Abstract
Pyrimidine nucleotides are of general importance for many aspects of cell function, but their role in the regulation of biosynthetic processes is still unclear. In this study, we investigate the influence of a decreased expression of UMP synthase (UMPS), a key enzyme in the pathway of de novo pyrimidine synthesis, on biosynthetic processes in growing potato (Solanum tuberosum) tubers. Transgenic plants were generated expressing UMPS in the antisense orientation under the control of the tuber-specific patatin promoter. Lines were selected with markedly decreased expression of UMPS in the tubers. Decreased expression of UMPS restricted the use of externally supplied orotate for de novo pyrimidine synthesis in tuber tissue, whereas the uridine-salvaging pathway was stimulated. This shift in the pathways of UMP synthesis was accompanied by increased levels of tuber uridine nucleotides, increased fluxes of [14C]sucrose to starch and cell wall synthesis, and increased amounts of starch and cell wall components in the tubers, whereas there were no changes in uridine nucleotide levels in leaves. Decreased expression of UMPS in tubers led to an increase in transcript levels of carbamoylphosphate synthase, uridine kinase, and uracil phosphoribosyltransferase, the latter two encoding enzymes in the pyrimidine salvage pathways. Thus, the results show that antisense inhibition of the de novo pathway of pyrimidine synthesis leads to a compensatory stimulation of the less energy-consuming salvage pathways, probably via increased expression and activity of uridine kinase and uracil phosphoribosyltransferase. This results in increased uridine nucleotide pool levels in tubers and improved biosynthetic performance.
INTRODUCTION
Throughout nature, purine and pyrimidine nucleotides are important cellular compounds that participate in many important biochemical processes. In addition to their pivotal role as building blocks for nucleic acid synthesis, they are also required for energy metabolism and for the continued synthesis of many biosynthetic products such as phospholipids or polysaccharides, for which specific nucleotides are required as cofactors in activating the appropriate precursor (Traut and Jones, 1996; Stasolla et al., 2003; Kafer et al., 2004). Uridine nucleotides are, for example, important cofactors involved in the use of sugars for glycogen synthesis in mammals (Stryer, 1996) and for starch and cell wall synthesis in plants (Heldt, 1997). However, molecular approaches have not yet been taken to assess the importance of changes in uridine nucleotide pool levels for biosynthetic performance (Stasolla et al., 2003).
The classical de novo pathway of pyrimidine synthesis terminates with the synthesis of UMP, whereas other divergent pathways lead to the formation of CTP and thymidine 5′-triphosphate (Neuhard and Nygaard, 1987). De novo UMP synthesis is highly conserved in both prokaryotes and eukaryotes, with the gene organization of the different steps of this pathway varying among organisms (Traut and Jones, 1996). In plants, the first three steps are catalyzed by separate enzymes—carbamoylphosphate synthase (CPS), aspartyl transcarbamoylase, and dihydroorotase—whereas the last two steps, converting orotate to UMP, are catalyzed by a single protein (UMP synthase [UMPS]) having two enzymatic activities, orotate phosphoribosyltransferase and orotidylate decarboxylase. UMPS is one of the key enzymes of de novo pyrimidine synthesis and is often regarded, both in mammalian and plant systems, as a key regulatory step of the pathway (Santoso and Thornburg, 1992; Traut and Jones, 1996).
In addition to the de novo pathway, several salvage pathways exist that allow cells to use preformed nucleotides as precursors, thereby avoiding the high metabolic cost of biosynthesis (Jones and Hann, 1979; Neuhard and Nygaard, 1987). Uridine kinase (UK) and uracil phosphoribosyltranferase (UPRT) are enzymes involved in two alternative salvage pathways for the synthesis of UMP that use uridine and uracil, respectively, as precursors. Studies have been performed in both mammals and plants in which the activities of de novo and salvage enzymes and the relative incorporation of exogenously supplied orotate, uridine, and uracil have been compared to assess the relative importance of these pathways. In mammalian tissues, both de novo and uridine salvage pathways are generally active, although often not to equivalent levels, with a decrease in the relative rate of the de novo pathway being observed in many adult tissues (Traut and Jones, 1996). In plants, most studies have been performed on actively growing potato (Solanum tuberosum) tubers, in which, similarly, high activities of both de novo and uridine salvage pathways were found (Katahira and Ashihara, 2002). The high salvage activity in tubers may be attributable to the rapid turnover of nucleotides and could be expected to contribute both to starch synthesis and to the maintenance of sufficient energy and substrate required for cell division and enlargement (Stasolla et al., 2003). In addition to this, the active salvage pathways observed in growing tubers would be a very efficient mechanism for generating nucleotides at low cost (Stasolla et al., 2003).
Despite the recent progress in establishing the pathways of pyrimidine synthesis in many organisms, little is known about the factors that control their interaction and coordination. Furthermore, molecular studies are lacking to assess the importance of changes in uridine nucleotide pool levels for various aspects of metabolic performance (Moffat and Ashihara, 2002; Stasolla et al., 2003). In one study, UMPS mutants were isolated from haploid cell suspensions of Nicotiana tabacum (Santoso and Thornburg, 1992). There was a reduced ability to regenerate plants from these mutant cells; however, given the somewhat cursory nature of that study, it is unclear whether this was a direct or a pleiotropic effect of the mutation. In this study, we generated transgenic potato plants with antisense inhibition of UMPS under the control of the tuber-specific B33 patatin promoter. Lines were selected with a 30 to 85% decrease in UMPS activity in growing tubers. Decreased UMPS activity restricted the use of orotate for uridine nucleotide synthesis in tuber tissue, whereas uridine salvage was increased. Unexpectedly, this resulted in higher uridine nucleotide levels accompanied by increased rates of starch and cell wall synthesis in growing tubers. There was an increase in the transcript levels of CPS, UK, and UPRT, the latter two encoding enzymes in the pyrimidine salvage pathways with activities that were also found to increase. Results show that antisense inhibition of the de novo pathway of pyrimidine synthesis leads to a compensatory stimulation of the less energy-consuming salvage pathways via increased expression of UK and UPRT, resulting in increased uridine nucleotide pool levels in tubers and improved biosynthetic performance.
RESULTS
Generation of Potato Plants with Decreased Expression of UMPS in Tubers
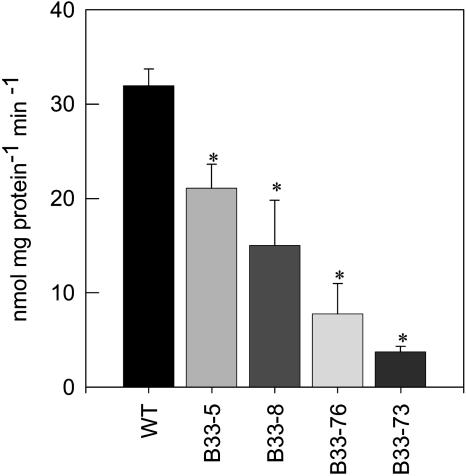
Potato plants (cv Desiree) were transformed with an antisense construct containing an Asp718/XbaI fragment of the cDNA encoding UMPS isolated from a potato tuber cDNA library, essentially as described by Kossmann et al. (1991). This gene fragment was expressed under the control of the B33 patatin promoter, which confers tuber-specific expression in parenchyma cells (Liu et al., 1990). After regeneration of plants, transformation was verified via kanamycin resistance and RNA gel blot analysis of UMPS transcription. After this primary screen, nine lines were selected, amplified, and grown in the greenhouse. Lines were selected from this harvest that showed a 30 to 80% decrease in UMPS activity in developing tubers (Figure 1).
Figure 1.
Antisense Inhibition of UMPS in Potato Tubers.
UMPS activities of tubers of transgenic plants with altered expression of StUMPS. Activity was measured in developing tubers isolated from 10-week-old potato plants. Data are presented as means ± se of determinations on six individual plants per line. Values marked with asterisks were determined to be significantly different from the wild-type value by t test.
Decreased Expression of UMPS Led to an Unexpected Increase in Uridine Nucleotide Pool Levels
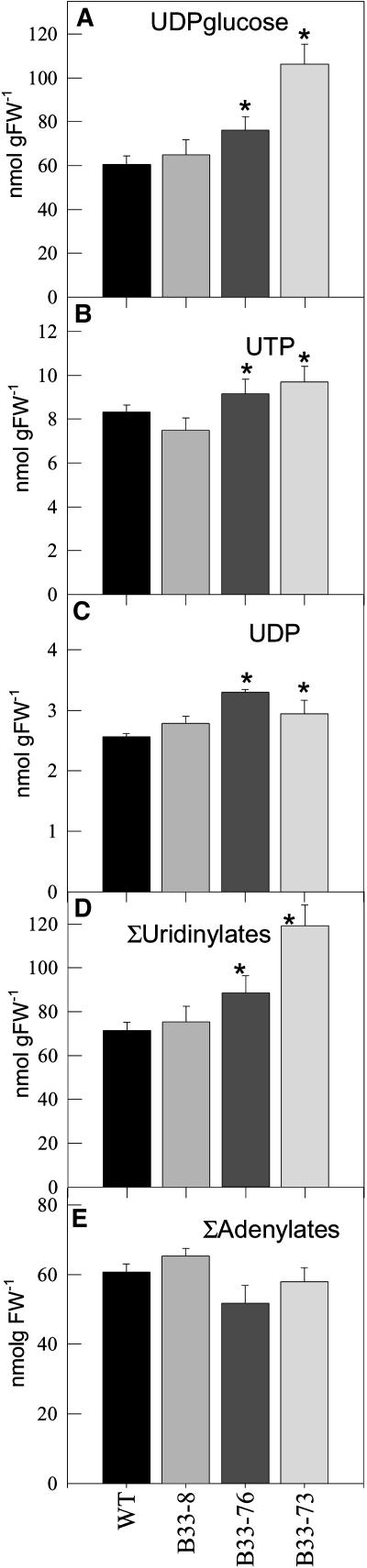
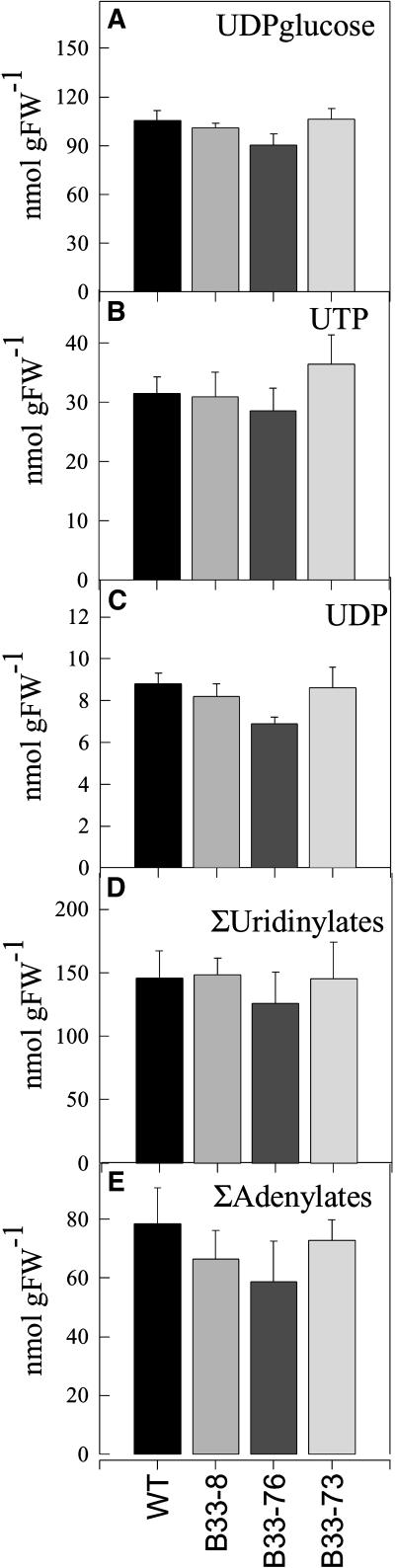
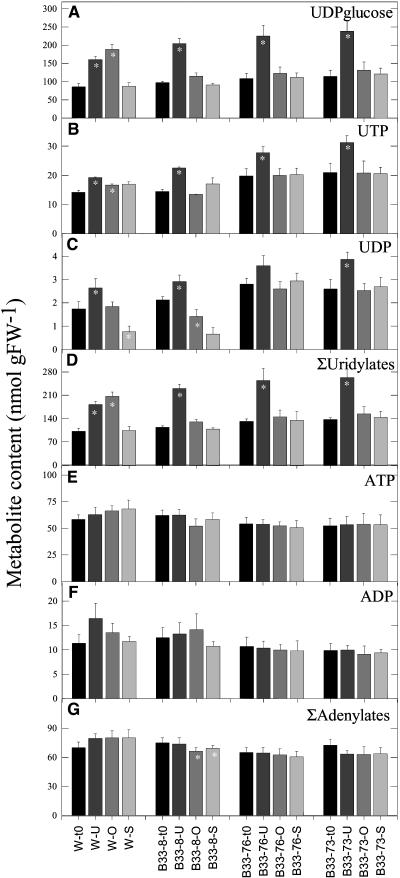
The influence of decreased expression of UMPS on the levels of uridine nucleotide pools was investigated by measuring UDP-Glc, UTP, and UDP levels in developing tubers (Figure 2). Unexpectedly, decreased UMPS activity led to an up to twofold increase in the total pool of uridine nucleotide levels (sum of UDP-Glc, UTP, and UDP; UMP was not detectable). UDP-Glc, representing the largest proportion of the uridine nucleotide pool, showed the strongest increase. No significant changes were observed in adenine nucleotide (ATP, ADP, and AMP) or guanine nucleotide (GTP and GDP) pools, revealing that the effect of decreased UMPS expression on nucleotide pool levels was specific to the uridine nucleotides. Analysis of nucleotide levels in the leaves of the transformants revealed that these were unchanged with respect to wild-type levels (Figure 3).
Figure 2.
Uridinylate and Adenylate Levels of Transgenic Potato Tubers.
(A) UDP-Glc.
(B) UTP.
(C) UDP.
(D) Total uridinylates.
(E) Total adenylates.
Data are presented as means ± se of determinations on six individual plants per line. Values marked with asterisks were determined to be significantly different from wild-type values by t test. FW, fresh weight.
Figure 3.
Uridinylate and Adenylate Levels of Transgenic Potato Leaves.
(A) UDP-Glc.
(B) UTP.
(C) UDP.
(D) Total uridinylates.
(E) Total adenylates.
Data are presented as means ± se of determinations on six individual plants per line.
Influence of Decreased Expression of UMPS on the Use of Sucrose for Biosynthetic Processes
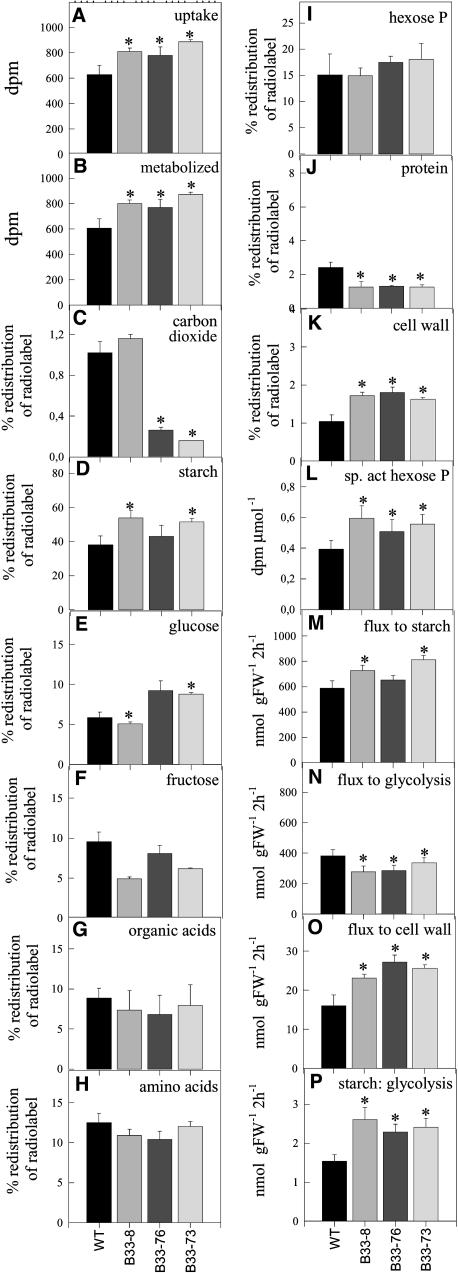
In potato tubers, sucrose is metabolized via sucrose synthase catalyzing the UDP-dependent conversion of sucrose to fructose and UDP-Glc. The latter is either directly used by cellulose synthase for cell wall synthesis or converted to Glc-1-P and UTP in a PPi-dependent reaction that is catalyzed by UGPase. Glc-1-P is subsequently used for glycolysis in the cytosol or for starch biosynthesis in the plastid. To investigate the influence of increased uridine nucleotide levels on sucrose metabolism, we incubated potato tuber discs from wild-type and transgenic lines with reduced UMPS activity for 2 h with [14C]sucrose and analyzed the way they metabolized the labeled sucrose (Figure 4). Decreased UMPS led to a stimulation of sucrose uptake (Figure 4A) and sucrose degradation (Figure 4B). An increased proportion of the label entered starch (Figure 4D) and cell walls (Figure 4K), whereas no changes were observed in label incorporation in hexose phosphates (Figure 4I), organic acids (Figure 4G), or amino acids (Figure 4H). There was an increase in label incorporation into protein (Figure 4J), and a strong decrease of label evolved as carbon dioxide (in lines B33-73 and B-33-76; Figure 4C). However, it should be noted that 14CO2 evolution after [14C]sucrose feeding is a very indirect measure of the rate of respiration. The specific activity of the hexose phosphate pool (Figure 4L) was used to calculate absolute fluxes. Decreased expression of UMPS led to an increase in the rates of starch (Figure 4M) and cell wall synthesis (Figure 4O), whereas glycolytic rates (Figure 4N) were decreased slightly. There was an increase in the ratio of starch to glycolysis (Figure 4P), indicating a shift in the partitioning of hexose phosphate away from respiration toward starch. Crucially, these alterations in biosynthetic activities were not accompanied by large changes in glycolytic intermediates, PPi, or organic acids such as citrate and malate (data not shown).
Figure 4.
Redistribution of Radiolabel and Calculation of Absolute Rates of Glycolysis and Starch and Cell Wall Biosynthesis after Incubation of Tuber Discs Excised from Wild-Type and UMPS Antisense Lines in 10 mM [U-14C]Sucrose.
Discs were cut from developing tubers of 10-week-old plants and washed three times in buffer and were then incubated in 10 mM 2-(N-morpholino)-ethane-sulfonic acid (Mes)-KOH, pH 6.5, containing [U-14C]sucrose to a final specific activity of 1.1 kBq/mol. The discs were incubated for 2 h, washed three times, extracted, and fractionated to determine the metabolic fate of the 14C. Absolute rates of glycolysis, starch, and cell wall biosynthesis within the tuber discs were calculated from the label incorporation data using the specific activity of the hexose phosphate pool to account for isotopic dilution factors. The specific activity was estimated by dividing the label retained in the hexose phosphate pool by the sum of carbon in phosphorylated sugars. Data are presented as means ± se of determinations on six individual plants per line. Values marked with asterisks were determined to be significantly different from wild-type values by t test.
Influence of Decreased Expression of UMPS on Metabolite Levels, Starch Accumulation, Yield, and Cell Wall Content in Tubers
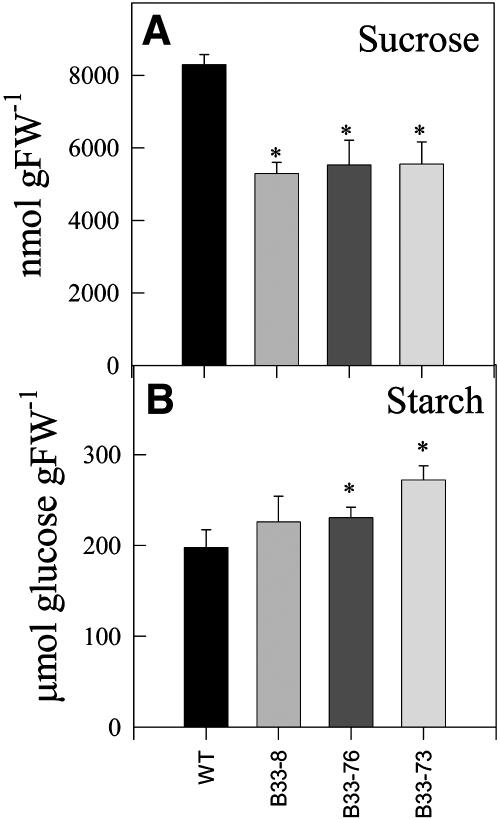
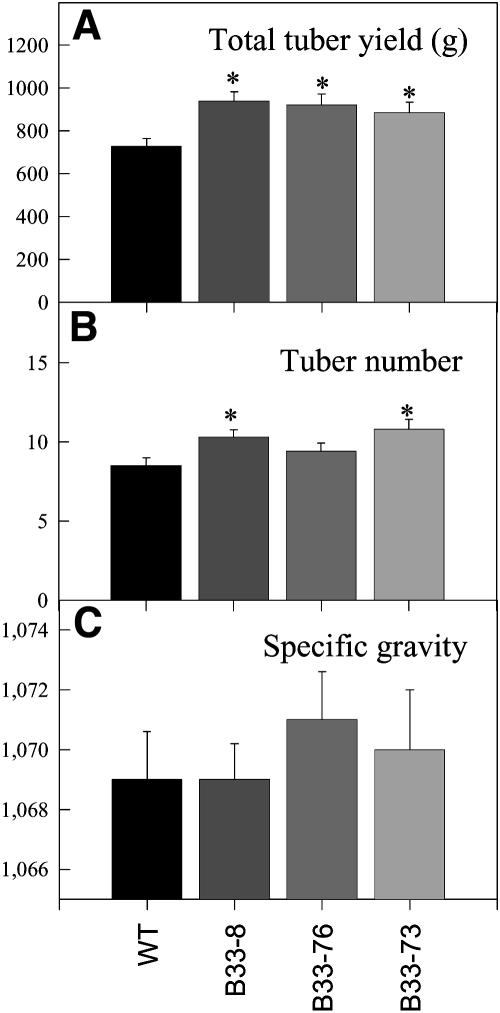
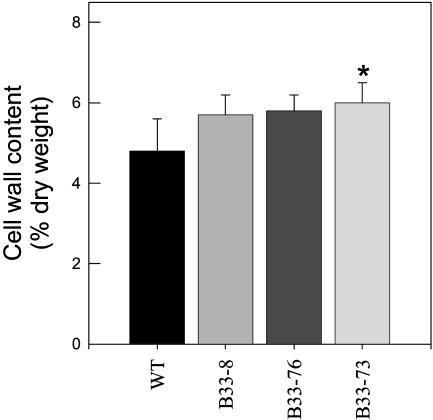
Tubers with decreased UMPS activity had decreased levels of sucrose (Figure 5A), whereas the levels of starch accumulation (Figure 5B) increased. This is consistent with the transgenic tubers having increased levels of uridine nucleotides (Figure 2) and increased fluxes of [14C]sucrose to starch (Figure 4). The increase in starch accumulation in response to decreased UMPS expression was also seen in other greenhouse trials (data not shown) as well as in field trials (Figure 6). In the latter, total tuber yield (Figure 6A), tuber number (Figure 6B), and specific gravity (Figure 6C) tendentially increased in the transgenic lines. As a consequence, the starch yield per plant is higher for all three transgenic lines (data not shown). Figure 7 shows that in transgenic tubers an increased percentage of the dry weight is conferred to cell walls. Again, this is consistent with the increase in uridine nucleotide levels (Figure 2) and increased fluxes of [14C]sucrose to cell walls, whereas fluxes to protein decreased (Figure 4).
Figure 5.
Sucrose and Starch Levels in Tubers of the Antisense Lines.
Carbohydrate levels (sucrose [A]; starch [B]) were measured in developing tubers isolated from 10-week-old potato plants. Data are presented as means ± se of determinations on six individual plants per line. Values marked with asterisks were determined to be significantly different from wild-type values by t test.
Figure 6.
Yield, Tuber Number, and Specific Gravity of the Antisense StUMPS Plants.
Potato plants were grown under field conditions in Golm, Brandenberg, Germany. Determinations were performed on fully senescent plants. Data are presented as means ± se of determinations on eight individual plants per line. Values marked with asterisks were determined to be significantly different from wild-type values by t test.
Figure 7.
Cell Wall Content of the StUMPS Plants.
Cell wall content was assessed as a percentage contribution to the total dry weight composition of the tuber parenchyma in the same samples used for the carbohydrate analysis presented in Figure 4. Data are presented as means ± se of determinations on six individual plants per line. The value marked with an asterisk was determined to be significantly different from the wild-type value by t test.
Influence of Decreased Expression of UMPS on the Use of Externally Supplied Orotate and Uridine for Uridine Nucleotide Synthesis in Potato Tuber Tissue Slices
External feeding of orotate and uridine has been used frequently to investigate the relative activities of the de novo and salvage pathways of uridine nucleotide synthesis in different organisms, including plants. To investigate the effect of decreased UMPS activity on de novo and savage pathways of uridine nucleotide synthesis, we incubated potato tuber slices from wild-type and three UMPS antisense lines with 10 mM orotate (intermediate of the de novo pathway and substrate of UMPS) and 10 mM uridine (precursor of the salvage pathway) in the presence of sucrose and measured the change in total uridine nucleotide pools in the tissue after 2 h (Figure 8). In discs of wild-type tubers, orotate and uridine were incorporated rapidly into internal uridine nucleotide pools, which increased by 100 and 75 nmol/g fresh weight, respectively, within 2 h, whereas no increase was observed in the osmotic control lacking the precursors. These findings are consistent with previous studies in which uridine and orotate were fed to wild-type tuber tissue (Loef et al., 1999) and document that both pathways are active in the wild type. In discs of all three UMPS antisense lines, no significant increases in internal uridine nucleotide pools were observed after feeding orotate, whereas feeding uridine led to a marked and significant increase in the uridine nucleotide pools; the levels of these metabolites were effectively unchanged in the control incubation. This decreased ability to use external orotate for uridine nucleotide synthesis is consistent with the decrease in UMPS activity, which is obviously compensated for by an increase in uridine-salvaging activity.
Figure 8.
Adenylate and Uridinylate Levels after Incubation of Tuber Discs Excised from Wild-Type Lines and UMPS Antisense Lines B33-8, B33-73, and B33-76 in 10 mM Orotate, Uridine, or Sucrose.
Discs were cut from developing tubers of 10-week-old plants and washed three times in buffer and were then incubated in 10 mM Mes-KOH, pH 6.5, containing 10 mM uridine (U), orotate (O), or sucrose (S). The discs were incubated for 2 h, washed three times, and extracted in trichloroacetic acid, and the levels of nucleotide and nucleoside were determined by HPLC. Data are presented as means ± se of determinations on six individual plants per line. Values marked with asterisks were determined to be significantly different from the time 0 control by t test.
Influence of Decreased UMPS Activity on the Expression of Other Genes Involved in Pyrimidine Synthesis
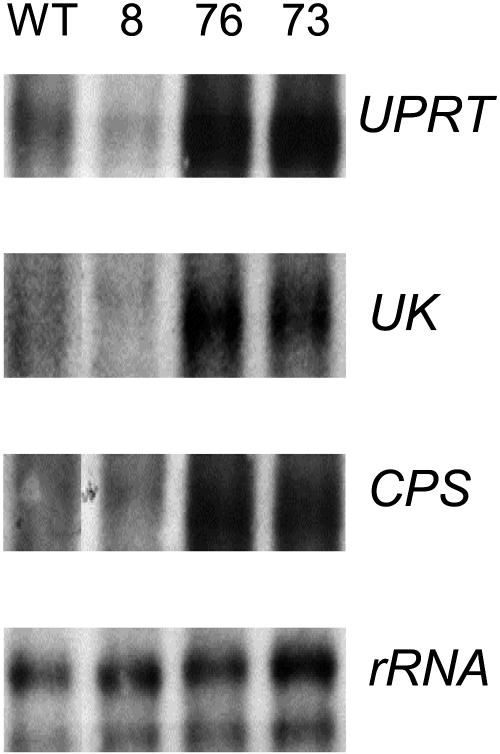
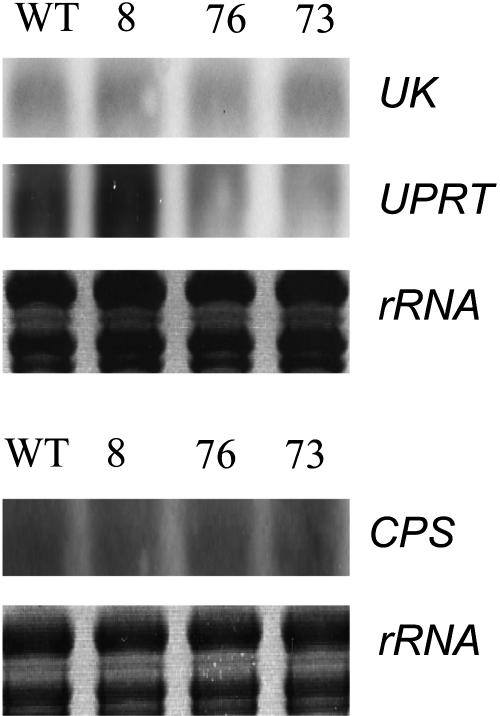
To investigate whether decreased expression of UMPS led to a compensatory increase in the expression of other genes of pyrimidine nucleotide synthesis, we investigated the steady state mRNA levels of CPS (encoding the first step of the de novo pathway), UK, and UPRT (encoding two alternative salvaging enzymes) in tubers of wild-type and transgenic lines (Figure 9). Compared with the wild type, expression of CPS, UK, and UPRT was increased markedly in lines 76 and 73, the lines with the strongest decrease in UMPS activity. Various studies demonstrate the transport of nucleosides and nucleobases between plant organs (Kombrink and Beevers, 1983; Ashihara and Crozier, 1999), and the recent identification and functional analysis of protein families that facilitate this process has demonstrated that such proteins are active in all plant organs (Gillissen et al., 2000; Möhlmann et al., 2001; Burkle et al., 2003; Li et al., 2003). Although our studies indicate that the steady state levels of uridine nucleotides were unaltered in leaves, we decided to investigate whether the levels of CPS, UK, and UPRT transcripts were increased in leaves of the transformants similar to tubers. In contrast to the situation in the tuber, these transcripts were essentially unaltered in leaves of the transformants and if anything decreased (Figure 10), suggesting that the transformants were unlikely to exhibit an increase in nucleoside and/or nucleobase transport between source and sink organs.
Figure 9.
RNA Gel Blot Analysis in Growing Tubers of Wild-Type and Transgenic Potato Plants.
RNA extracts were prepared from the same samples used for the metabolite measurements presented in Figures 4 and 6. Transcript levels for UPRT, UK, and CPS are presented above their RNA loading control values.
Figure 10.
RNA Gel Blot Analysis in Fully Expanded, Nonsenescent Leaves of Wild-Type and Transgenic Potato Plants.
Transcript levels for UPRT, UK, and CPS are presented above their respective RNA loading control values.
To establish whether the altered transcription of the salvage pathway in the tubers led to corresponding increases in enzyme activity, we next determined the activity of UK and UPRT in the same samples. Increased enzyme activities of UK and UPRT were observed in the transformants, with lines 8, 76, and 73 exhibiting 151% ± 30%, 133% ± 18%, and 169% ± 50% of the wild-type UK activity and 145% ± 35%, 216% ± 52%, and 172% ± 32% of the wild-type UPRT activity, respectively.
DISCUSSION
Uridine nucleotides are cofactors in the use of sugars for various biosynthetic processes in such different organisms as mammals and plants. However, studies of uridine nucleotide synthesis and the role of uridine nucleotide levels in primary metabolism have been somewhat limited (Stasolla et al., 2003). One reason for this is the fact that the presence of multiple routes of nucleotide synthesis and degradation renders biochemical studies difficult to perform. A second reason is the sparse use of molecular studies, such as reversed genetic approaches, to alter specific steps of uridine nucleotide synthesis. A further reason is the breadth of biochemical pathways in which uridine nucleotides participate, including synthesis of the nucleic acids that are central for cell division and growth. Therefore, it is a major challenge to find tools to dissect these different roles of uridine nucleotides in different tissues and developmental stages.
In this study, we used a molecular approach to specifically manipulate uridine nucleotide synthesis in potato plants. We expressed a gene coding for UMPS in the antisense orientation using the tuber parenchyma–specific patatin promoter. We chose UMPS because on the basis of previous biochemical studies in a range of organisms it has been suggested as a rate-limiting step of de novo uridine nucleotide biosynthesis (Traut and Jones, 1996). We used a tissue-specific promoter to avoid the possible side effects that might be associated with constitutive expression. Furthermore, the confined expression in storage parenchyma cells of tubers should allow investigation of the role of uridine nucleotide synthesis specifically in the context of carbon storage metabolism. Our results show that decreased expression of UMPS leads to a shift in the pathways of pyrimidine synthesis in favor of uridine salvaging, resulting in increased uridine nucleotide levels, stimulation of sucrose degradation, and increased use of sucrose for biosynthetic processes. Increased uridine nucleotide levels were accompanied by an increase in starch accumulation and cell wall content in growing tubers and increased yield. This finding provides direct evidence that the prevailing levels of uridine nucleotide pools are limiting for the use of sucrose for starch and cell wall synthesis in developing sinks and suggests a novel route for genetic engineering of crops. These results are consistent with previous physiological studies with wild-type potato tubers, wherein increasing uridine nucleotide levels by the feeding of orotate and uridine to isolated tuber discs led to increased rates of sucrose degradation and starch synthesis (Loef et al., 1999).
Intriguingly, the effects of reducing the expression of UMPS on biosynthetic fluxes to starch and cell walls were apparent with a reduction of only 40% of its activity. A more severe reduction of UMPS led to a further stimulation of carbon fluxes in biosynthetic pathways, whereas less carbon was allocated to glycolysis. Moreover, only parameters that are likely to be dependent on the supply of uridine, in one form or another, seem to be correlatively changed with respect to the change in activity imposed by the transformation, suggesting that certain observed changes, such as the altered flux to glucose and carbon dioxide, may be pleiotropic effects of the manipulation. That possibility aside, the observations made in this study suggest that there is an increase in biosynthesis despite a minor decrease in the rate of glycolysis. In the case of cell wall synthesis, which does not have such a great energy demand, it is not difficult to reconcile these facts. Because several recent studies, however, have highlighted the importance of ATP for starch synthesis within the tuber (Tjaden et al., 1998; Regierer et al., 2002), the increased starch synthesis is at first glance somewhat puzzling. However, the levels of adenylate pools and the adenylate energy state were not altered in response to an increase in UMPS expression, suggesting that they are present at high enough levels to support the levels of biosynthesis observed. Moreover, similar shifts in metabolism to those reported here were observed previously after increasing uridine nucleotide levels by feeding of precursors to tuber discs (Loef et al., 1999). Stimulation of starch synthesis was shown by the increased rate of [14C]sucrose incorporation into starch, whereas the rate of glycolysis and the rate of respiratory oxygen consumption remained unaltered. Together, the results from this and previous studies (Heldt, 1997; Fernie et al., 2004) thus provide further support for the notion that the respiratory pathways of plants are not necessarily coupled to ATP production.
Early studies provided correlative evidence that uridine nucleotide metabolism is regulated in parallel with changes in sucrose and starch metabolism, indicating that increased sink activity involves a coordinated increase in uridine nucleotide levels (Ross and Cole, 1968; Merlo et al., 1993; Sowokinos et al., 1993). More recent physiological studies demonstrated that the rates of sucrose breakdown and starch synthesis can be modified by altering the overall uridine nucleotide levels in response to short-term precursor feeding to tuber discs (Loef et al., 1999). In our experiments, we were able to alter the uridine nucleotide levels in planta using a transgenic approach, showing that the endogenous uridine nucleotide levels are limiting for the conversion of sucrose to starch during the major tuber-bulking phase. Stimulation of sucrose breakdown is shown by the decrease in the levels of sucrose compared with hexose phosphates and by the increased rates of [14C]sucrose metabolism. On the basis of the results presented here, uridine nucleotide metabolism clearly exerts a large influence on sucrose metabolism in vivo, because sucrose metabolism was one of the parameters that changed dramatically in all three transgenic lines. This effect may be caused by an increased rate of nucleotide turnover or by an increase in the absolute levels of uridine nucleotide pools. The latter option is supported by the fact that the cytosolic concentration of UDP in tubers is below the Km (UDP) of sucrose synthase and therefore restricts sucrose degradation. Moreover, sucrose degradation was stimulated after increasing the levels of uridine nucleotide pools by feeding precursors to tuber discs (Loef et al., 1999). The biotechnological importance of these findings was demonstrated in a field trial in which transgenic plants with decreased expression of UMPS showed a higher yield and as such an increased total amount of starch per plant. Given that the repression of UMPS has such beneficial effects on plant performance, it is puzzling that neither evolution nor plant breeding provided mechanisms to switch off the de novo synthesis of uridinylates in growing potato tubers. It is tempting to speculate that the de novo pathway must confer an advantage to the tuber under certain physiological conditions that we have not studied here.
Uridine nucleotides are also important precursors for cell wall synthesis, with UDP-Glc serving as the immediate substrate for cellulose synthase. Despite the fact that UDP-Glc has long been established as a major precursor of matrix polysaccharides (Feingold and Avigad, 1980; Reiter and Vanzin, 2001), it has been a matter of debate whether in planta UDP-Glc levels are limiting for the synthesis of cellulose, the major constituent of cell walls in plants (Haigler et al., 2001). Our results show that transgenic tubers with increased UDP-Glc levels had increased rates of [14C]sucrose incorporation into cell walls, and cell walls also made up an increased proportion of tuber dry matter. In growing potato tubers, starch synthesis and growth occur simultaneously, the latter requiring the synthesis of cell wall material in expanding and newly formed cells. These are processes with a great demand for UDP-Glc, and our results show that UDP-Glc levels are limiting for both cell wall and starch synthesis. Interestingly, metabolic processes that do not rely on uridine nucleotides, such as protein synthesis, glycolysis, and respiration, were not affected or were even decreased in the transgenic lines.
In growing potato tubers, there are high activities of both the de novo and uridine-salvaging pathways of pyrimidine synthesis (Katahira and Ashihara, 2002). Our results show that a strong reduction of UMPS activity, catalyzing the conversion of orotate to UMP at the end of the de novo pathway, led to a marked restriction in the use of orotate for uridine nucleotide synthesis in potato tubers. This finding shows that UMPS activity makes a large contribution to the control of de novo pyrimidine synthesis. In contrast to this, the use of uridine for uridine nucleotide synthesis was increased, showing that an increase in the rate of uridine salvaging via UK compensates for the inhibition of de novo synthesis. This compensatory interaction between the de novo and salvage pathways of uridine nucleotide synthesis was accompanied by changes in the transcript levels of genes involved in these pathways. Reduction of UMPS activity led to increased expression of UK and UPRT, which are catalyzing steps of the uridine and uracil salvage pathways, respectively. The upregulation of UK is consistent with the stimulation of uridine salvaging when UMPS is reduced. There was also an increased expression of CPS, catalyzing the first step of the de novo route, which can be regarded as a compensatory response to induce the de novo pathway. The mechanisms leading to these coordinated changes in gene expression are currently unknown. However, it is tempting to speculate that they might be linked to metabolic imbalances between intermediates of the de novo pathway, such as an accumulation of orotate, signaling an impairment of de novo pathway function. In this context, it is interesting that the increase in the levels of these transcripts was greatest in the lines that showed a strong increase in the steady state levels of uridine nucleotides. Despite this fact, measurement of the maximal catalytic activities of UK and UPRT revealed that these were increased markedly in all three transformants, suggesting the possibility that mechanisms of metabolic regulation other than transcriptional regulation are also involved in the upregulation of the salvage pathway. The regulatory interactions between the pathways combine to provide enough flexibility to prevent a decrease in the nucleotide levels if one of the pathways is compromised.
The shift in the pathways in favor of the uridine salvage pathway allows both greater uridine nucleotide levels and improved biosynthetic performance. It has been suggested previously that nucleotide salvage pathways are an efficient way to make nucleotides at low cost in situations in which preformed nucleotides are available (Stasolla et al., 2003). As discussed above, understanding of the transport of nucleosides and nucleobases in plants is developing with the cloning and characterization of several transport proteins in recent years (Gillissen et al., 2000; Möhlmann et al., 2001; Burkle et al., 2003; Li et al., 2003). Because large amounts of uridine nucleotides are transported in the phloem vessels connecting tubers and leaves (Geigenberger et al., 1993), it is possible that some nucleosides and nucleotides produced in leaves are translocated and symplasmically unloaded into growing tubers. This is consistent with the finding that interruption of phloem transport by detaching tubers (Geigenberger et al., 1994) or incubation of tuber slices without nucleotide precursor supply (Geiger et al., 1998; Loef et al., 1999) leads to a decrease in uridine nucleotide pool levels within tuber tissue. Uridine nucleotide levels also decrease in growing tubers when phloem transport is decreased during day/night transitions (Geigenberger and Stitt, 2000). The use of preformed nucleosides and/or nucleobases would provide a more energy-efficient means of uridine nucleotide synthesis. This is especially important in the context of growing tubers, which have a low energy state because of restricted oxygen availability that is limiting for biosynthetic processes (Geigenberger et al., 2000; Geigenberger, 2003). In this situation, a shift to more energy-conserving pathways of nucleotide synthesis would allow more nucleotides to be made and thus more energy to be available for other biosynthetic processes.
An interesting analogy to this system is the use of two alternative routes of sucrose degradation in plants, sucrose synthase and invertase, which differ in their energy costs. In growing potato tubers, invertase is repressed and sucrose is degraded predominantly via the less energy-consuming sucrose synthase route. Interestingly, overexpression of a high energy-consuming invertase in the potato tuber leads to an inhibition in the rate of starch synthesis and a decrease in tuber starch accumulation (Bologa et al., 2003). The results of our study show that inhibition of the more energy-consuming de novo pathway of uridine nucleotide synthesis is beneficial for storage metabolism in growing tubers. In tuber tissue, the availability of preformed nucleosides and/or nucleobases is obviously sufficient to drive high rates of nucleotide salvage. It will be interesting to determine the roles of de novo and salvaging pathways of pyrimidine synthesis in tissues within different metabolic and developmental contexts. Two recent studies have implicated the metabolic regulation of these pathways under phosphate stress (Hewitt et al., 2005) and programmed cell death (Stasolla et al., 2004). It is likely that the analysis of additional biological circumstances will allow the relative importance of these alternative pathways to be assessed and thus enhance our understanding of their complex regulation.
METHODS
Plant Material
Potato (Solanum tuberosum cv Desiree) was obtained from Saatzucht Lange (Bad Schwartau, Germany). Plants were maintained in tissue culture as described in the literature (Tauberger et al., 2000) with a 16 h light/8 h dark regime on MS medium (Sigma-Aldrich, St. Louis, MO) containing 2% (w/v) sucrose. In the greenhouse, plants were grown under the same light regime with a minimum of 250 mmol·m−2·s−1 at 22°C. In this article, the term “developing tubers” is used for tubers (>10 g fresh weight) harvested from healthy 10-week-old plants; the term “mature tubers” refers to tubers from senescent plants.
Chemicals
The starch determination kit, enzymes used for biochemical assays, and all enzymes used for the modification and restriction of DNA were obtained from Boehringer Mannheim (Mannheim, Germany). All other chemicals were purchased from Sigma-Aldrich or Merck (Darmstadt, Germany). Radiolabeled substrates were from Amersham International (Braunschweig, Germany).
Cloning of StUMPS and Generation of Transgenic Plants
A full-length cDNA encoding StUMPS (for S. tuberosum UMPS) was isolated from a potato cDNA library (Kossmann et al., 1991) by screening with radiolabeled (Multiprime labeling system; Amersham International) oligonucleotide primers designed with respect to conserved UMPS sequences deduced from a range of UMPS sequences available from GenBank. Standard procedures were performed according to Sambrook et al. (1989). The following oligonucleotide probes were used: 5′-TCTCTAGATTCAAGCTGAAATCCGGGATCTCTTCC-3′ and 5′-CCGGTACCGGCTTTATAATTCCACGGCCAACTAT-3′ Positive plaques were selected, and purified λZAPII clones were excised in vivo before DNA sequencing. A clone with a full-length UMPS was then subcloned into the plasmid pBluescript SK+ (Stratagene, La Jolla, CA). A 1300-bp Asp718/XbaI fragment from the StUMPS cDNA was cloned into the vector pBinAR-Kan (Liu et al., 1990) between the tuber-specific B33 patatin promoter and the octopine synthase terminator. This construct was introduced into potato by an Agrobacterium tumefaciens–mediated transformation protocol (Rocha-Sosa et al., 1989). Transgenic plants were selected on kanamycin-containing medium (Dietze et al., 1995). Initial screening of ∼80 lines was performed by determining the StUMPS transcript level in tubers of plants grown in 2-liter pots under greenhouse conditions. A second screen was then performed at the enzyme activity level using tubers from six plants per line for the nine preselected lines in the greenhouse.
Analysis of Enzyme Activities
Enzyme extraction was performed exactly as described previously (Tauberger et al., 2000). The activity of UMPS was analyzed by radioactive assay according to the procedure of Yablonski et al. (1996). UK and UPRT activities were determined essentially according to the procedure of Katahira and Ashihara (2002), with the following modifications: longer incubation times were used after it had been determined that the reaction was linear for at least 4 h, and the reactions were heat-inactivated (95°C for 5 min) rather than perchlorate-inactivated. Finally, the eluting radioactive products were quantified using a thin layer chromatography scanner.
Extraction of RNA and RNA Gel Blot Experiments
Total RNA was isolated from 2 g fresh weight of tuber leaf tissue as described by Logemann et al. (1987). Standard conditions were used for the transfer of RNA to membranes and for the subsequent hybridizations. To check transcript levels of selected enzymes, the membranes were hybridized with tomato ESTs from the Clemson State University (Clemson, SC) collection using conditions described previously (Roessner-Tunali et al., 2003). The following ESTs were used: cTOD11G2 (for CPS), cLED31H23 (for UK), and cLEM5E22 (for UPRT).
Metabolite Analyses
The levels of soluble sugars and starch were assessed as detailed previously Fernie et al. (2001b). The levels of glycolytic intermediates were analyzed as described by Geigenberger et al. (2000), whereas the levels of adenylates and uridinylates were analyzed by HPLC according to the method defined by Regierer et al. (2002). The recovery of these metabolites through the processes of storage, extraction, and assay has been documented previously (Geigenberger et al., 1994; Fernie et al., 2001c).
Feeding and Labeling Experiments
Tuber discs (8 mm diameter, 1 to 2 mm thick) were cut directly from growing tubers attached to the fully photosynthesizing mother plant, washed three times with 10 mM Mes-KOH, pH 6.5, and then incubated (eight discs in a volume of 4 mL in a 100-mL Erlenmeyer flask shaken at 90 rpm) in 10 mM Mes-KOH, pH 6.5, containing the substrate specified in the text for 2 h. Kinetic experiments have previously documented that the wounding response is relatively slow in cv Desiree, being well in excess of 2 h (Roessner-Tunali et al., 2004). In the case of the radiolabel experiments, the media also contained 10 mM [U-14C]sucrose (specific activity, 1.1 kBq/mol). After this period, the discs were harvested, washed three times in buffer (100 mL per eight discs), and frozen in liquid nitrogen.
Fractionation of Radiolabeled Tissue Extracts
Tuber discs were extracted with 80% (v/v) ethanol at 80°C (1 mL per two discs) and reextracted in two subsequent steps with 50% (v/v) ethanol (1 mL per two discs at each step), the combined supernatants were dried under an air stream at 40°C and taken up in 1 mL of water, and label was separated by ion-exchange chromatography and thin layer chromatography as described by Fernie et al. (2001a). The insoluble material left after ethanol extraction was analyzed for label in starch, cell wall, and protein as described by Fernie et al. (2002).
Analysis of the Cell Wall Content of Tubers
Cell wall material was prepared as described by York et al. (1985). In brief, frozen potato tuber tissue was ground and lyophilized, and dry weight was determined. Proteins, metabolites, and membranes were extracted using 100 mM sodium phosphate buffer, pH 7, 70% ethanol, a chloroform:methanol mixture (1:1, v/v), and acetone. After every extraction step, the plant material was centrifuged and the supernatant was discarded. Starch was enzymatically digested as described by Geigenberger et al. (1998). The dry weight of the residual plant material was measured to determine the amount of cell wall material.
Yield Trials
To assess the yield parameters of these plants, they were grown in high sample number under both glasshouse (n = 15) and field (n = 8) conditions exactly as defined by Regierer et al. (2002). The number of tubers per plant, the total tuber yield, and the specific gravity of the tubers (ρ) were determined. In the case of the latter, this was deduced according to the formula ρ = mass of tubers in air/(mass of tubers in air − mass of tubers in water). All measurements were made on mature tubers.
Statistical Analyses
Where differences are described in the text as significant, a t test was performed using the two-tailed equal variance algorithm incorporated into Microsoft Excel 7.0 (Microsoft, Seattle, WA) that yielded P < 0.05.
Supplementary Material
Acknowledgments
We thank Karin Koehl for organizing and supervising the field trials described in this article. This work was supported in part by the Deutsche Forschungsgemeinschaft (P.G., J.T.v.D., and A.R.F.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Alisdair R. Fernie (fernie@mpimp-golm.mpg.de).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.105.033548.
References
- Ashihara, H., and Crozier, A. (1999). Biosynthesis and metabolism of caffeine and related purine alkaloids in plants. Adv. Bot. Res. 30, 117–205. [Google Scholar]
- Bologa, K.L., Fernie, A.R., Leisse, A., Ehlers Loureiro, M., and Geigenberger, P. (2003). A bypass of sucrose synthase leads to low internal oxygen and impaired metabolic performance in growing potato tubers. Plant Physiol. 132, 2058–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkle, L., Cedzich, A., Dopke, C., Stransky, H., Okumoto, S., Gillissen, B., Kuhn, C., and Frommer, W.B. (2003). Transport of cytokinins mediated by purine transporters of the PUP family expressed in phloem, hydathodes, and pollen of Arabidopsis. Plant J. 34, 13–26. [DOI] [PubMed] [Google Scholar]
- Dietze, J., Blau, A., and Willmitzer, L. (1995). Agrobacterium-mediated transformation of potato (Solanum tuberosum). In Gene Transfer to Plants XXII, I. Potrykus and G. Spangenberg, eds (Berlin: Springer-Verlag), pp. 24–29.
- Feingold, D.S., and Avigad, G. (1980). Sugar nucleotide transformations in plants. In The Biochemistry of Plants: A Comprehensive Treatise, P.K. Stumpf, E.E. Conn, eds (New York: Academic Press), pp. 101–170.
- Fernie, A.R., Carrari, F., and Sweetlove, L.J. (2004). Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 7, 254–261. [DOI] [PubMed] [Google Scholar]
- Fernie, A.R., Roessner, U., and Geigenberger, P. (2001. a). The sucrose analog palatinose leads to a stimulation of sucrose degradation and starch synthesis when supplied to discs of growing potato tubers. Plant Physiol. 125, 1967–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie, A.R., Roessner, U., Trethewey, R.N., and Willmitzer, L. (2001. b). The contribution of plastidial phosphoglucomutase to the control of starch synthesis within the potato tuber. Planta 213, 418–426. [DOI] [PubMed] [Google Scholar]
- Fernie, A.R., Roscher, A., Ratcliffe, R.G., and Kruger, N.J. (2001. c). Fructose 2,6-bisphosphate activates pyrophosphate:fructose 6-phosphate 1-phosphotransferase and increases triose phosphate to hexose phosphate cycling in heterotrophic cells. Planta 212, 250–263. [DOI] [PubMed] [Google Scholar]
- Fernie, A.R., Roscher, A., Ratcliffe, R.G., and Kruger, N.J. (2002). Activation of pyrophosphate:fructose 6-phosphate 1-phosphotransferase by fructose 2,6-bisphosphate stimulates conversion of hexose phosphates to triose phosphates but does not influence accumulation of carbohydrates in phosphate-deficient tobacco cells. Physiol. Plant. 114, 172–181. [DOI] [PubMed] [Google Scholar]
- Geigenberger, P. (2003). Response of plant metabolism to too little oxygen. Curr. Opin. Plant Biol. 6, 247–256. [DOI] [PubMed] [Google Scholar]
- Geigenberger, P., and Stitt, M. (2000). Diurnal changes in sucrose, nucleotides, starch synthesis, and AGPS transcript in growing potato tubers that are suppressed by decreased expression of sucrose phosphate synthase. Plant J. 23, 795–806. [DOI] [PubMed] [Google Scholar]
- Geigenberger, P., Fernie, A.R., Gibon, Y., Christ, M., and Stitt, M. (2000). Metabolic activity decreases as an adaptive response to low internal oxygen in growing potato tubers. Biol. Chem. 381, 723–740. [DOI] [PubMed] [Google Scholar]
- Geigenberger, P., Hajirezaei, M., Geiger, M., Deiting, U., Sonnewald, U., and Stitt, M. (1998). Overexpression of pyrophosphatase leads to increased sucrose degradation and starch synthesis, increased activities of enzymes for sucrose-starch interconversions, and increased levels of nucleotides in growing potato tubers. Planta 205, 428–437. [DOI] [PubMed] [Google Scholar]
- Geigenberger, P., Langenberger, S., Wilke, I., Heineke, D., Heldt, H.W., and Stitt, M. (1993). Sucrose is metabolised by sucrose synthase and glycolysis within the phloem complex of Ricinus communis L. seedlings. Planta 190, 446–453. [Google Scholar]
- Geigenberger, P., Merlo, L., Reimholz, R., and Stitt, M. (1994). When growing potato tubers are detached from their mother plant there is a rapid inhibition of starch synthesis, involving inhibition of ADP-glucose pyrophosphorylase. Planta 193, 486–493. [Google Scholar]
- Geiger, M., Stitt, M., and Geigenberger, P. (1998). Metabolism in potato tuber slices responds differently after addition of sucrose and glucose. Planta 206, 245–252. [Google Scholar]
- Gillissen, B., Burkle, L., Andre, B., Kühn, C., Rentsch, D., Brandl, B., and Frommer, W.B. (2000). A new family of high-affinity transporters for adenine, cytosine and purine derivatives in Arabidopsis. Plant Cell 12, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler, C.H., Ivanova-Datcheva, I., Hogan, P.S., Salnikov, V.V., Hwang, S., Martin, K., and Delmer, D.P. (2001). Carbon partitioning to cellulose synthesis. Plant Mol. Biol. 47, 29–51. [PubMed] [Google Scholar]
- Heldt, H.W. (1997). Plant Biochemistry. (Oxford: Oxford University Press).
- Hewitt, M.M., Carr, J.M., Williamson, C.L., and Slocum, R.D. (2005). Effects of phosphate limitation on expression of genes involved in pyrimidine synthesis and salvaging in Arabidopsis. Plant Physiol. Biochem. 43, 91–99. [DOI] [PubMed] [Google Scholar]
- Jones, G.E., and Hann, J. (1979). Haplopappus gracilis cell strains resistant to pyrimidine analogues. Theor. Appl. Genet. 54, 81–87. [DOI] [PubMed] [Google Scholar]
- Kafer, C., Zhou, L., Santoso, D., Guirgis, A., Weers, B., Park, S., and Thornburg, R. (2004). Regulation of pyrimidine metabolism in plants. Front. Biosci. 9, 1611–1625. [DOI] [PubMed] [Google Scholar]
- Katahira, R., and Ashihara, H. (2002). Profiles of pyrimidine biosynthesis, salvage and degradation in disks of potato (Solanum tuberosum L.) tubers. Planta 215, 821–828. [DOI] [PubMed] [Google Scholar]
- Kombrink, E., and Beevers, H. (1983). Transport of purine and pyrimidine bases and nucleosides from endosperm to cotyledons in germinating castor bean seedlings. Plant Physiol. 73, 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossmann, J., Visser, R.G.F., Müller-Röber, B., Willmitzer, L., and Sonnewald, U. (1991). Cloning and expression analysis of a potato cDNA that encodes branching enzyme: Evidence for co-expression of starch biosynthetic genes. Mol. Gen. Genet. 230, 39–44. [DOI] [PubMed] [Google Scholar]
- Li, G.Y., Liu, K.F., Baldwin, S.A., and Wang, D.W. (2003). Equilibrative nucleoside transporters of Arabidopsis thaliana: cDNA cloning, expression pattern, and analysis of transport activities. J. Biol. Chem. 278, 35732–35742. [DOI] [PubMed] [Google Scholar]
- Liu, X.J., Prat, S., Willmitzer, L., and Frommer, W.B. (1990). Cis regulatory elements directing tuber-specific and sucrose-inducible expression of a chimeric class I patatin promoter-GUS fusion. Mol. Gen. Genet. 223, 401–406. [DOI] [PubMed] [Google Scholar]
- Loef, I., Stitt, M., and Geigenberger, P. (1999). Feeding orotate leads to a specific increase in uridine nucleotide levels, resulting in a stimulation of sucrose degradation and starch synthesis in discs of growing potato tubers. Planta 209, 314–323. [DOI] [PubMed] [Google Scholar]
- Logemann, J., Schell, J., and Willmitzer, L. (1987). Improved method for the isolation of RNA from plant tissues. Anal. Biochem. 163, 16–20. [DOI] [PubMed] [Google Scholar]
- Merlo, L., Geigenberger, P., Hajirezaei, M., and Stitt, M. (1993). Changes of carbohydrates, metabolites and enzyme activities in potato tubers during development, and within a single tuber along a stolon-apex gradient. J. Plant Physiol. 142, 392–402. [Google Scholar]
- Moffat, B.A., and Ashihara, H. (2002) Purine and pyrimidine nucleotide synthesis and metabolism. In The Arabidopsis Book, 2nd ed., C.R. Somerville and E.M. Meyerowitz, eds (Rockville, MD: American Society of Plant Biologists), 10.1199/tab.0018, http://www.aspb.org/publications/arabidopsis/. [DOI] [PMC free article] [PubMed]
- Möhlmann, T., Mezher, Z., Schwerdtfeger, G., and Neuhaus, H.E. (2001). Characterisation of a concentrative type of adenosine transporter from Arabidopsis thaliana (ENT1, At). FEBS Lett. 509, 370–374. [DOI] [PubMed] [Google Scholar]
- Neuhard, J., and Nygaard, P. (1987). Biosynthesis and conversions of nucleotides. In Purines and Pyrimidines, F.C. Neidhard, ed (Washington, DC: American Society of Microbiology), pp. 580–599.
- Regierer, B., Fernie, A.R., Springer, F., Perez-Melis, A., Leisse, A., Koehl, K., Willmitzer, L., Geigenberger, P., and Kossmann, J. (2002). Starch content and yield increase as a result of altering adenylate pools in transgenic plants. Nat. Biotechnol. 20, 1256–1260. [DOI] [PubMed] [Google Scholar]
- Reiter, W.D., and Vanzin, G.R. (2001). Molecular genetics of nucleotide sugar interconversion in plants. Plant Mol. Biol. 47, 95–113. [PubMed] [Google Scholar]
- Rocha-Sosa, M., Sonnewald, U., Frommer, W., Stratmann, M., Schell, J., and Willmitzer, L. (1989). Both developmental and metabolic signals activate the promoter of the class I patatin gene. EMBO J. 8, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner-Tunali, U., Liu, J., Leisse, A., Balbo, I., Perez-Melis, A., Willmitzer, L., and Fernie, A.R. (2004). Kinetics of labelling of organic and amino acids in potato tubers by gas chromatography-mass spectrometry following incubation in 13C labelled isotopes. Plant J. 39, 668–679. [DOI] [PubMed] [Google Scholar]
- Roessner-Tunali, U., Urbanczyk-Wochniak, E., Czechowski, T., Kolbe, A., Willmitzer, L., and Fernie, A.R. (2003). De novo amino acid biosynthesis in potato tubers is regulated by sucrose levels. Plant Physiol. 133, 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, C., and Cole, C.V. (1968). Metabolism of cytidine and uridine in bean leaves. Plant Physiol. 40, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Santoso, D., and Thornburg, R.W. (1992). Isolation and characterization of UMP synthase mutants from haploid cell suspensions of Nicotiana tabacum. Plant Physiol. 99, 1216–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos, J.R., Soychalla, J.P., and Desborough, S.L. (1993). Pyrophosphorylase in Solanum tuberosum. IV. Purification, tissue localisation, and physiochemical properties of UDPglucose pyrophosphorylase. Plant Physiol. 101, 1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasolla, C., Katahira, R., Thorpe, T.A., and Ashihara, H. (2003). Purine and pyrimidine nucleotide metabolism in higher plants. J. Plant Physiol. 160, 1271–1295. [DOI] [PubMed] [Google Scholar]
- Stasolla, C., Loukanina, N., Yeung, E.C., and Thorpe, T.A. (2004). Alterations in pyrimidine metabolism as an early signal during the execution of programmed cell death in tobacco BY-2 cells. J. Exp. Bot. 55, 2513–2522. [DOI] [PubMed] [Google Scholar]
- Stryer, L. (1996). Biochemistry. (Weinheim, Germany: VCR Weinheim).
- Tauberger, E., Fernie, A.R., Emmermann, M., Renz, A., Kossmann, J., Willmitzer, L., and Trethewey, R.N. (2000). Antisense inhibition of plastidial phosphoglucomutase provides compelling evidence that potato tuber amyloplasts import carbon from the cytosol in the form of glucose 6-phosphate. Plant J. 23, 43–53. [DOI] [PubMed] [Google Scholar]
- Tjaden, J., Möhlmann, T., Kampfenkel, K., Henrichs, G., and Neuhaus, H.E. (1998). Altered plastidic ATP/ADP-transporter activity influences potato (Solanum tuberosum L.) tuber morphology, yield and composition of tuber starch. Plant J. 16, 531–540. [Google Scholar]
- Traut, T.W., and Jones, M.E. (1996). Uracil metabolism–UMP synthesis from orotic acid or uridine and conversion of uracil to beta-alanine: Enzymes and cDNAs. Prog. Nucleic Acid Res. Mol. Biol. 53, 1–78. [DOI] [PubMed] [Google Scholar]
- Yablonski, M.S., Pasek, D.A., Han, B.D., Jones, M.E., and Traut, T.W. (1996). Intrinsic activity and stability of bifunctional human UMP synthase and its two separate catalytic domains, orotate phosphoribosyltransferase and orotidine-5′-phosphate decarboxylase. J. Biol. Chem. 271, 10704–10708. [DOI] [PubMed] [Google Scholar]
- York, W.S., Darvill, A.G., McNeil, T., Stevenson, T.T., and Albersheim, P. (1985). Isolation and characterization of plant cell walls and cell wall components. Methods Enzymol. 118, 3–40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.