Abstract
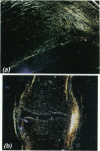
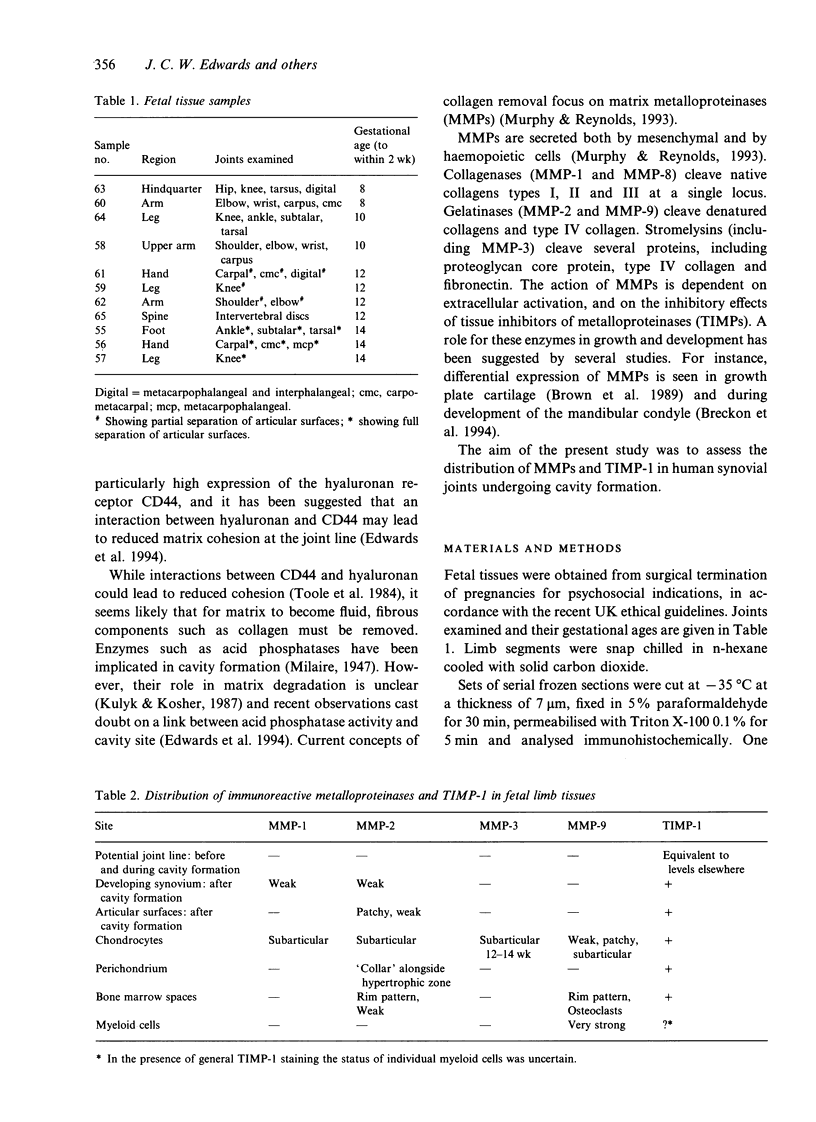
Matrix metalloproteinases (MMPs) have been implicated in tissue remodelling in growth and development. A histochemical study of human fetal limbs was undertaken to assess the presence, and consequently the possible role, of MMPs and their inhibitor TIMP-1 (tissue inhibitor of metalloproteinases-1) in synovial joint cavity formation. Cryostat sections of fetal limbs from 7 to 14 wk gestation were stained with specific antibodies to collagenase (MMP-1), gelantinases A (MMP-2) and B (MMP-9), stromelysin (MMP-3) and TIMP-1. Immunoreactive (IR) MMP-1, MMP-2 and MMP-3 were seen chiefly in chondrocytes, but in all cases in zones distant from the joint line before cavity formation. IR-MMP-1 and MMP-2 were also localised both in synovium and on the articular surfaces of joints after cavity formation. In addition IR-MMP-2 was seen in a "collar' of perichondrium alongside the hypertrophic zone of chondrocytes and weakly in bone marrow spaces. IR-MMP-9 was seen in neutrophil leucocytes and in bone marrow spaces. IR-TIMP-1 was generally distributed in connective tissue cells. No IR-MMP (1, 2,3 or 9) was seen along potential joint lines before or at the time of cavity formation, nor was there aspecific decrease in IR-TIMP-1 at this site. These findings confirm a role for metalloproteinases in developmental processes such as cartilage remodelling and bone marrow space formation. MMP-1 and MMP-2 may be involved in the remodelling of developing synovial tissue and the articular surfaces subsequent to cavity formation. However, we have failed to find evidence to indicate that the loss of tissue strength at the joint line which allows synovial joint cavity formation relates to high local levels of MMPS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN H. DEVELOPMENT, MORPHOLOGY AND HISTOCHEMISTRY OF THE EARLY SYNOVIAL TISSUE IN HUMAN FOETUSES. Acta Anat (Basel) 1964;58:90–115. doi: 10.1159/000142577. [DOI] [PubMed] [Google Scholar]
- Allan J. A., Hembry R. M., Angal S., Reynolds J. J., Murphy G. Binding of latent and high Mr active forms of stromelysin to collagen is mediated by the C-terminal domain. J Cell Sci. 1991 Aug;99(Pt 4):789–795. doi: 10.1242/jcs.99.4.789. [DOI] [PubMed] [Google Scholar]
- Archer C. W., Morrison H., Pitsillides A. A. Cellular aspects of the development of diarthrodial joints and articular cartilage. J Anat. 1994 Jun;184(Pt 3):447–456. [PMC free article] [PubMed] [Google Scholar]
- Breckon J. J., Hembry R. M., Reynolds J. J., Meikle M. C. Regional and temporal changes in the synthesis of matrix metalloproteinases and TIMP-1 during development of the rabbit mandibular condyle. J Anat. 1994 Feb;184(Pt 1):99–110. [PMC free article] [PubMed] [Google Scholar]
- Brown C. C., Hembry R. M., Reynolds J. J. Immunolocalization of metalloproteinases and their inhibitor in the rabbit growth plate. J Bone Joint Surg Am. 1989 Apr;71(4):580–593. [PubMed] [Google Scholar]
- Craig F. M., Bayliss M. T., Bentley G., Archer C. W. A role for hyaluronan in joint development. J Anat. 1990 Aug;171:17–23. [PMC free article] [PubMed] [Google Scholar]
- Craig F. M., Bentley G., Archer C. W. The spatial and temporal pattern of collagens I and II and keratan sulphate in the developing chick metatarsophalangeal joint. Development. 1987 Mar;99(3):383–391. doi: 10.1242/dev.99.3.383. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Wilkinson L. S., Jones H. M., Soothill P., Henderson K. J., Worrall J. G., Pitsillides A. A. The formation of human synovial joint cavities: a possible role for hyaluronan and CD44 in altered interzone cohesion. J Anat. 1994 Oct;185(Pt 2):355–367. [PMC free article] [PubMed] [Google Scholar]
- Hembry R. M., Bagga M. R., Reynolds J. J., Hamblen D. L. Immunolocalisation studies on six matrix metalloproteinases and their inhibitors, TIMP-1 and TIMP-2, in synovia from patients with osteo- and rheumatoid arthritis. Ann Rheum Dis. 1995 Jan;54(1):25–32. doi: 10.1136/ard.54.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembry R. M., Murphy G., Cawston T. E., Dingle J. T., Reynolds J. J. Characterization of a specific antiserum for mammalian collagenase from several species: immunolocalization of collagenase in rabbit chondrocytes and uterus. J Cell Sci. 1986 Mar;81:105–123. doi: 10.1242/jcs.81.1.105. [DOI] [PubMed] [Google Scholar]
- Hembry R. M., Murphy G., Reynolds J. J. Immunolocalization of tissue inhibitor of metalloproteinases (TIMP) in human cells. Characterization and use of a specific antiserum. J Cell Sci. 1985 Feb;73:105–119. doi: 10.1242/jcs.73.1.105. [DOI] [PubMed] [Google Scholar]
- Hipps D. S., Hembry R. M., Docherty A. J., Reynolds J. J., Murphy G. Purification and characterization of human 72-kDa gelatinase (type IV collagenase). Use of immunolocalisation to demonstrate the non-coordinate regulation of the 72-kDa and 95-kDa gelatinases by human fibroblasts. Biol Chem Hoppe Seyler. 1991 Apr;372(4):287–296. doi: 10.1515/bchm3.1991.372.1.287. [DOI] [PubMed] [Google Scholar]
- Kulyk W. M., Kosher R. A. Temporal and spatial analysis of hyaluronidase activity during development of the embryonic chick limb bud. Dev Biol. 1987 Apr;120(2):535–541. doi: 10.1016/0012-1606(87)90256-9. [DOI] [PubMed] [Google Scholar]
- MUNARON G. Osservazioni istofisiche ed istochimiche sul mesenchima intermedio delle articolazioni embrionali e sui suoi derivati. Boll Soc Ital Biol Sper. 1954 Jul;30(7):919–922. [PubMed] [Google Scholar]
- Murphy G., Ward R., Hembry R. M., Reynolds J. J., Kühn K., Tryggvason K. Characterization of gelatinase from pig polymorphonuclear leucocytes. A metalloproteinase resembling tumour type IV collagenase. Biochem J. 1989 Mar 1;258(2):463–472. doi: 10.1042/bj2580463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsillides A. A., Archer C. W., Prehm P., Bayliss M. T., Edwards J. C. Alterations in hyaluronan synthesis during developing joint cavitation. J Histochem Cytochem. 1995 Mar;43(3):263–273. doi: 10.1177/43.3.7868856. [DOI] [PubMed] [Google Scholar]
- Prehm P. Synthesis of hyaluronate in differentiated teratocarcinoma cells. Mechanism of chain growth. Biochem J. 1983 Apr 1;211(1):191–198. doi: 10.1042/bj2110191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill C., Dorfman A. The role of hyaluronic acid in intercellular adhesion of cultured mouse cells. Exp Cell Res. 1978 Nov;117(1):155–164. doi: 10.1016/0014-4827(78)90438-x. [DOI] [PubMed] [Google Scholar]
- Whitham S. E., Murphy G., Angel P., Rahmsdorf H. J., Smith B. J., Lyons A., Harris T. J., Reynolds J. J., Herrlich P., Docherty A. J. Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J. 1986 Dec 15;240(3):913–916. doi: 10.1042/bj2400913. [DOI] [PMC free article] [PubMed] [Google Scholar]