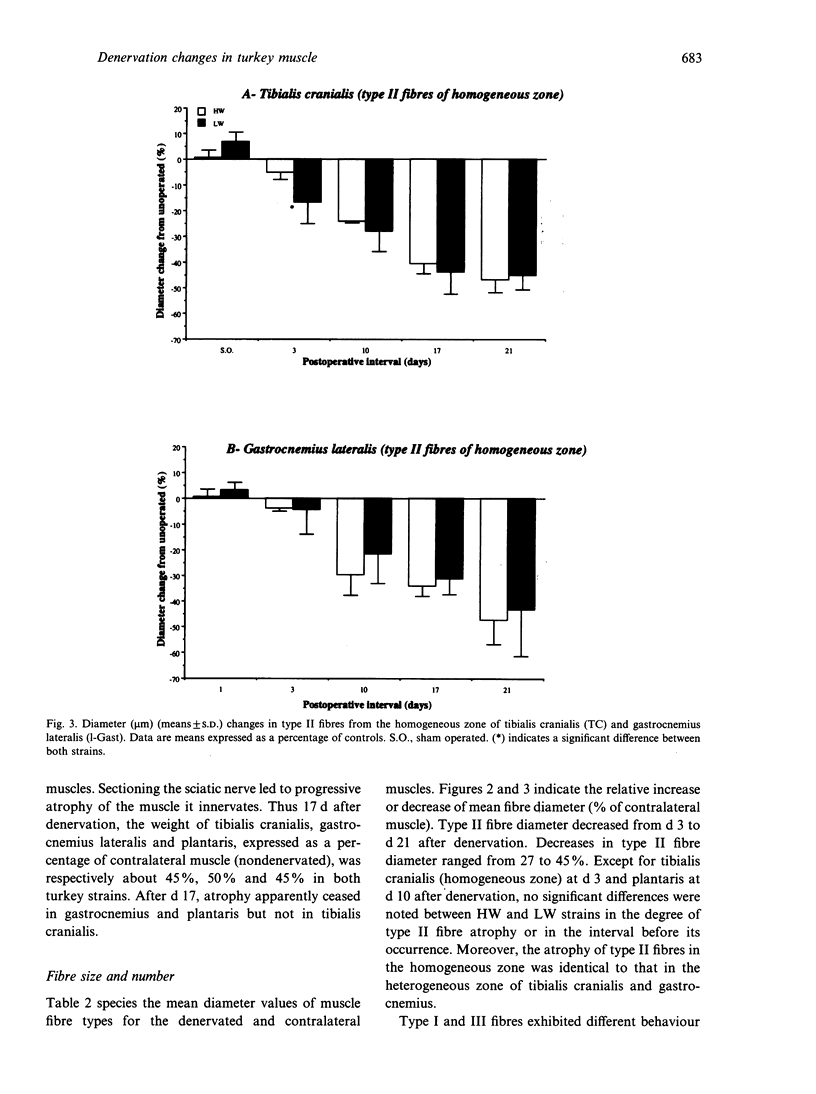
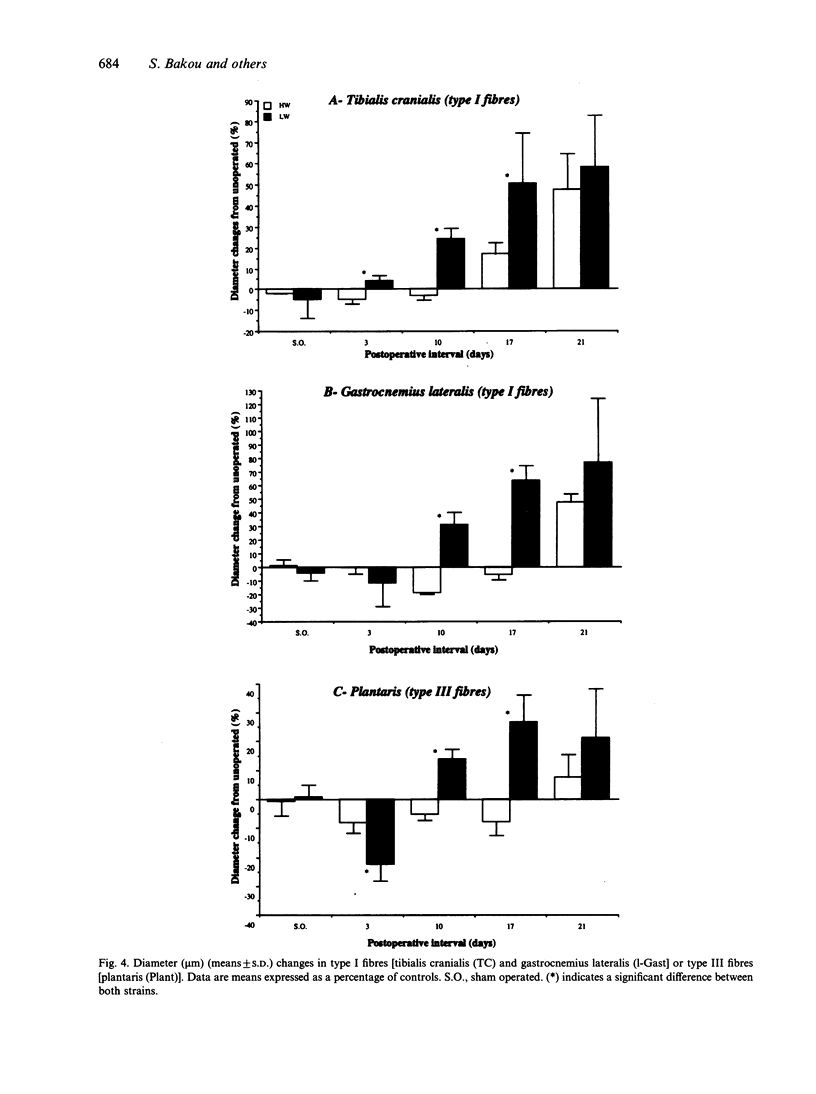
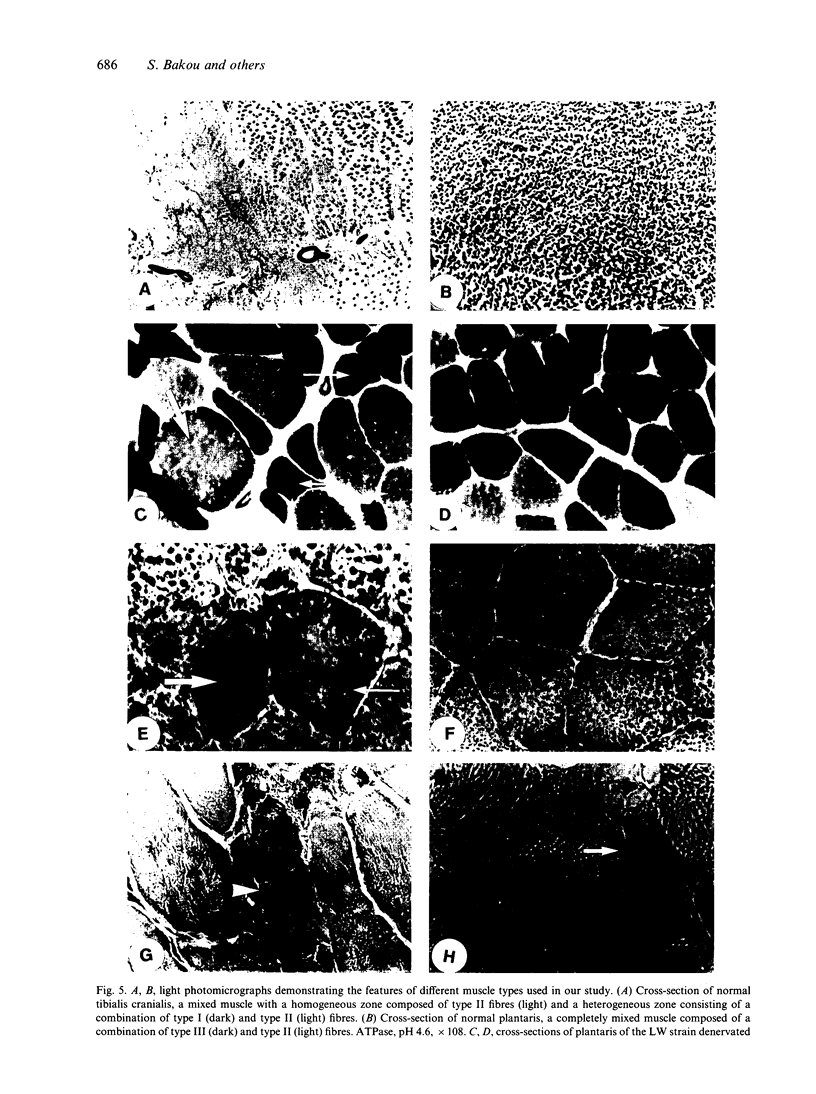
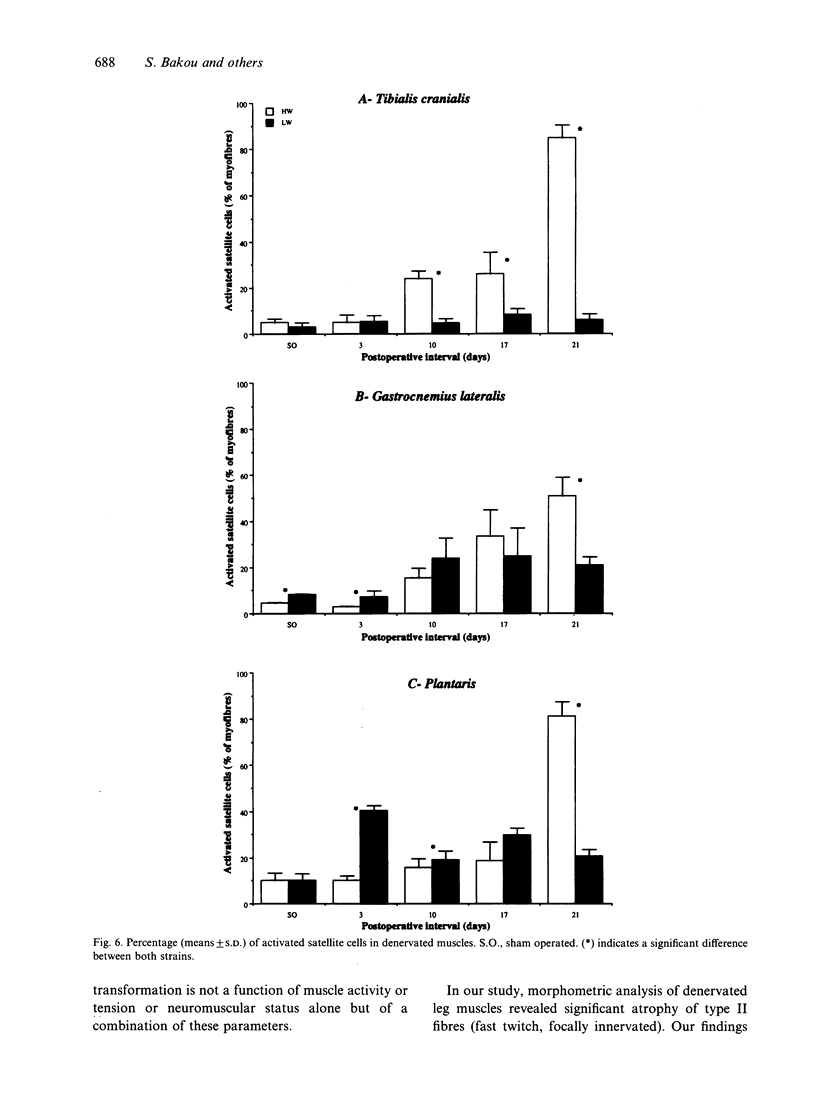
Morphological features and the chronology of muscle changes after denervation were studied over a 21 d period in 2 heavy (HW) and light-weight (LW) strains of 6-wk-old male turkeys. The atrophy of tibialis cranialis, gastrocnemius lateralis and plantaris muscles was apparent at d 3 after denervation. By d 21 the weight of these muscles had reached 45-60% of that of nondenervated contralateral muscle. Cellular lesions, such as irregularities in mitochondrial distribution or coagulative necrosis with fragmentation and lysis associated with moderate infiltration of inflammatory cells, were similar in both strains. Ten days after denervation, immunolabelling of a proliferating cell nuclear antigen (PCNA) expressed during the G1 and S phase of the cell cycle revealed satellite cell activation in denervated muscles. The number of satellite cells activated at d 21 was markedly greater in the HW than LW strain. Morphometric analysis revealed that fast twitch (type II) fibres were atrophied after denervation, whereas slow-twitch (type I) and slow tonic (type III) fibres were hypertrophied from d 10. Hypertrophy occurred more rapidly in the LW than HW strain.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asmussen G., Kiessling A. Hypertrophy and atrophy of mammalian extraocular muscle fibres following denervation. Experientia. 1975 Oct 15;31(10):1186–1188. doi: 10.1007/BF02326784. [DOI] [PubMed] [Google Scholar]
- BAJUSZ E. "RED" SKELETAL MUSCLE FIBERS: RELATIVE INDEPENDENCE OF NEURAL CONTROL. Science. 1964 Aug 28;145(3635):938–939. doi: 10.1126/science.145.3635.938. [DOI] [PubMed] [Google Scholar]
- Barnard E. A., Lyles J. M., Pizzey J. A. Fibre types in chicken skeletal muscles and their changes in muscular dystrophy. J Physiol. 1982 Oct;331:333–354. doi: 10.1113/jphysiol.1982.sp014375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr R. J. Selective denervation of the Musculus pectoralis muscle in the chicken. Poult Sci. 1990 Jan;69(1):124–132. doi: 10.3382/ps.0690124. [DOI] [PubMed] [Google Scholar]
- Cherel Y., Hurtrel M., Gardahaut M. F., Merly F., Magras-Resch C., Fontaine-Perus J., Wyers M. Comparison of postnatal development of anterior latissimus dorsi (ALD) muscle in heavy- and light-weight strains of turkey (Meleagris gallopavo). Growth Dev Aging. 1994 Fall;58(3):157–165. [PubMed] [Google Scholar]
- Christiansen S. P., Baker R. S., Madhat M., Terrell B. Type-specific changes in fiber morphometry following denervation of canine extraocular muscle. Exp Mol Pathol. 1992 Apr;56(2):87–95. doi: 10.1016/0014-4800(92)90026-8. [DOI] [PubMed] [Google Scholar]
- Connold A. L., Kamel-Reid S., Vrbová G., Zak R. Inactivity induces muscle hypertrophy and redistribution of myosin isozymes in chicken anterior latissimus dorsi muscle. Pflugers Arch. 1993 Apr;423(1-2):34–40. doi: 10.1007/BF00374958. [DOI] [PubMed] [Google Scholar]
- Cullen M. J., Harris J. B., Marshall M. W., Ward M. R. An electrophysiological and morphological study of normal and denervated chicken latissimus dorsi muscles. J Physiol. 1975 Feb;245(2):371–385. doi: 10.1113/jphysiol.1975.sp010851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhoot G. K., Perry S. V. The effect of denervation on the distribution of the polymorphic forms of troponin components in fast and slow muscles of the adult rat. Cell Tissue Res. 1982;225(1):201–215. doi: 10.1007/BF00216229. [DOI] [PubMed] [Google Scholar]
- Feng T. P., Lu D. X. New lights on the phenomenon of transient hypertrophy in the denervated hemidiaphragm of the rat. Sci Sin. 1965 Dec;14(12):1772–1784. [PubMed] [Google Scholar]
- Fischbach G. D., Robbins N. Changes in contractile properties of disused soleus muscles. J Physiol. 1969 Apr;201(2):305–320. doi: 10.1113/jphysiol.1969.sp008757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F. The effects of denervation on protein turnover of rat skeletal muscle. Biochem J. 1976 Apr 15;156(1):71–80. doi: 10.1042/bj1560071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T., Vrbová G. The influence of innervation on the differentiation of contractile speeds of developing chick muscles. Pflugers Arch. 1975 Nov 14;360(3):199–218. doi: 10.1007/BF00583716. [DOI] [PubMed] [Google Scholar]
- Gosselin L. E., Brice G., Carlson B., Prakash Y. S., Sieck G. C. Changes in satellite cell mitotic activity during acute period of unilateral diaphragm denervation. J Appl Physiol (1985) 1994 Sep;77(3):1128–1134. doi: 10.1152/jappl.1994.77.3.1128. [DOI] [PubMed] [Google Scholar]
- HESS A. Structural differences of fast and slow extrafusal muscle fibres and their nerve endings in chickens. J Physiol. 1961 Jul;157:221–231. doi: 10.1113/jphysiol.1961.sp006717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins D., Manchester K. L., Gregory M. Histochemical and biochemical characteristics of the transient hypertrophy of the denervated rat hemidiaphragm. Exp Neurol. 1983 Aug;81(2):279–293. doi: 10.1016/0014-4886(83)90263-7. [DOI] [PubMed] [Google Scholar]
- Jaweed M. M., Herbison G. J., Ditunno J. F. Denervation and reinnervation of fast and slow muscles. A histochemical study in rats. J Histochem Cytochem. 1975 Nov;23(11):808–827. doi: 10.1177/23.11.127809. [DOI] [PubMed] [Google Scholar]
- Jirmanová I., Zelená J. Effect of denervation and tenotomy on slow and fast muscles of the chicken. Z Zellforsch Mikrosk Anat. 1970;106(3):333–347. doi: 10.1007/BF00335777. [DOI] [PubMed] [Google Scholar]
- Kelly A. M. Satellite cells and myofiber growth in the rat soleus and extensor digitorum longus muscles. Dev Biol. 1978 Jul;65(1):1–10. doi: 10.1016/0012-1606(78)90174-4. [DOI] [PubMed] [Google Scholar]
- Lieber R. L., Fridén J. O., Hargens A. R., Feringa E. R. Long-term effects of spinal cord transection on fast and slow rat skeletal muscle. II. Morphometric properties. Exp Neurol. 1986 Mar;91(3):435–448. doi: 10.1016/0014-4886(86)90042-7. [DOI] [PubMed] [Google Scholar]
- Madarame H., Fujimoto Y., Moriguchi R. Ultrastructural studies on muscular atrophy in Marek's disease. I. Denervation atrophy in chicken skeletal muscle. A light and electron microscopic study. Jpn J Vet Res. 1986 Jan;34(1):25–49. [PubMed] [Google Scholar]
- Mayer R. F., Burke R. E., Toop J., Walmsley B., Hodgson J. A. The effect of spinal cord transection on motor units in cat medial gastrocnemius muscles. Muscle Nerve. 1984 Jan;7(1):23–31. doi: 10.1002/mus.880070105. [DOI] [PubMed] [Google Scholar]
- McFarland D. C., Pesall J. E., Gilkerson K. K., Swenning T. A. Comparison of the proliferation and differentiation of myogenic satellite cells derived from Merriam's and commercial varieties of turkeys. Comp Biochem Physiol Comp Physiol. 1993 Mar;104(3):455–460. doi: 10.1016/0300-9629(93)90446-b. [DOI] [PubMed] [Google Scholar]
- McGeachie J. K. Sustained cell proliferation in denervated skeletal muscle of mice. Cell Tissue Res. 1989 Aug;257(2):455–457. doi: 10.1007/BF00261848. [DOI] [PubMed] [Google Scholar]
- McGeachie J. K. The fate of proliferating cells in skeletal muscle after denervation or tenotomy: an autoradiographic study. Neuroscience. 1985 Jun;15(2):499–506. doi: 10.1016/0306-4522(85)90228-3. [DOI] [PubMed] [Google Scholar]
- McGeachie J., Allbrook D. Cell proliferation in skeletal muscle following denervation or tenotomy. A series of autoradiographic studies. Cell Tissue Res. 1978 Oct 17;193(2):259–267. doi: 10.1007/BF00209039. [DOI] [PubMed] [Google Scholar]
- Melichna J., Gutmann E. Stimulation and immobilization effects on contractile and histochemical properties of denervated muscle. Pflugers Arch. 1974;352(2):165–178. doi: 10.1007/BF00587515. [DOI] [PubMed] [Google Scholar]
- Murray M. A., Robbins N. Cell proliferation in denervated muscle: time course, distribution and relation to disuse. Neuroscience. 1982 Jul;7(7):1817–1822. doi: 10.1016/0306-4522(82)90039-2. [DOI] [PubMed] [Google Scholar]
- Niederle B., Mayr R. Course of denervation atrophy in type I and type II fibres of rat extensor digitorum longus muscle. Anat Embryol (Berl) 1978 May 31;153(1):9–21. doi: 10.1007/BF00569846. [DOI] [PubMed] [Google Scholar]
- O'Brien R. A., Vrbová G. Acetylcholine synthesis in nerve endings to slow and fast muscles of developing chicks: effect of muscle activity. Neuroscience. 1978;3(12):1227–1230. doi: 10.1016/0306-4522(78)90142-2. [DOI] [PubMed] [Google Scholar]
- Ontell M. Evidence for myoblastic potential of satellite cells in denervated muscle. Cell Tissue Res. 1975 Jul 16;160(3):345–353. doi: 10.1007/BF00222044. [DOI] [PubMed] [Google Scholar]
- Ontell M. Muscle satellite cells: a validated technique for light microscopic identification and a quantitative study of changes in their population following denervation. Anat Rec. 1974 Feb;178(2):211–227. doi: 10.1002/ar.1091780206. [DOI] [PubMed] [Google Scholar]
- Porter J. D., Burns L. A., McMahon E. J. Denervation of primate extraocular muscle. A unique pattern of structural alterations. Invest Ophthalmol Vis Sci. 1989 Aug;30(8):1894–1908. [PubMed] [Google Scholar]
- Reid S. K., Kennedy J. M., Shimizu N., Stewart A., Vrbova G., Zak R. Regulation of expression of avian slow myosin heavy-chain isoforms. Biochem J. 1989 Jun 1;260(2):449–454. doi: 10.1042/bj2600449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanul F. C., Hogan E. L. Enzymatic changes in denervated muscle. I. Histochemical studies. Arch Neurol. 1965 Sep;13(3):263–273. doi: 10.1001/archneur.1965.00470030043003. [DOI] [PubMed] [Google Scholar]
- Rubinstein N. A., Kelly A. M. Myogenic and neurogenic contributions to the development of fast and slow twitch muscles in rat. Dev Biol. 1978 Feb;62(2):473–485. doi: 10.1016/0012-1606(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Shindo M. L., Herzon G. D., Hanson D. G., Cain D. J., Sahgal V. Effects of denervation on laryngeal muscles: a canine model. Laryngoscope. 1992 Jun;102(6):663–669. doi: 10.1288/00005537-199206000-00012. [DOI] [PubMed] [Google Scholar]
- Snow M. H. A quantitative ultrastructural analysis of satellite cells in denervated fast and slow muscles of the mouse. Anat Rec. 1983 Dec;207(4):593–604. doi: 10.1002/ar.1092070407. [DOI] [PubMed] [Google Scholar]
- Sola O. M., Christensen D. L., Martin A. W. Hypertrophy and hyperplasia of adult chicken anterior latissimus dorsi muscles following stretch with and without denervation. Exp Neurol. 1973 Oct;41(1):76–100. doi: 10.1016/0014-4886(73)90182-9. [DOI] [PubMed] [Google Scholar]
- Stewart D. M. Effect of age on the response of four muscles of the rat to denervation. Am J Physiol. 1968 May;214(5):1139–1146. doi: 10.1152/ajplegacy.1968.214.5.1139. [DOI] [PubMed] [Google Scholar]
- Sun S. S., McFarland D. C. Interaction of fibroblast growth factor with turkey embryonic myoblasts and myogenic satellite cells. Comp Biochem Physiol Comp Physiol. 1993 May;105(1):85–89. doi: 10.1016/0300-9629(93)90177-6. [DOI] [PubMed] [Google Scholar]
- Turner L. V., Manchester K. L. Effects of denervation hypertrophy in rat diaphragm muscle on the activity of ribosomes and sap fractions in protein synthesis. Biochim Biophys Acta. 1973 Apr 11;299(4):612–620. doi: 10.1016/0005-2787(73)90234-7. [DOI] [PubMed] [Google Scholar]
- Zhan W. Z., Farkas G. A., Schroeder M. A., Gosselin L. E., Sieck G. C. Regional adaptations of rabbit diaphragm muscle fibers to unilateral denervation. J Appl Physiol (1985) 1995 Sep;79(3):941–950. doi: 10.1152/jappl.1995.79.3.941. [DOI] [PubMed] [Google Scholar]
- Zhan W. Z., Sieck G. C. Adaptations of diaphragm and medial gastrocnemius muscles to inactivity. J Appl Physiol (1985) 1992 Apr;72(4):1445–1453. doi: 10.1152/jappl.1992.72.4.1445. [DOI] [PubMed] [Google Scholar]