Abstract
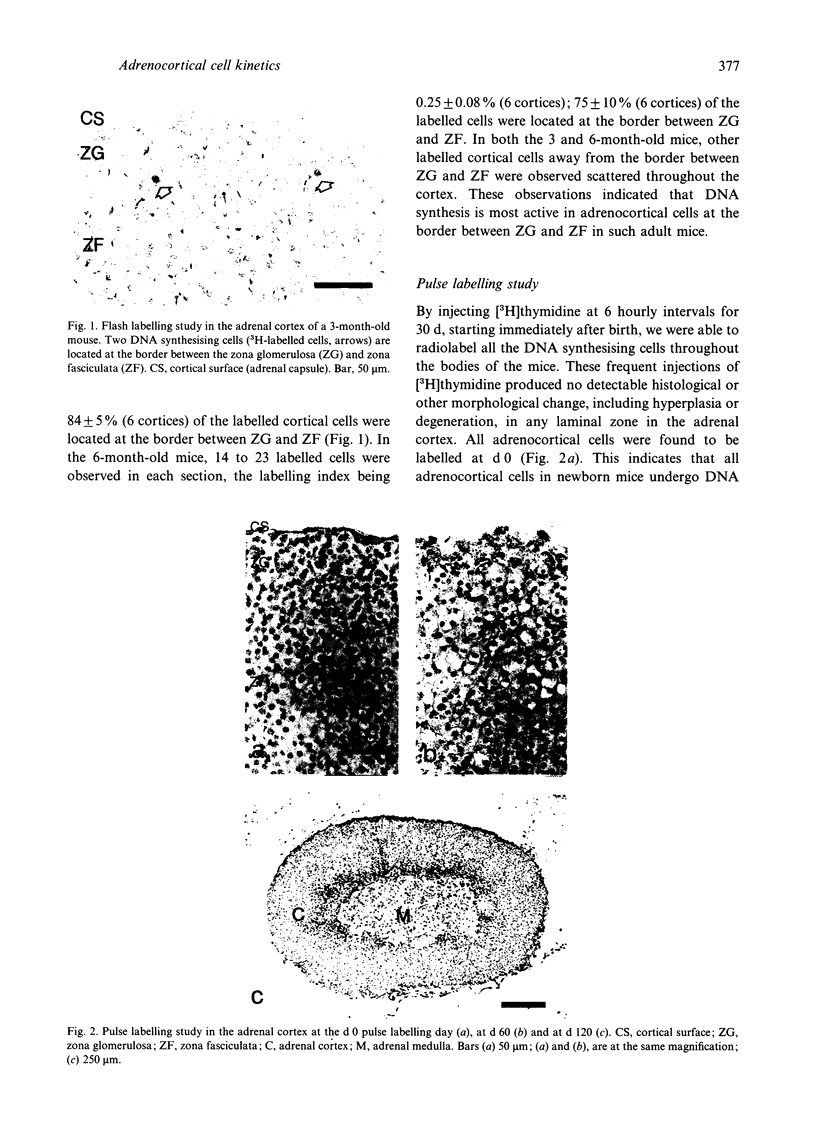
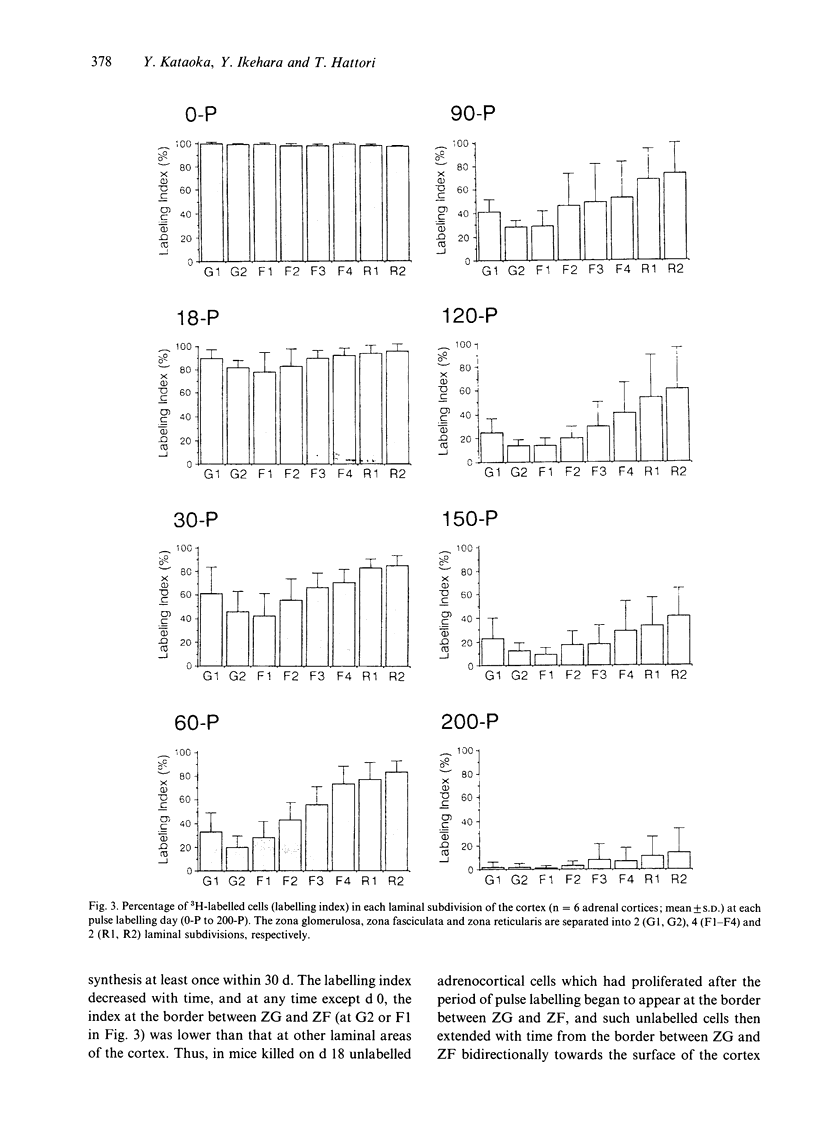
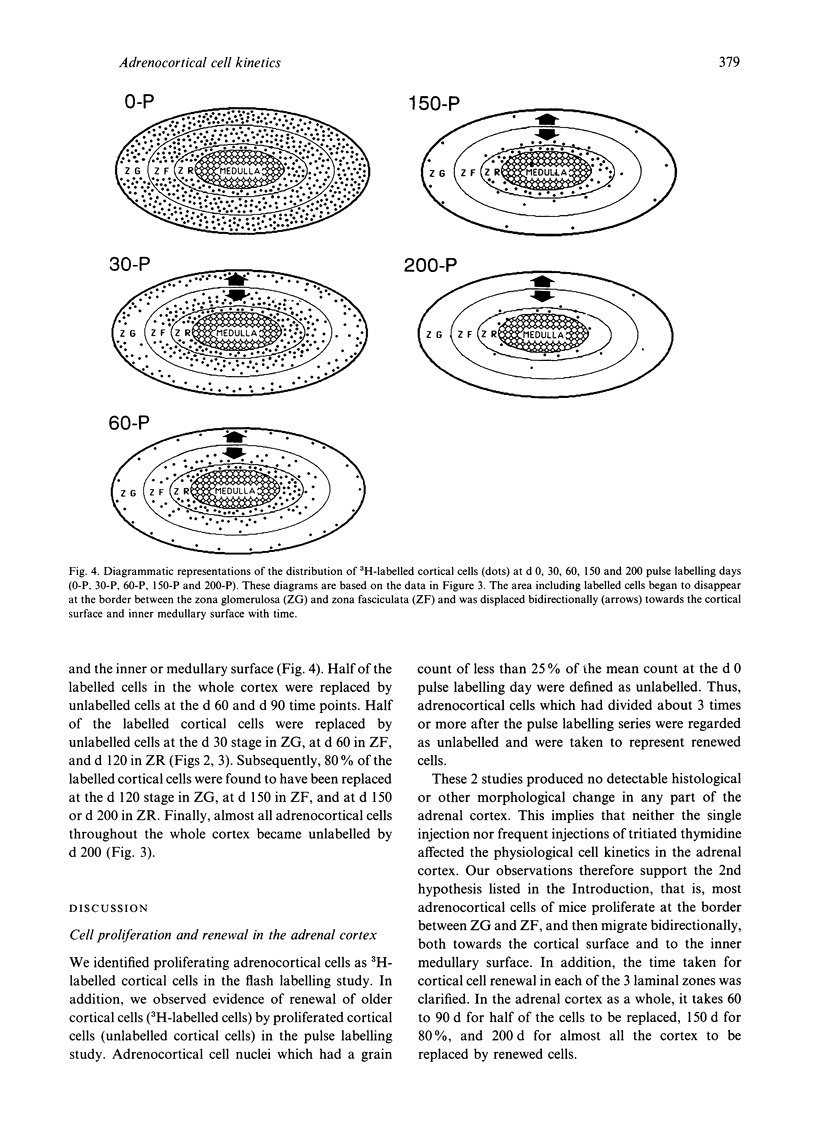
Although many hypotheses concerning cell proliferation and renewal in the adrenal cortex of mammals have been proposed, this topic has so far not been elucidated. Adrenocortical cells of adult mammals have low proliferative activity and take a considerable length of time to be renewed. This makes it difficult to investigate the dynamic features of their proliferation. To clarify the cell kinetics, we undertook a long term study in mice using an autoradiographic technique. We radiolabelled almost all the cells throughout the body in newborn mice with the exception of the neurons in central nervous system by the frequent subcutaneous injections of [3H]thymidine every 6 h for 30 d (pulse labelling). After this sequence of pulse labelling, we observed autoradiographically a decrease in the number of 3H-labelled cells in the adrenal cortex as a result of replacement with proliferated unlabelled cells (renewed cells). Single injections of [3H]thymidine (flash labelling) was also performed to examine DNA synthesis in the adrenal cortex. The investigations indicated that the adrenocortical cells proliferate at the border between the zona glomerulosa and the zona fasciculata, and that renewed cells which proliferated in that region move with time bidirectionally towards the cortical surface and the inner (medullary) surface. Half of the cortical cells in the zona glomerulosa, zona fasciculata and zona reticularis were replaced by renewed cells in 30, 60 and 120 d respectively. It took 200 d for almost all cortical cells to be replaced by renewed cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAIN A. J., HARRISON R. G. Cytological and histochemical variations in the adrenal cortex of the albino rat. J Anat. 1950 Apr;84(2):196–226. [PMC free article] [PubMed] [Google Scholar]
- DIDERHOLM H., HELLMAN B. The cell migration in the adrenal cortex of rats studied with tritiated thymidine. Acta Physiol Scand. 1960 Dec 30;50:197–202. doi: 10.1111/j.1748-1716.1960.tb00175.x. [DOI] [PubMed] [Google Scholar]
- FORD J. K., YOUNG R. W. Cell proliferation and displacement in the adrenal cortex of young rats injected with tritiated thymidine. Anat Rec. 1963 Jun;146:125–137. doi: 10.1002/ar.1091460206. [DOI] [PubMed] [Google Scholar]
- GRABER A. L., NEY R. L., NICHOLSON W. E., ISLAND D. P., LIDDLE G. W. NATURAL HISTORY OF PITUITARY-ADRENAL RECOVERY FOLLOWING LONG-TERM SUPPRESSION WITH CORTICOSTEROIDS. J Clin Endocrinol Metab. 1965 Jan;25:11–16. doi: 10.1210/jcem-25-1-11. [DOI] [PubMed] [Google Scholar]
- Hattori T., Fujita S. Tritiated thymidine autoradiographic study of cell migration and renewal in the pyloric mucosa of golden hamsters. Cell Tissue Res. 1976 Nov 24;175(1):49–57. doi: 10.1007/BF00220822. [DOI] [PubMed] [Google Scholar]
- MESSIER B., LEBLOND C. P. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am J Anat. 1960 May;106:247–285. doi: 10.1002/aja.1001060305. [DOI] [PubMed] [Google Scholar]
- Mitani F., Suzuki H., Hata J., Ogishima T., Shimada H., Ishimura Y. A novel cell layer without corticosteroid-synthesizing enzymes in rat adrenal cortex: histochemical detection and possible physiological role. Endocrinology. 1994 Jul;135(1):431–438. doi: 10.1210/endo.135.1.8013381. [DOI] [PubMed] [Google Scholar]
- Ochi M., Sawada T., Kusunoki T., Hattori T. Morphology and cell dynamics of adipose tissue in hypothalamic obese mice. Am J Physiol. 1988 May;254(5 Pt 2):R740–R745. doi: 10.1152/ajpregu.1988.254.5.R740. [DOI] [PubMed] [Google Scholar]
- Ogishima T., Suzuki H., Hata J., Mitani F., Ishimura Y. Zone-specific expression of aldosterone synthase cytochrome P-450 and cytochrome P-45011 beta in rat adrenal cortex: histochemical basis for the functional zonation. Endocrinology. 1992 May;130(5):2971–2977. doi: 10.1210/endo.130.5.1572304. [DOI] [PubMed] [Google Scholar]
- Sasano H., Mason J. I., Sasano N. Immunohistochemical study of cytochrome P-45017 alpha in human adrenocortical disorders. Hum Pathol. 1989 Feb;20(2):113–117. doi: 10.1016/0046-8177(89)90174-3. [DOI] [PubMed] [Google Scholar]
- Shinzawa K., Ishibashi S., Murakoshi M., Watanabe K., Kominami S., Kawahara A., Takemori S. Relationship between zonal distribution of microsomal cytochrome P-450s (P-450(17)alpha,lyase and P-450C21) and steroidogenic activities in guinea-pig adrenal cortex. J Endocrinol. 1988 Nov;119(2):191–200. doi: 10.1677/joe.0.1190191. [DOI] [PubMed] [Google Scholar]
- Wright N. A. Cell proliferation in the prepubertal male rat adrenal cortex: an autoradiographic study. J Endocrinol. 1971 Apr;49(4):599–609. doi: 10.1677/joe.0.0490599. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Voncina D., Morley A. R. An attempt to demonstrate cell migration from the zona glomerulosa in the prepubertal male rat adrenal cortex. J Endocrinol. 1973 Dec;59(3):451–459. doi: 10.1677/joe.0.0590451. [DOI] [PubMed] [Google Scholar]
- Zajicek G., Ariel I., Arber N. The streaming adrenal cortex: direct evidence of centripetal migration of adrenocytes by estimation of cell turnover rate. J Endocrinol. 1986 Dec;111(3):477–482. doi: 10.1677/joe.0.1110477. [DOI] [PubMed] [Google Scholar]