Abstract
Objective
Sleep disorders are common in Alzheimer’s disease (AD) patients and can impair the glymphatic system, leading to cognitive decline. This study aimed to investigate whether AD patients with sleep disorders exhibit worse glymphatic function and more severe cognitive impairment compared to those without sleep disorders and to explore the underlying molecular imaging mechanisms.
Methods
This study included 40 AD patients with sleep disorders (ADSD), 39 cognitively matched AD patients without sleep disorders (ADNSD), and 25 healthy middle-aged and elderly controls (NC). Participants underwent functional magnetic resonance imaging (fMRI), and cognitive and sleep assessments. The ALPS (Along the Perivascular Space) index was calculated, followed by intergroup comparisons, correlation analyses, and mediation analyses. The diagnostic utility of the ALPS index was assessed using a receiver operating characteristic (ROC) curve.
Results
The ALPS index was lower in the ADNSD and ADSD groups compared to the NC group. In the ADSD group, PSQI scores were negatively correlated with MMSE scores. The ALPS index was positively correlated with MMSE scores and negatively with PSQI scores. Mediation analyses indicated that the ALPS index partially mediated the effect of sleep disturbances on cognitive impairment (indirect effect = −0.134; mediation effect = 30.505%). The area under the ROC curve (AUROC) for distinguishing ADSD from ADNSD was 0.86, with a cutoff ALPS index value 1.309.
Conclusion
Sleep disorders worsen glymphatic function and cognitive impairment in AD patients. The ALPS index partially mediates the impact of sleep disorders on cognitive function and shows moderate accuracy in distinguishing between patients with ADSD and ADNSD.
Keywords: Alzheimer’s disease, sleep disorders, glymphatic system, diffusion tensor imaging analysis along the perivascular space (DTI-ALPS), mediation analysis
Introduction
As is well known, Alzheimer’s Disease (AD) is a neurodegenerative disease characterized by the deposition of amyloid beta (Aβ) and excessive phosphorylation of tau protein.1,2 Among the several high-risk factors for AD, sleep disruptions (SD) are prevalent in over 50% of AD patients3 and can increase the risk of AD by promoting Aβ deposition and abnormal phosphorylation of tau protein, etc.4 The impact of sleep disorders on brain functional activity in AD patients has always been a research hotspot. According to structural magnetic resonance imaging (sMRI) research, AD patients with sleep disorders (ADSD) had lower brainstem and pineal gland volume than AD patients without sleep disorders (ADNSD),5,6 both of which are important brain regions for regulating circadian rhythms. Functional magnetic resonance imaging (fMRI) studies revealed a decreased static amplitude of low-frequency fluctuation (sALFF) in the precentral gyrus and a decreased percent amplitude of fluctuation (PerAF) in both the precentral and postcentral gyrus in the ADSD group.7,8 Weakened activation of these areas may be related to adverse sleep outcomes such as decreased slow-wave sleep and more overnight awakenings. In addition, a recent study has found that the functional connections between the sensorimotor network with the posterior central gyrus as the critical node and the primary visual network with the cuneus and calcarine as the critical nodes are impaired in the ADSD group.9 Furthermore, a Single-photon emission computed tomography (SPECT) study found abnormal activation of the dorsal lateral prefrontal cortex (DLPFC) in the ADSD group, which may be related to impaired cholinergic pathways, leading to sleep deprivation and cognitive dysfunction.10 However, the above studies focus on structural and functional aspects. In recent years, research has confirmed that the human brain’s perivascular space (PVS) network can function as glymphatic circulation,11 providing new ideas for further exploring the impact of sleep disorders on AD.
The glymphatic system (GS) is a vital waste clearance system in the brain and plays a crucial role in cerebrospinal fluid (CSF) circulation and the exchange of substances between the cerebrospinal fluid and interstitial fluid (ISF). It is closely related to the transport and clearance of Aβ and tau proteins in the central nervous system.12,13 The glymphatic system is closely related to the sleep state, becoming more active during sleep, especially during stage 3 of slow-wave activity, which is conducive to the excretion of pathological proteins.14,15 Research has shown that interruption of slow-wave sleep increases levels of Aβ in cerebrospinal fluid.16 Notably, Aβ concentrations in the right hippocampus, which is the critical brain region for memory, significantly increase after only one night of sleep deprivation in adults.17 Recent studies have found that middle-aged and elderly patients with chronic insomnia have significant glymphatic dysfunction, and glymphatic dysfunction is significantly correlated with cognitive decline.18 This shows that SD causes damage to the glymphatic system and interfere Aβ metabolism, and affect cognitive function. According to research, aging itself also leads to a decline in glymphatic circulation in the human brain.19 In middle-aged and elderly individuals, chronic insomnia further exacerbates glymphatic dysfunction, leading to the accumulation of Aβ and tau proteins, which increases the risk of dementia.20
In recent years, the use of magnetic resonance imaging (MRI) to evaluate glymphatic system function has been widely applied in clinical and scientific research, among which the most commonly used method is the diffusion tensor image analysis along the perivascular space (DTI-ALPS) proposed by Taoka et al.21 The ALPS index is calculated to reflect the integrity of the glymphatic system by separating the diffusivity of anteriorly and posteriorly oriented water molecules in the region of the projection and association fibers at the level of the lateral ventricle. The reliability of the ALPS index as a measure of glymphatic activity has been confirmed by a recent study, which demonstrated a significant correlation between the ALPS index and glymphatic clearance measured using intrathecal angiography,22 providing new insights into human glymphatic function.
It has been shown that SD accelerates the pathological process of AD.9 However, whether SD worsens cognitive impairment in AD and the relationship between SD, ALPS index, and cognitive status remains worth exploring. In this study, we considered sleep status an independent AD risk factor. We examined the differences in glymphatic system function between Alzheimer’s disease with sleep disorders (ADSD) and Alzheimer’s disease without sleep disorders (ADNSD). We hypothesized that SD not only exacerbates glymphatic dysfunction but also accelerates cognitive decline in patients with AD.
Methods
Participants
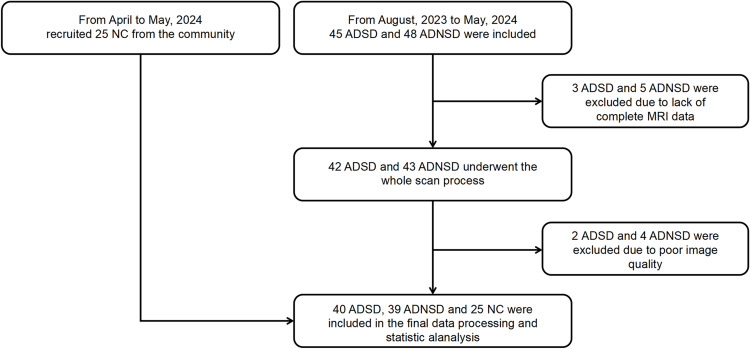
This study prospectively recruited 40 patients with ADSD and 39 patients with ADNSD from the Neurology Clinic of The First Affiliated Hospital of Soochow University between August 2023 and May 2024. Additionally, 25 healthy middle-aged and elderly volunteers were enrolled from the community, as illustrated in the flowchart in Figure 1. All participants were right-handed, and the diagnosis of AD was confirmed by an experienced neurologist according to the 2011 National Institute on Aging-Alzheimer’s Association (NIA-AA) criteria.23 A neurologist also assessed healthy volunteers using the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MOCA) to evaluate their cognitive status. Sleep quality was assessed using the Chinese version of the Pittsburgh Sleep Quality Index (PSQI), with a score of 7 or higher indicating the presence of a sleep disorder.24 We try our best to control the influence of confounding factors on the research results. In the process of including patients, we first carefully inquired about the onset time of sleep disorders in the ADSD group to ensure that all included patients developed sleep disorders after exhibiting symptoms of cognitive impairment and that the sleep disorders lasted for at least one month. Secondly, to minimize the impact of hypnotic drugs on brain function, all ADSD patients underwent rs-MRI examinations before any clinical intervention for sleep disorders. Finally, we carefully inquired about the patients’ daily routines, and excluded patients who had insufficient sleep due to personal lifestyle reasons. Exclusion criteria included: a Fazekas score of ≥3, other neurological disorders (eg, Lewy body dementia, frontotemporal lobe dementia, epilepsy, encephalitis, brain tumors, and Parkinson’s disease), a history of psychiatric disorders, contraindications for magnetic resonance scanning, or poor-quality MRI scans. The study complied with the Declaration of Helsinki and received approval from the Ethics Committee of the First Affiliated Hospital of Soochow University (number 2024–317th). All participants adhered to the principle of complete voluntary participation, we obtained the consent of the patient’s legal guardians and signed a written informed consent form prior to participating in the study.
Figure 1.
Research flowchart.
Abbreviations: ADSD, AD patients with sleep disorders; ADNSD, AD patients without sleep disorders; NC, Normal control.
Image Acquisition
A 3.0 T MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) was used to acquire NODDI data from the subjects. During the scan, an 8-channel coil was used for the head, with sponge pads to secure the subjects’ heads. The scanning parameters for the NODDI sequence were as follows: field of view (FOV) = 240 mm × 240 mm, layer thickness = 2 mm, repetition time (TR) = 5000 ms, echo time (TE) = 95.0 ms, voxel size = 2 × 2×2 mm, diffusion-sensitive gradient directions = 64, diffusion sensitivity coefficients b = 0, b = 1000s/mm2, b = 2000s/ mm2. A total of 66 layers were scanned in each direction, with the total scan time lasting 11 minutes and 21 seconds. Additionally, conventional T1- and T2-weighted images, as well as T2WI FLAIR sequences, were also collected to exclude organic lesions. Throughout the scan, participants were instructed to keep their eyes closed, remain awake, and minimize any movement. At the end of the scan, an experienced radiologist performed an initial image quality assessment, excluding any patients with significant motion artifacts.
Preprocessing of DTI Data and Calculation of ALPS Indexes
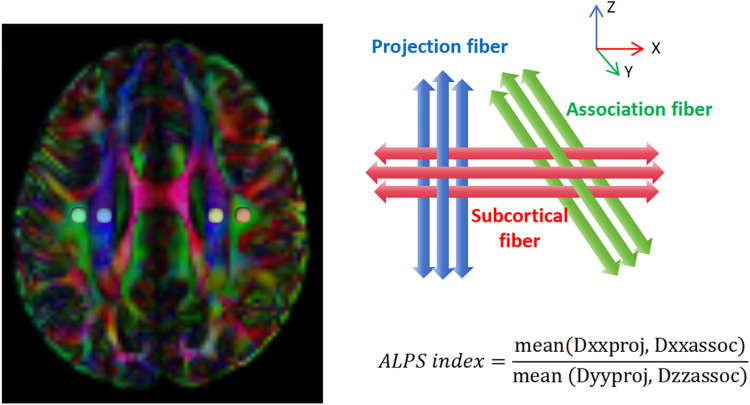
In this study, we used DSI Studio software (version “Chen” build January 13, 2022, http://dsi-studio.labsolver.org) to preprocess the images and calculate the ALPS index. The specific steps are as follows: File format conversion: The original DICOM files were converted to NIFTI format using MRIcroN software (https://www.nitrc.org/projects/mricron). The NIFTI files were then imported into DSI Studio and converted to SRC format. Image correction: Use TOPUP/EDDY to perform phase distortion and eddy current correction on images to correct distortions caused by magnetization and eddy currents; Use motion programs to perform motion correction on images, aligning all images in the time series with the b0 image at the first time point to correct involuntary head movements of subjects during the acquisition process; Set up masks to improve reconstruction efficiency: Use the Q-space differential reconstruction (QSDR) method to convert the images into MNI space and and align them with the ICBM152_adult template; Estimate and output color-coded FA maps and x-axis, y-axis, and z-axis diffusivity maps; Place region of interest (ROI): Place a spherical ROI with a radius of 3mm at the positions of projection fibers represented by the superior corona radia (SCR) and association fibers represented by the superior longitudinal fasciculus (SLF) on both sides of the lateral ventricle body level. A radiologist who is unaware of clinical information checks the position of the ROI, ensuring that it is placed in the projection fibers and association fibers, and that there is no overlap between each ROI. If necessary, slight manual correction can be performed. The four central coordinates for the ROIs were as follows: left projection fiber (53,46,40), left association fiber (59,46,40), right projection fiber (27,46,40), and right association fiber (21,46,40). Calculation of the ALPS index: Calculate the ALPS index according to the formula in Figure 2, where Dxxproj is the diffusion rate along the X-axis in the projection fiber, Dxxassoc is the diffusion rate along the X-axis in the association fiber, Dyyproj is the diffusion rate along the Y-axis in the projection fiber, and Dzzassoc is the diffusion rate along the Z-axis in the association fiber.
Figure 2.
Calculation of Alps index. Dxxproj is the diffusion rate along the X-axis in the projection fiber, Dxxassoc is the diffusion rate along the X-axis in the association fiber, Dyyproj is the diffusion rate along the Y-axis in the projection fiber, and Dzzassoc is the diffusion rate along the Z-axis in the association fiber.
Statistical Analysis
Demographic and clinical data were analyzed using SPSS 27.0. The Shapiro–Wilk test was employed to assess the normality of continuous variables. For normally distributed continuous variables, between-group comparisons were conducted using one-way ANOVA. For variables that did not conform to a normal distribution, the Kruskal–Wallis test was used, with post hoc two-by-two comparisons adjusted using the Bonferroni correction. Categorical variables were compared using the chi-squared test. Pearson’s correlation analysis is used to calculate the correlation between the ALPS index and clinical scales (MMSE, MOCA, PSQI), as well as the correlation between the ALPS index and age and education level. In addition, we used a two-sample t-test to analyze whether there were differences in the ALPS index among different genders and APOE4 carrier states. ROC curves were used to assess the diagnostic performance of the ALPS index in differentiating between ADSD and ADNSD. Finally, we included the ALPS index as a mediating variable in a structural equation model. Mediation effects were tested using Model 4 in the SPSS PROCESS macro, and the Bootstrap method provided by Hayes was explored for verification. We included age, gender, and APOE4 carrying status as covariates. All tests were two-tailed, and p < 0.05 was considered statistically significant.
Results
Demographic and Neuropsychological Data
The study included 40 participants with ADSD, 39 with ADNSD, and 25 healthy controls (NC). Demographic analysis showed the following: 26 females in the ADSD group, with a mean age of 68.63 ± 6.19 years; 23 females in the ADNSD group, with a mean age of 69.90±6.28 years; and 13 females in the NC group, with a mean age of 67.36±7.13 years. There were no significant differences between ADSD and ADNSD patients in demographic and clinical characteristics other than the PSQI scores (p < 0.001). The detailed baseline demographic data are presented in Table 1.
Table 1.
Demographic and Clinical Data
| ADSD (n=40) | ADNSD (n=39) | NC (n=25) | P | Post Hoc Tests P value | |||
|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | |||||
| Age (year) | 68.63±6.19 | 69.90±6.28 | 67.36±7.13 | 0.306 | – | – | – |
| Gender (F/M) | 26/14 | 23/16 | 13/12 | 0.580 | – | – | – |
| Education (year) | 6.15±3.84 | 6.38±4.08 | 6.84±3.30 | 0.875 | – | – | – |
| APOE4, n (%) | 22 (55.00%) | 23 (58.97%) | – | 0.721 | – | – | – |
| MMSE | 18.18±3.13 | 19.08±2.91 | 29.24±0.97 | <0.001 | 0.925 | 0.000 | 0.000 |
| MOCA | 14.88±4.01 | 14.56±2.65 | 27.72±1.62 | <0.001 | 0.969 | 0.000 | 0.000 |
| PSQI | 11.95±3.43 | 3.77±1.84 | 3.12±1.72 | <0.001 | 0.000 | 0.000 | 1.000 |
Abbreviations: ADSD, AD patients with sleep disorders; ADNSD, AD patients without sleep disorders; NC, Normal control; MMSE, Mini-Mental state examination; MOCA, Montreal Cognitive Assessment Scale; PQSI, Pittsburgh Sleep Quality Index.
Comparison of ALPS Index Between Groups
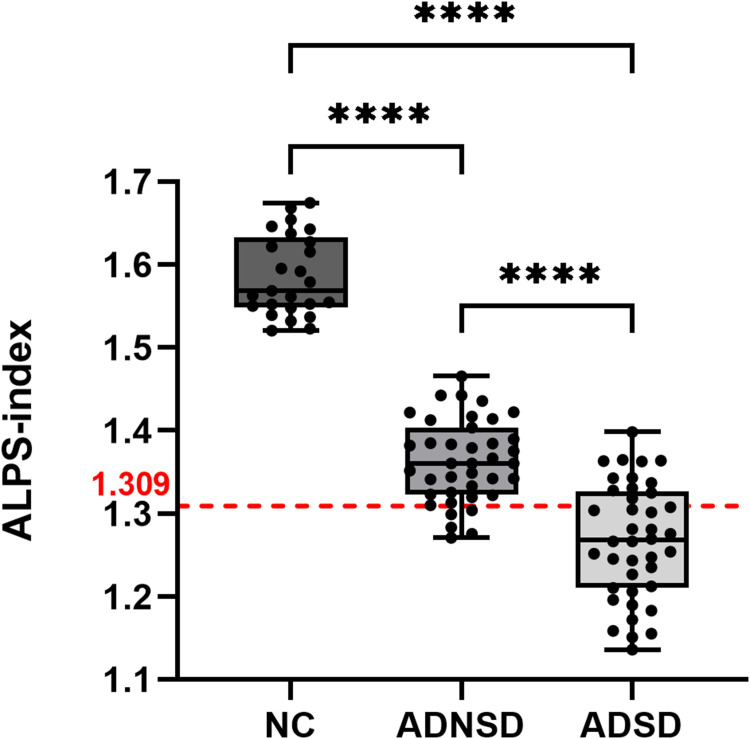
The ALPS index for both hemispheres and the mean ALPS index showed significant differences among the three groups. Specifically, the ALPS index was significantly lower in both the ADNSD and ADSD groups compared to the NC group (1.586 ± 0.048 vs 1.363 ± 0.049 vs 1.268 ± 0.069, p < 0.001), as illustrated in Figure 3. Additionally, there were significant differences in ALPS index between the three groups in both the left hemisphere (1.587 ± 0.060 vs 1.359 ± 0.056 vs 1.249 ± 0.092, p < 0.001) and the right hemisphere (1.586 ± 0.054 vs 1.367 ± 0.056 vs 1.287 ± 0.062, p < 0.001) (Table 2).
Figure 3.
Difference of the Alps-index between groups. ALPS-index cut-off of 1.309; ****p < 0.0001.
Abbreviation: ALPS index, The index of diffusivity along the perivascular space.
Table 2.
Differences of the ALPS Index Between Groups
| ADSD (n=40) | ADNSD (n=39) | NC (n=25) | P | Post Hoc Tests P value | |||
|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | |||||
| Left ALPS index | 1.249±0.092 | 1.359±0.056 | 1.587±0.060 | <0.001 | <0.001 | 0.000 | 0.000 |
| Right ALPS index | 1.287±0.062 | 1.367±0.056 | 1.586±0.054 | <0.001 | <0.001 | 0.000 | 0.000 |
| Mean ALPS index | 1.268±0.069 | 1.363±0.049 | 1.586±0.048 | <0.001 | <0.001 | 0.000 | 0.000 |
Abbreviations: ADSD, AD patients with sleep disorders; ADNSD, AD patients without sleep disorders; NC, Normal control; ALPS index, The index of diffusivity along the perivascular space.
Correlation of the ALPS Index with Neuropsychological Scales
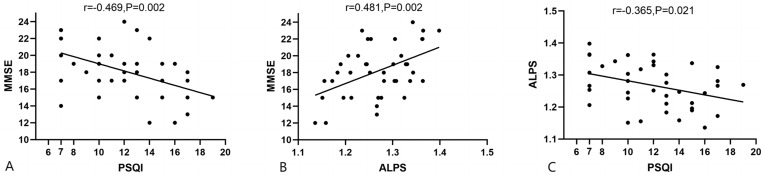
Correlation analyses revealed that in the ADSD group, PSQI scores were significantly negatively correlated with MMSE scores (r = −0.469, P = 0.002) (Figure 4A), ALPS and MMSE, MOCA scores were significantly positively correlated (r = 0.481, P = 0.002) (r=0.397, P=0.011) (Figure 4B, Supplementary Figure 1 and Supplementary Table 1). PSQI scores and ALPS index were negatively correlated (r = −0.365, P = 0.021) (Figure 4C). In addition, we did not find a significant correlation between ALPS index and age or education level in the ADSD group (Supplementary Table 1); There is no significant difference in ALPS between different genders and APOE4 carrier states (Supplementary Tables 2 and 3).
Figure 4.
Correlation Analysis of Alps-index with neuropsychological scales in ADSD patients. (A) Correlation between PSQI score and MMSE score; (B) Correlation between ALPS index and MMSE score; (C) Correlation between PSQI score and ALPS index.
Abbreviations: ALPS index, The index of diffusivity along the perivascular space; MMSE, Mini-Mental state examination; PQSI, Pittsburgh Sleep Quality Index.
Mediation Analysis and ROC Analysis
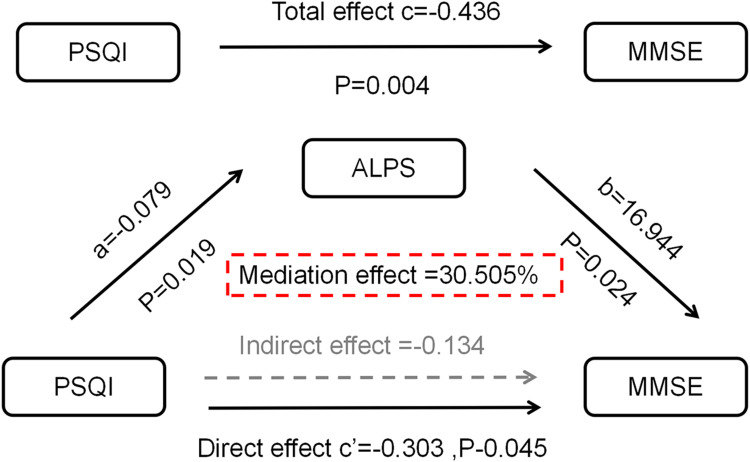
To further explore the relationships between PSQI, MMSE, and the ALPS index and to understand the mechanism of cognitive impairment due to sleep disorders, we conducted a mediation analysis. The mediating analysis showed that the ALPS index partially mediated the effect of sleep disturbance on cognitive impairment in AD patients (indirect effect = −0.134, mediated effect = 30.505%) (Figure 5 and Table 3).
Figure 5.
Mediation analysis. A mediation analysis based on SPSS Model 4, with gender, age, years of education, and APOE4 gene carrier status as covariates.
Abbreviations: ALPS index, The index of diffusivity along the perivascular space; MMSE, Mini-Mental state examination; PQSI, Pittsburgh Sleep Quality Index.
Table 3.
Detailed Table of Total Effects, Direct Effects, and Indirect Effects
| Effect | Se | LLCI | ULCI | Effect Quantity | |
|---|---|---|---|---|---|
| Total effect | −0.436 | 0.143 | −0.727 | −0.146 | – |
| Direct effect | −0.303 | 0.146 | −0.598 | −0.067 | 69.495% |
| Indirect effect | −0.134 | 0.098 | −0.391 | −0.076 | 30.505% |
Abbreviations: Se, Standard error of indirect effects; LLCI, Lower limit of 95% CI; ULC, Upper limit of 95% CI.
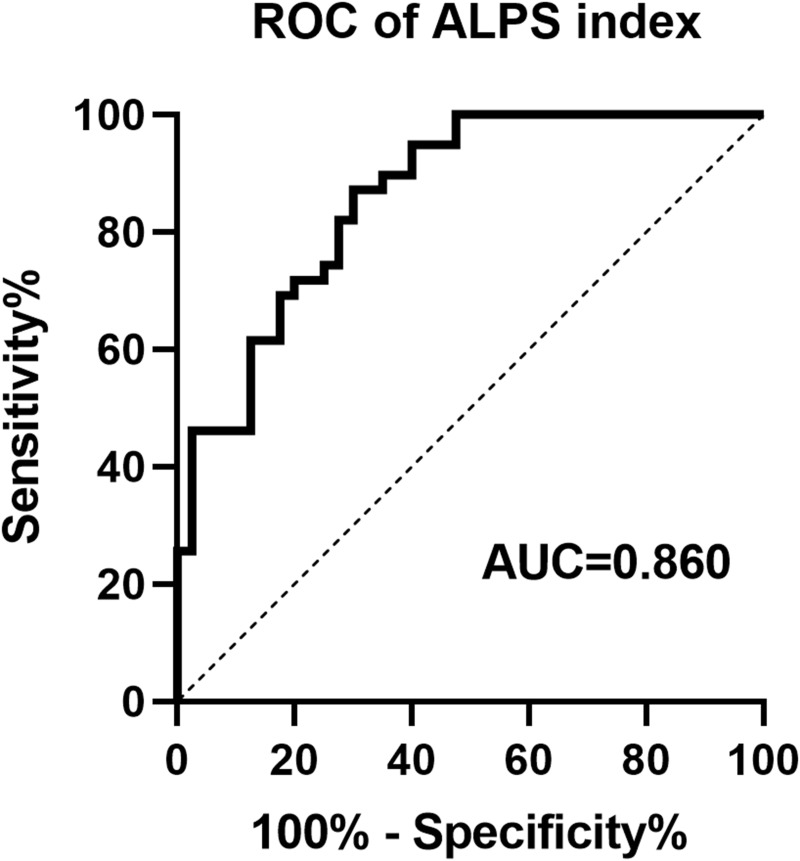
ROC analysis indicated that the ALPS-index equivocally and moderately differentiated between sleep disorders and normal sleep in AD patients, with an area under the curve (AUC) of 0.86 (95% CI: 0.78 to 0.93) (Figure 6). The ALPS-index demonstrated high sensitivity (87.2%) and relatively low specificity (70.0%) for predicting sleep disorders, with a cutoff value of 1.309.
Figure 6.
The ROC curve of Alps index distinguishing ADSD and ADNSD.
Abbreviation: ROC, Receiver Operating Characteristic.
Discussion
This study examined sleep disorders as independent risk factors for AD. It introduced the ALPS index to investigate the effect of sleep disorders on AD development from the perspective of glymphatic circulation. The results revealed that the ALPS index in the ADSD group was significantly lower compared to the ADNSD group. Furthermore, the ALPS index was significantly positively correlated with MMSE scores and significantly negatively correlated with PSQI scores in the ADSD group, suggesting that the ALPS index mediates the cognitive impairment induced by sleep disorders. This study suggests that sleep disorders exacerbate glymphatic function and cognitive decline, with the effects of sleep disorders on cognition being partially mediated by the ALPS index.
Both sleep disorders and Alzheimer’s disease impair glymphatic system function. Our findings of a progressive and significant decrease in the ALPS index among patients with ADNSD and ADSD compared to NC suggest two things: first, that glymphatic function is impaired in patients with Alzheimer’s disease, and second, that sleep disorders exacerbate the deterioration of their glymphatic function. Previous studies have confirmed that glymphatic dysfunction persists throughout the AD spectrum, from subjective cognitive decline (SCD) to mild cognitive impairment (MCI), becoming increasingly severe. This is reflected in the ALPS index, which shows a decreasing trend as the disease progresses, reaching its lowest levels in AD.25 The animal model of AD revealed the relationship between AD pathology and glymphatic circulation disorders. The absence of aquaporin-4 (AQP4) in astrocytes, which glymphatic exchange depends on, slows down the rate of CSF-CSF exchange, affects the clearance of Aβ, and accelerates the formation of Aβ plaques.12,26,27 Glymphatic clearance is significantly enhanced during sleep;28 Hablitz et al have found that there is a circadian rhythm in mice glymphatic clearance rate, which is regulated by AQP4 polarization;29 SD mice exhibit AQP4 polarization disorder and glymphatic clearance inhibition;30,31 Zhang et al also found that AQP4 deficiency leads to impaired glymphatic function in mice with chronic sleep interruption, resulting in the accumulation of Aβ and tau proteins in the brain, ultimately leading to working memory impairment.32 From this, we might speculate that AQP4 may be an essential factor in the interaction of SD, AD, and pathological protein deposition and could be an important target for future interventions. However, unfortunately, this study did not collect Aβ and tau proteins in the CSF of AD patients, nor could it evaluate their AQP4 status. Therefore, we can consider including such indicators in future studies. In addition, there is an interaction between sleep disorders and glymphatic damage. Sleep disorders can inhibit glymphatic system function, and glymphatic system dysfunction can also lead to the deposition of orexins (A and B) in the brain, mainly in the dorsal raphe and locus coeruleus, resulting in fragmented sleep and low sleep efficiency.33 Various sleep disorders, such as REM sleep behavior disorder (RBD) and obstructive sleep apnea (OSA), can impair glymphatic function.34,35 Therefore, although this study did not differentiate between types of sleep disorders in detail, it does not affect the conclusion that sleep disorders exacerbate AD glymphatic function damage.
Our correlation analysis showed that PSQI scores were significantly negatively correlated with MMSE scores in the ADSD group (r = −0.469, P = 0.002), indicating that sleep disorders aggravate cognitive impairment in AD patients. Studies have shown that acute and chronic sleep deprivation affects the metabolism of Aβ and tau proteins,36 leading to their abnormal accumulation in the brain, often resulting in cognitive dysfunction.28 The mammalian sleep cycle includes two core stages: non-rapid eye movement (NREM) and rapid eye movement (REM) sleep. Slow-wave sleep (SWS), is the third stage of NREM sleep. The memory consolidation effect of sleep occurs mainly at this stage, and REM sleep can strengthen and consolidate this process through different molecular pathways.37 Studies have documented significant alterations in the sleep structure of AD patients, with a significant reduction in slow-wave and REM sleep. These changes predominantly affect brain regions such as the parietal-temporal and frontal regions, which are vital for memory consolidation and cognitive functions.38,39 Additionally, sleep fragmentation, characterized by frequent awakenings and reduced sleep efficiency, has been linked to an increased risk of AD and cognitive decline.40 We also found a significant negative correlation between PSQI scores and the ALPS index in the ADSD group (r = −0.365, P = 0.021) and a significant positive correlation between the ALPS index and MMSE scores (r = 0.481, P = 0.002). These findings align with previous studies.21,41,42 Saito et al found a significant negative correlation between the ALPS index and the use of hypnotic drugs.41 Therefore, in order to exclude the influence of hypnotic drugs on the research results, this study included patients who had not yet undergone drug intervention. Li et al found that a lower ALPS index was predictive of increased cognitive impairment,25 while Kamagata et al reported that a lower ALPS index was associated with reduced CSF Aβ42 levels and deficits across several cognitive domains in patients with AD and MCI.43 These results highlight the intricate relationship between sleep disorders, glymphatic impairment, and cognitive decline.
To explore these complex relationships further, mediation analyses were conducted. The results showed that the ALPS index partially mediated the exacerbation of cognitive impairment by sleep disorders in the ADSD group, accounting for 30.5% of the total effect. This suggests that sleep disorders exacerbate cognitive impairment in AD, in part because of their impaired glymphatic function. Animal experiments have found that significantly enhanced glymphatic activity can reduce Aβ load and improve memory in AD mouse models.44 Hsu et al found that in AD, the ALPS index was negatively correlated with brain Aβ and tau protein deposition and positively correlated with cognitive scores. They also noted that ALPS index mediated cognitive dysfunction related to Aβ and tau deposition in some brain regions (eg, precuneus), which are at a high risk of being affected by sleep disorder.27 Their research indirectly supports our results, and in our future research, we should also consider adopting a multimodal approach and incorporating diverse imaging parameters. Additionally, Jin et al found that glymphatic dysfunction appears early in middle-aged and older individuals and plays a crucial role in the progression from chronic insomnia to cognitive impairment.18 Our findings elucidate the interactions between sleep disorders, the glymphatic system, and cognitive function and deepen the understanding of the influence of sleep on cognition. ROC analysis showed that the ALPS index was moderately accurate (AUC = 0.86) and highly sensitive (87.2%) in distinguishing AD patients with and without sleep disorders, though its specificity was relatively low (70.0%). This suggests that while the ALPS index shows potential as a marker for detecting sleep disorders in patients with AD, it should be used alongside other indicators to improve diagnostic accuracy.
Our study has several limitations. First, the sample size was relatively small, and all were collected from the same hospital, which may introduce selection bias and limit the generalizability of our findings. Second, this study used only the PSQI to assess sleep-related disorders without incorporating objective measures such as sleep polysomnography, which could provide more comprehensive information about sleep disorders. Third, we did not include a detailed classification of sleep disorders in our current study, such as OSA, RBD, difficulty falling asleep, sleep fragmentation, and circadian rhythm disorders. Fourth, we did not assess the pathological burden in the AD patients so we could not provide direct evidence linking the ALPS index to specific biomarkers of AD. Moreover, PET scans were not performed in this study, therefore the dynamics of central Aβ or tau proteins cannot be resolved. In future research, we will collect more comprehensive imaging indicators. In addition, using only the MMSE to assess cognitive status is limited; future studies should include more comprehensive neuropsychological assessment. Finally, this study is a cross-sectional study with certain limitations in demonstrating causal relationships. In future research, we will consider using longitudinal studies to further explore the relationship between sleep disorders and cognition in the AD patients.
In conclusion, our study confirms that sleep disorders exacerbate glymphatic impairment and worsen cognitive deficits in patients with AD. The combination of the ALPS index and our findings sheds light on the mechanisms by which sleep disorders contribute to cognitive impairment. This highlights the importance of sleep in the progression of patients with AD and provides a basis for future clinician decision-making and targeted interventions.
Acknowledgments
The author would like to express their gratitude to the National Natural Science Foundation of China (grant number 81971573) and the Suzhou Gusu Medical Youth Talent (grant number GSWS2020019) for providing funding support for this study. In addition, thank you to all volunteers for their active participation in this study. An unauthorized version of the Chinese MMSE was used by the study team without permission, however, this has now been corrected with PAR. The MMSE is a copyrighted instrument and may not be used or reproduced in whole or in part, In any form or language, or by any means without written permission of PAR (www.parinc. com).
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant number 81971573) and the Suzhou Gusu Medical Youth Talent (grant number GSWS2020019).
Abbreviations
AD, Alzheimer’s Disease; ADSD, Alzheimer’s Disease with Sleep Disorders; ADNSD, Alzheimer’s Disease without Sleep Disorders; NC, Normal Controls; SD, Sleep disturbances; SCD, subjective cognitive decline; MCI, mild cognitive impairment; PSQI, Pittsburgh Sleep Quality Index; MMSE, Mini-Mental state examination; MOCA, Montreal Cognitive Assessment Scale; sALFF, static Amplitude of Low-Frequency Fluctuation; PerAF, Percent Amplitude of Fluctuation; SPECT, Single-Photon Emission Computed Tomography; DLPFC, Dorsal Lateral Prefrontal Cortex; NREM, Non-Rapid Eye Movement; REM, Rapid Eye Movement; SWS, Slow-Wave Sleep; OSA, Obstructive Sleep Apnea; RBD, Rapid Eye Movement Sleep Behavior Disorder; ROC, Receiver Operating Characteristic; DTI-ALPS, Diffusion Tensor Image Analysis along the Perivascular Space; AUROC, Area Under the Receiver Operating Characteristic Curve; ISF, Interstitial Fluid; GS, Glymphatic System; SCR, Superior Corona Radia; SLF, Superior Longitudinal Fasciculus; QSDR, Q-space Differential Reconstruction; NODDI, Neurite Orientation Dispersion and Density Imaging; ROI, Region of Interest; MNI, Montreal Neurological Institute; ICBM152, International Consortium for Brain Mapping 152.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Khan S, Barve KH, Kumar MS. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr Neuropharmacol. 2020;18(11):1106–1125. PMID: 32484110; PMCID: PMC7709159. doi: 10.2174/1570159X18666200528142429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rostagno AA. Pathogenesis of Alzheimer’s disease. Int J Mol Sci. 2022;24(1):107. PMID: 36613544; PMCID: PMC9820480. doi: 10.3390/ijms24010107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chappel-Farley MG, Lui KK, Dave A, Chen IY, Mander BA. Candidate mechanisms linking insomnia disorder to Alzheimer’s disease risk. Curr Opin Behav Sci. 2020;33:92–98. doi: 10.1016/j.cobeha.2020.01.010 [DOI] [Google Scholar]
- 4.Sadeghmousavi S, Eskian M, Rahmani F, Rezaei N. The effect of insomnia on development of Alzheimer’s disease. J Neuroinflammation. 2020;17(1):289. PMID: 33023629; PMCID: PMC7542374. doi: 10.1186/s12974-020-01960-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JH, Jung WS, Choi WH, Lim HK. Aberrant brain stem morphometry associated with sleep disturbance in drug-naïve subjects with Alzheimer’s disease. Neuropsychiatr Dis Treat. 2016;12:2089–2093. PMID: 27601903; PMCID: PMC5003099. doi: 10.2147/NDT.S114383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J, Suh SW, Kim GE, et al. Smaller pineal gland is associated with rapid eye movement sleep behavior disorder in Alzheimer’s disease. Alzheimers Res Ther. 2020;12(1):157. PMID: 33220712; PMCID: PMC7680594. doi: 10.1186/s13195-020-00725-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li K, Luo X, Zeng Q, et al. Interactions between sleep disturbances and Alzheimer’s disease on brain function: a preliminary study combining the static and dynamic functional MRI. Sci Rep. 2019;9(1):19064. PMID: 31836777; PMCID: PMC6911090. doi: 10.1038/s41598-019-55452-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Peng D. Altered intrinsic brain activity in mild Alzheimer’s disease patients with sleep disturbances. Neuroreport. 2021;32(11):942–948. PMID: 34132706. doi: 10.1097/WNR.0000000000001689 [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Zhu R, Zhou X, Zhang Z, Peng D. Altered local and remote functional connectivity in mild Alzheimer’s disease patients with sleep disturbances. Front Aging Neurosci. 2023;15:1269582. PMID: 37920381; PMCID: PMC10619161. doi: 10.3389/fnagi.2023.1269582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ismail Z, Herrmann N, Francis PL, et al. A SPECT study of sleep disturbance and Alzheimer’s disease. Dement Geriatr Cognit Disord. 2009;27(3):254–259. PMID: 19246910. doi: 10.1159/000203889 [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17(11):1016–1024. PMID: 30353860; PMCID: PMC6261373. doi: 10.1016/S1474-4422(18)30318-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. PMID: 22896675; PMCID: PMC3551275. doi: 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishida K, Yamada K, Nishiyama R, et al. Glymphatic system clears extracellular tau and protects from tau aggregation and neurodegeneration. J Exp Med. 2022;219(3):e20211275. PMID: 35212707; PMCID: PMC8932543. doi: 10.1084/jem.20211275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hablitz LM, Vinitsky HS, Sun Q, et al. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv. 2019;5(2):eaav5447. PMID: 30820460; PMCID: PMC6392807. doi: 10.1126/sciadv.aav5447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. PMID: 24136970; PMCID: PMC3880190. doi: 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsson M, Ärlig J, Hedner J, Blennow K, Zetterberg H. Sleep deprivation and cerebrospinal fluid biomarkers for Alzheimer’s disease. Sleep. 2018;41(5). PMID: 29425372. doi: 10.1093/sleep/zsy025 [DOI] [PubMed] [Google Scholar]
- 17.Shokri-Kojori E, Wang GJ, Wiers CE, et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A. 2018;115(17):4483–4488. PMID: 29632177; PMCID: PMC5924922. doi: 10.1073/pnas.1721694115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin Y, Zhang W, Yu M, et al. Glymphatic system dysfunction in middle-aged and elderly chronic insomnia patients with cognitive impairment evidenced by diffusion tensor imaging along the perivascular space (DTI-ALPS). Sleep Med. 2024;115:145–151. PMID: 38364456. doi: 10.1016/j.sleep.2024.01.028 [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Cai J, Zhang W, et al. Impairment of the glymphatic pathway and putative meningeal lymphatic vessels in the aging human. Ann Neurol. 2020;87(3):357–369. PMID: 31916277. doi: 10.1002/ana.25670 [DOI] [PubMed] [Google Scholar]
- 20.Sabia S, Fayosse A, Dumurgier J, et al. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. 2021;12(1):2289. PMID: 33879784; PMCID: PMC8058039. doi: 10.1038/s41467-021-22354-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol. 2017;35(4):172–178. PMID: 28197821. doi: 10.1007/s11604-017-0617-z [DOI] [PubMed] [Google Scholar]
- 22.Taoka T, Ito R, Nakamichi R, et al. Reproducibility of diffusion tensor image analysis along the perivascular space (DTI-ALPS) for evaluating interstitial fluid diffusivity and glymphatic function: cHanges in Alps index on multiple conditiON acquIsition eXperiment (CHAMONIX) study. Jpn J Radiol. 2022;40(2):147–158. doi: 10.1007/s11604-021-01187-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. PMID: 21514250; PMCID: PMC3312024. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilz LK, Keller LK, Lenssen D, Roenneberg T. Time to rethink sleep quality: PSQI scores reflect sleep quality on workdays. Sleep. 2018;41(5). PMID: 29420828. doi: 10.1093/sleep/zsy029 [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Wang L, Zhong J, et al.,;. Impaired glymphatic function as a biomarker for subjective cognitive decline: an exploratory dual cohort study. Alzheimers Dement. 2024;20:6542–6555. PMID: 39107995. doi: 10.1002/alz.14149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon M, Wang MX, Ismail O, et al. Loss of perivascular aquaporin-4 localization impairs glymphatic exchange and promotes amyloid β plaque formation in mice. Alzheimers Res Ther. 2022;14(1):59. PMID: 35473943; PMCID: PMC9040291. doi: 10.1186/s13195-022-00999-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu JL, Wei YC, Toh CH, et al. Magnetic resonance images implicate that glymphatic alterations mediate cognitive dysfunction in Alzheimer disease. Ann Neurol. 2023;93(1):164–174. PMID: 36214568; PMCID: PMC10091747. doi: 10.1002/ana.26516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klostranec JM, Vucevic D, Bhatia KD, et al. Current concepts in intracranial interstitial fluid transport and the glymphatic system: part I-Anatomy and physiology. Radiology. 2021;301(3):502–514. PMID: 34665028. doi: 10.1148/radiol.2021202043 [DOI] [PubMed] [Google Scholar]
- 29.Hablitz LM, Plá V, Giannetto M, et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun. 2020;11(1):4411. PMID: 32879313; PMCID: PMC7468152. doi: 10.1038/s41467-020-18115-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achariyar TM, Li B, Peng W, et al. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener. 2016;11(1):74. Erratum in: Mol Neurodegener. 2017 Jan 12;12(1):3. doi: 10.1186/s13024-016-0147-7. PMID: 27931262; PMCID: PMC5146863. doi: 10.1186/s13024-016-0138-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu DX, He X, Wu D, et al. Continuous theta burst stimulation facilitates the clearance efficiency of the glymphatic pathway in a mouse model of sleep deprivation. Neurosci Lett. 2017;653:189–194. PMID: 28576566. doi: 10.1016/j.neulet.2017.05.064 [DOI] [PubMed] [Google Scholar]
- 32.Zhang R, Liu Y, Chen Y, et al. Aquaporin 4 deletion exacerbates brain impairments in a mouse model of chronic sleep disruption. CNS Neurosci Ther. 2020;26(2):228–239. PMID: 31364823; PMCID: PMC6978250. doi: 10.1111/cns.13194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christensen J, Yamakawa GR, Shultz SR, Mychasiuk R. Is the glymphatic system the missing link between sleep impairments and neurological disorders? Examining the implications and uncertainties. Prog Neurobiol. 2021;198:101917. PMID: 32991958. doi: 10.1016/j.pneurobio.2020.101917 [DOI] [PubMed] [Google Scholar]
- 34.Bae YJ, Kim JM, Choi BS, et al. Altered brain glymphatic flow at diffusion-tensor MRI in rapid eye movement sleep behavior disorder. Radiology. 2023;307(5):e221848. PMID: 37158722. doi: 10.1148/radiol.221848 [DOI] [PubMed] [Google Scholar]
- 35.Lee HJ, Lee DA, Shin KJ, Park KM. Glymphatic system dysfunction in obstructive sleep apnea evidenced by DTI-ALPS. Sleep Med. 2022;89:176–181. PMID: 35030357. doi: 10.1016/j.sleep.2021.12.013 [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Holtzman DM. Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacology. 2020;45(1):104–120. PMID: 31408876; PMCID: PMC6879647. doi: 10.1038/s41386-019-0478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. PMID: 20046194. doi: 10.1038/nrn2762 [DOI] [PubMed] [Google Scholar]
- 38.Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev. 2015;19:29–38. PMID: 24846773. doi: 10.1016/j.smrv.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 39.Hassainia F, Petit D, Nielsen T, Gauthier S, Montplaisir J. Quantitative EEG and statistical mapping of wakefulness and REM sleep in the evaluation of mild to moderate Alzheimer’s disease. Eur Neurol. 1997;37(4):219–224. PMID: 9208261. doi: 10.1159/000117446 [DOI] [PubMed] [Google Scholar]
- 40.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36(7):1027–1032. doi: 10.5665/sleep.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saito Y, Hayakawa Y, Kamagata K, et al. Glymphatic system impairment in sleep disruption: diffusion tensor image analysis along the perivascular space (DTI-ALPS). Jpn J Radiol. 2023;41(12):1335–1343. PMID: 37368182. doi: 10.1007/s11604-023-01463-6 [DOI] [PubMed] [Google Scholar]
- 42.Steward CE, Venkatraman VK, Lui E, et al. Assessment of the DTI-ALPS parameter along the perivascular space in older adults at risk of dementia. J Neuroimaging. 2021;31(3):569–578. PMID: 33556226. doi: 10.1111/jon.12837 [DOI] [PubMed] [Google Scholar]
- 43.Kamagata K, Andica C, Takabayashi K, et al.; Alzheimer’s Disease Neuroimaging Initiative. Association of MRI indices of glymphatic system with amyloid deposition and cognition in mild cognitive impairment and Alzheimer disease. Neurology. 2022;99(24):e2648–e2660. PMID: 36123122; PMCID: PMC9757870. doi: 10.1212/WNL.0000000000201300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee Y, Choi Y, Park EJ, et al. Improvement of glymphatic-lymphatic drainage of beta-amyloid by focused ultrasound in Alzheimer’s disease model. Sci Rep. 2020;10(1):16144. PMID: 32999351; PMCID: PMC7527457. doi: 10.1038/s41598-020-73151-8 [DOI] [PMC free article] [PubMed] [Google Scholar]