Abstract
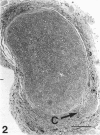
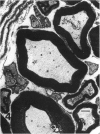
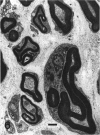
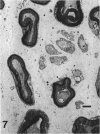
The morphological changes were examined proximal and distal to crush and transection injuries of the lingual/chorda tympani nerve. Under general anaesthesia the nerve was transected unilaterally in 6 adult cats and crushed with watchmakers forceps in 6 others. After 12 wk, again under general anaesthesia, the injured and contralateral (control) nerves were removed, fixed and embedded for histological examination. Sections were cut from sites proximal and distal to the injury and from a site equivalent to that of the injury on the control side. Using systematic randomised sampling techniques the number of nonmyelinated axons and the number and size of myelinated axons in each nerve at each location was estimated. In addition, the mean number of nonmyelinated axons in each Schwann cell unit was determined. The only significant difference between control and injured nerves proximal to either injury was a reduction in the number of myelinated axons in the chorda tympani after transection, and an increase in their mean size. This indicates a selective loss of smaller fibres and is consistent with the poor recovery of gustatory and thermosensitive fibres previously reported (Robinson, 1989). Distal to both types of injury there was an increase in the number of fascicles. The mean number of myelinated axons was reduced distal to a crush injury but unchanged distal to transection. The number of nonmyelinated axons distal to a transection injury was 5 times control counts and after a crush injury double. These findings suggest that sprouting persists 12 wk after both injuries but is much greater after transection.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baranowski A. P., Priestley J. V., McMahon S. Substance P in cutaneous primary sensory neurons--a comparison of models of nerve injury that allow varying degrees of regeneration. Neuroscience. 1993 Aug;55(4):1025–1036. doi: 10.1016/0306-4522(93)90316-8. [DOI] [PubMed] [Google Scholar]
- Blackburn C. W., Bramley P. A. Lingual nerve damage associated with the removal of lower third molars. Br Dent J. 1989 Aug 5;167(3):103–107. doi: 10.1038/sj.bdj.4806922. [DOI] [PubMed] [Google Scholar]
- CAMMERMEYER J. DIFFERENTIAL RESPONSE OF TWO NEURON TYPES TO FACIAL NERVE TRANSECTION IN YOUNG AND OLD RABBITS. J Neuropathol Exp Neurol. 1963 Oct;22:594–616. doi: 10.1097/00005072-196310000-00003. [DOI] [PubMed] [Google Scholar]
- CRAGG B. G., THOMAS P. K. Changes in conduction velocity and fibre size proximal to peripheral nerve lesions. J Physiol. 1961 Jul;157:315–327. doi: 10.1113/jphysiol.1961.sp006724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael F. A., McGowan D. A. Incidence of nerve damage following third molar removal: a West of Scotland Oral Surgery Research Group study. Br J Oral Maxillofac Surg. 1992 Apr;30(2):78–82. doi: 10.1016/0266-4356(92)90074-s. [DOI] [PubMed] [Google Scholar]
- Carter D. A., Lisney S. J. The numbers of unmyelinated and myelinated axons in normal and regenerated rat saphenous nerves. J Neurol Sci. 1987 Sep;80(2-3):163–171. doi: 10.1016/0022-510x(87)90152-3. [DOI] [PubMed] [Google Scholar]
- Friede R. L., Bischhausen R. How are sheath dimensions affected by axon caliber and internode length? Brain Res. 1982 Mar 11;235(2):335–350. doi: 10.1016/0006-8993(82)91012-5. [DOI] [PubMed] [Google Scholar]
- Haftek J., Thomas P. K. Electron-microscope observations on the effects of localized crush injuries on the connective tissues of peripheral nerve. J Anat. 1968 Sep;103(Pt 2):233–243. [PMC free article] [PubMed] [Google Scholar]
- Hoffer J. A., Stein R. B., Gordon T. Differential atrophy of sensory and motor fibers following section of cat peripheral nerves. Brain Res. 1979 Dec 14;178(2-3):347–361. doi: 10.1016/0006-8993(79)90698-x. [DOI] [PubMed] [Google Scholar]
- Holland G. R., Robinson P. P. Axon populations in cat lingual and chorda tympani nerves. J Dent Res. 1992 Aug;71(8):1468–1472. doi: 10.1177/00220345920710080201. [DOI] [PubMed] [Google Scholar]
- Holland G. R., Robinson P. P. The number and size of axons central and peripheral to inferior alveolar nerve injuries in the cat. J Anat. 1990 Dec;173:129–137. [PMC free article] [PubMed] [Google Scholar]
- Horch K. W., Lisney S. J. On the number and nature of regenerating myelinated axons after lesions of cutaneous nerves in the cat. J Physiol. 1981;313:275–286. doi: 10.1113/jphysiol.1981.sp013664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenq C. B., Coggeshall R. E. Numbers of regenerating axons in parent and tributary peripheral nerves in the rat. Brain Res. 1985 Feb 4;326(1):27–40. doi: 10.1016/0006-8993(85)91381-2. [DOI] [PubMed] [Google Scholar]
- Korthals J. K., Gieron M. A., Wisniewski H. M. Nerve regeneration patterns after acute ischemic injury. Neurology. 1989 Jul;39(7):932–937. doi: 10.1212/wnl.39.7.932. [DOI] [PubMed] [Google Scholar]
- LUBINSKA L. Demyelination and remyelination in the proximal parts of regenerating nerve fibers. J Comp Neurol. 1961 Dec;117:275–289. doi: 10.1002/cne.901170302. [DOI] [PubMed] [Google Scholar]
- Mason D. A. Lingual nerve damage following lower third molar surgery. Int J Oral Maxillofac Surg. 1988 Oct;17(5):290–294. doi: 10.1016/s0901-5027(88)80005-5. [DOI] [PubMed] [Google Scholar]
- Mayhew T. M., Sharma A. K. Sampling schemes for estimating nerve fibre size. I. Methods for nerve trunks of mixed fascicularity. J Anat. 1984 Aug;139(Pt 1):45–58. [PMC free article] [PubMed] [Google Scholar]
- Morris J. H., Hudson A. R., Weddell G. A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. IV. Changes in fascicular microtopography, perineurium and endoneurial fibroblasts. Z Zellforsch Mikrosk Anat. 1972;124(2):165–203. doi: 10.1007/BF00335678. [DOI] [PubMed] [Google Scholar]
- Peyronnard J. M., Charron L., Lavoie J., Messier J. P. Differences in horseradish peroxidase labeling of sensory, motor and sympathetic neurons following chronic axotomy of the rat sural nerve. Brain Res. 1986 Jan 29;364(1):137–150. doi: 10.1016/0006-8993(86)90994-7. [DOI] [PubMed] [Google Scholar]
- Pogrel M. A., Kaban L. B. Injuries to the inferior alveolar and lingual nerves. J Calif Dent Assoc. 1993 Jan;21(1):50–54. [PubMed] [Google Scholar]
- Povlsen B., Hildebrand C., Wiesenfeld-Hallin Z., Stankovic N. Functional projection of regenerated rat sural nerve axons to the hindpaw skin after sciatic nerve lesions. Exp Neurol. 1993 Jan;119(1):99–106. doi: 10.1006/exnr.1993.1010. [DOI] [PubMed] [Google Scholar]
- Robinson P. P. The effect of injury on the properties of afferent fibres in the lingual nerve. Br J Oral Maxillofac Surg. 1992 Feb;30(1):39–45. doi: 10.1016/0266-4356(92)90135-6. [DOI] [PubMed] [Google Scholar]
- Robinson P. P. The reinnervation of the tongue and salivary glands after lingual nerve injuries in cats. Brain Res. 1989 Apr 3;483(2):259–271. doi: 10.1016/0006-8993(89)90170-4. [DOI] [PubMed] [Google Scholar]
- Rood J. P. Lingual split technique. Damage to inferior alveolar and lingual nerves during removal of impacted mandibular third molars. Br Dent J. 1983 Jun 25;154(12):402–403. doi: 10.1038/sj.bdj.4805103. [DOI] [PubMed] [Google Scholar]
- Rood J. P. Permanent damage to inferior alveolar and lingual nerves during the removal of impacted mandibular third molars. Comparison of two methods of bone removal. Br Dent J. 1992 Feb 8;172(3):108–110. doi: 10.1038/sj.bdj.4807777. [DOI] [PubMed] [Google Scholar]
- Smith E. E., Harn S. D. Presence of nerve cell bodies in the lingual nerve in the third molar area. J Oral Maxillofac Surg. 1989 Sep;47(9):931–935. doi: 10.1016/0278-2391(89)90376-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. K., Jones D. G. The cellular response to nerve injury. II. Regeneration of the perineurium after nerve section. J Anat. 1967 Jan;101(Pt 1):45–55. [PMC free article] [PubMed] [Google Scholar]
- Waite P. M. Rearrangement of neuronal responses in the trigeminal system of the rat following peripheral nerve section. J Physiol. 1984 Jul;352:425–445. doi: 10.1113/jphysiol.1984.sp015301. [DOI] [PMC free article] [PubMed] [Google Scholar]