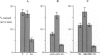Abstract
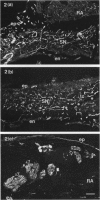
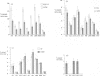
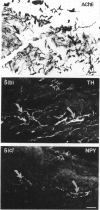
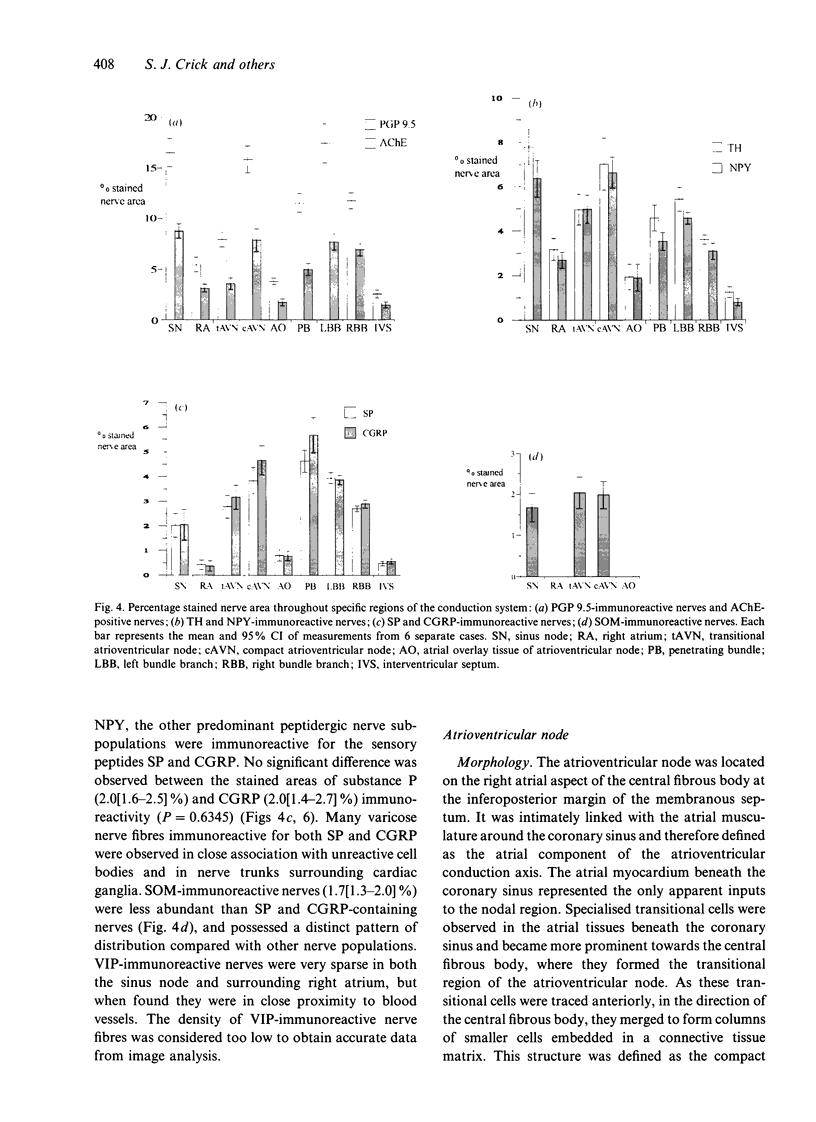
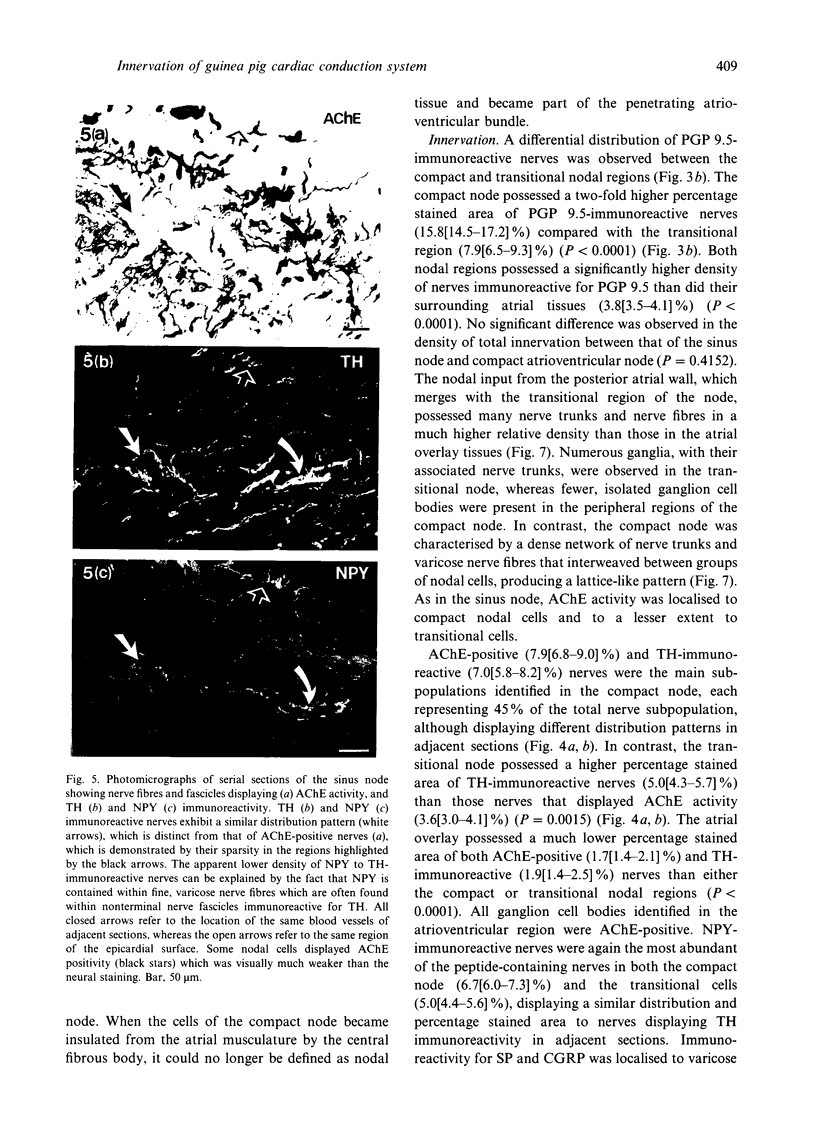
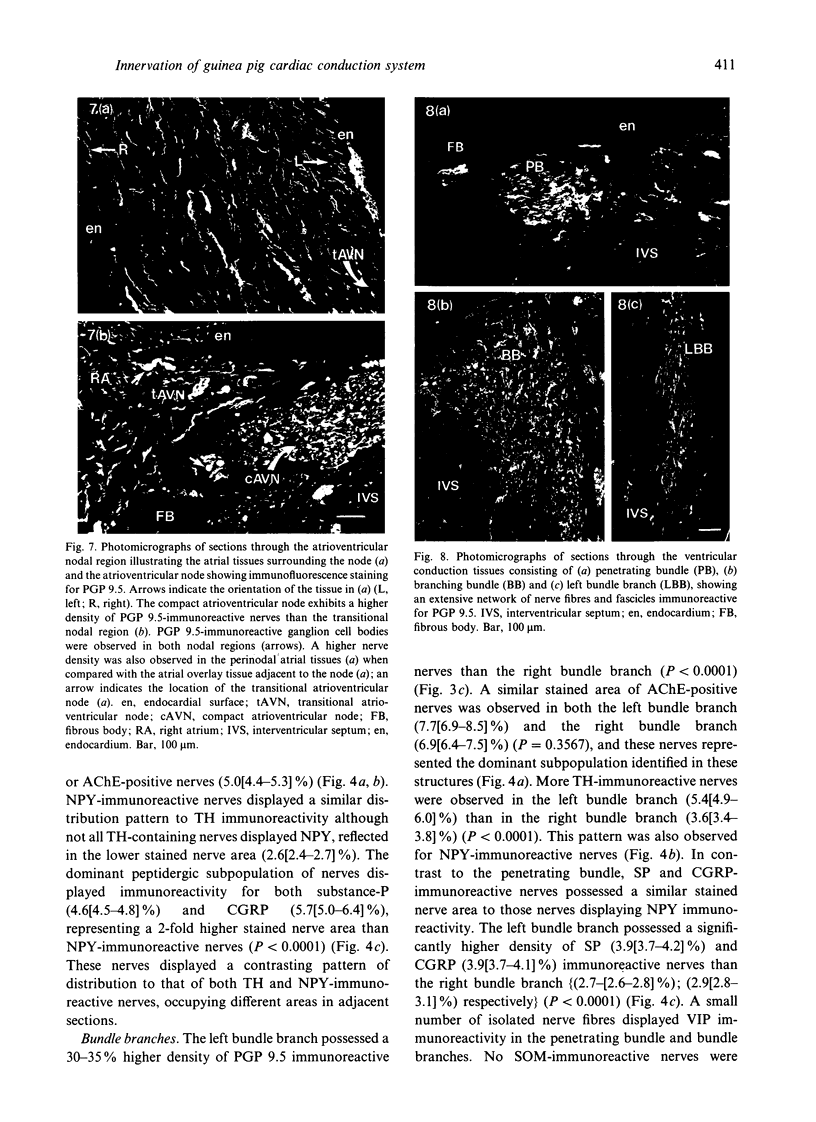
Quantitative measurements of relative nerve density were achieved using computer-assisted image analysis of immunohistochemically and histochemically defined nerves in the conduction system of the guinea pig heart. All regions of the conduction system possessed a similar density of nerve fibres and fascicles displaying immunoreactivity for the general neuronal marker protein gene product 9.5 (PGP 9.5), and this was 3 to 4-fold higher than in the adjacent myocardium. Acetylcholinesterase (AChE) positive and tyrosine hydroxylase (TH)-immunoreactive nerves were the main subtypes identified in the sinus and atrioventricular nodes, representing 40-45% of the stained area occupied by PGP 9.5-immunoreactive nerves. AChE-positive nerves were the dominant subtype identified in the left and right bundle branches, but were equal in proportion to TH-immunoreactive nerves in the penetrating bundle. Neuropeptide Y-immunoreactive nerves represented the main peptide-containing subpopulation in the nodal tissues, displaying a similar pattern of distribution and relative density to those nerves demonstrating TH immunoreactivity. Substance P and calcitonin gene-related polypeptide immunoreactive nerves were present throughout the conduction system and represented the main peptide-containing subpopulation in the ventricular conduction tissues. Nerve fibres showing immunoreactivity for either somatostatin or vasoactive intestinal polypeptide exhibited distinct patterns of distribution and comprised a relatively minor component of the innervation. The innervation of the guinea pig conduction tissues thus exhibits a uniform distribution and it comprises putative parasympathetic nerves and intrinsic neurons (AChE positive), sympathetic efferent nerves (NPY and TH-immunoreactive nerves) as well as other peptide-containing nerves, some of which (substance P and calcitonin gene-related polypeptide) are considered to represent afferent nerves. The distribution and density of nerve subpopulations in the guinea pig conduction system differ from those observed in the human conduction system, which suggests that the guinea pig may be an inappropriate model for comparative functional studies.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. M., Bircham P. M., Edwards A. V., Tatemoto K., Bloom S. R. Neuropeptide Y (NPY) reduces myocardial perfusion and inhibits the force of contraction of the isolated perfused rabbit heart. Regul Pept. 1983 Jul;6(3):247–253. doi: 10.1016/0167-0115(83)90143-x. [DOI] [PubMed] [Google Scholar]
- Allen T. G., Burnstock G. Intracellular studies of the electrophysiological properties of cultured intracardiac neurones of the guinea-pig. J Physiol. 1987 Jul;388:349–366. doi: 10.1113/jphysiol.1987.sp016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson F. L., Wynn J. R., Kimball J., Hanson G. R., Hammond E., Hershberger R., Kralios A. C. Vasoactive intestinal peptide in canine hearts: effect of total cardiac denervation. Am J Physiol. 1992 Feb;262(2 Pt 2):H598–H602. doi: 10.1152/ajpheart.1992.262.2.H598. [DOI] [PubMed] [Google Scholar]
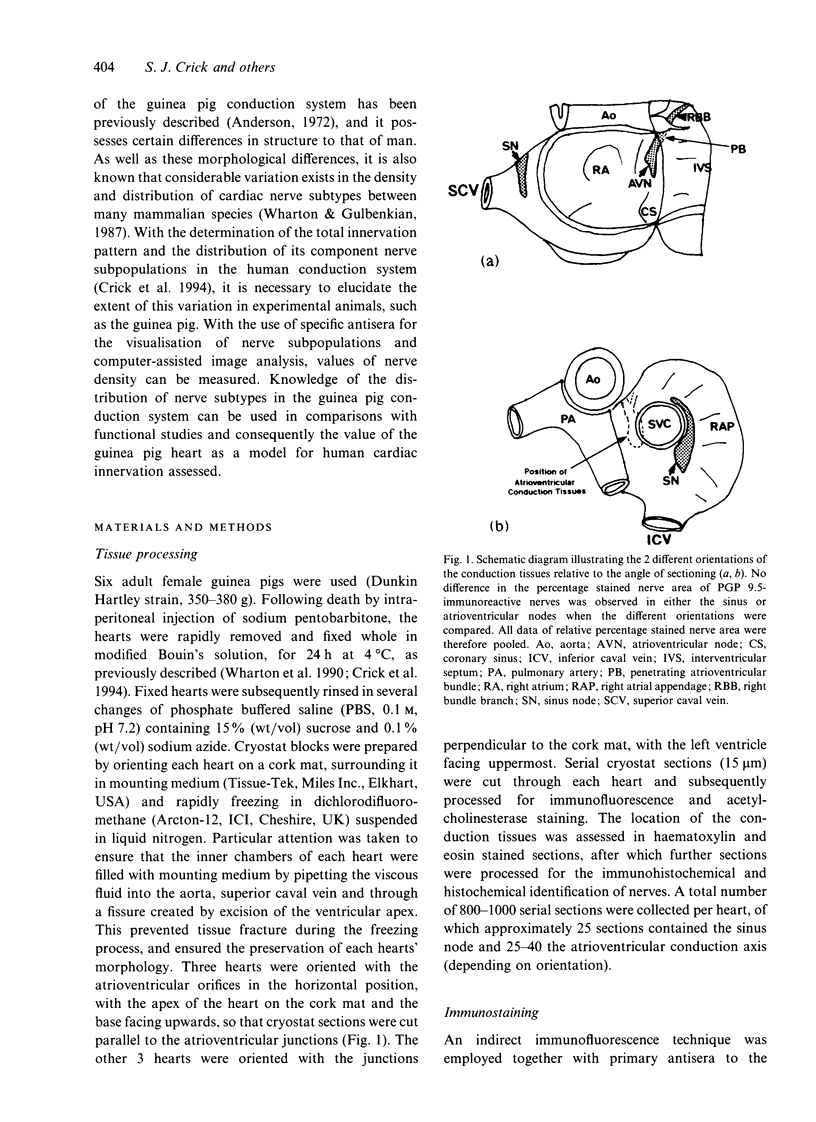
- Anderson R. H. The disposition, morphology and innervation of cardiac specialized tissue in the guinea-pig. J Anat. 1972 Apr;111(Pt 3):453–468. [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam A., Grupp I., Matlib M. A., Benza R., Jackson R. L., Fischer J. E., Grupp G. Comparison of the effects of neuropeptide Y (NPY) and 4-norleucine-NPY on isolated perfused rat hearts; effects of NPY on atrial and ventricular strips of rat heart and on rabbit heart mitochondria. Regul Pept. 1988 Jun;21(3-4):289–299. doi: 10.1016/0167-0115(88)90012-2. [DOI] [PubMed] [Google Scholar]
- Brown O. M., Salata J. J., Graziani L. A. The distribution of acetylcholine and choline in guinea pig heart. Life Sci. 1985 Jan 28;36(4):383–389. doi: 10.1016/0024-3205(85)90125-0. [DOI] [PubMed] [Google Scholar]
- Crick S. J., Wharton J., Sheppard M. N., Royston D., Yacoub M. H., Anderson R. H., Polak J. M. Innervation of the human cardiac conduction system. A quantitative immunohistochemical and histochemical study. Circulation. 1994 Apr;89(4):1697–1708. doi: 10.1161/01.cir.89.4.1697. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Franco-Cereceda A., Saria A., Lundberg J. M., Theodorsson-Norheim E., Hökfelt T. Distribution and origin of substance P- and neuropeptide Y-immunoreactive nerves in the guinea-pig heart. Cell Tissue Res. 1986;243(3):477–485. doi: 10.1007/BF00218054. [DOI] [PubMed] [Google Scholar]
- Day S. M., Gu J., Polak J. M., Bloom S. R. Somatostatin in the human heart and comparison with guinea pig and rat heart. Br Heart J. 1985 Feb;53(2):153–157. doi: 10.1136/hrt.53.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez J., Tamargo J., Valenzuela C. Negative inotropic effect of somatostatin in guinea-pig atrial fibres. Br J Pharmacol. 1985 Nov;86(3):547–555. doi: 10.1111/j.1476-5381.1985.tb08930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Badawi A., Schenk E. A. Histochemical methods for separate, consecutive and simultaneous demonstration of acetylcholinesterase and norepinephrine in cryostat sections. J Histochem Cytochem. 1967 Oct;15(10):580–588. doi: 10.1177/15.10.580. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A., Henke H., Lundberg J. M., Petermann J. B., Hökfelt T., Fischer J. A. Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin. Peptides. 1987 Mar-Apr;8(2):399–410. doi: 10.1016/0196-9781(87)90117-3. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A., Lundberg J. M. Calcitonin gene-related peptide (CGRP) and capsaicin-induced stimulation of heart contractile rate and force. Naunyn Schmiedebergs Arch Pharmacol. 1985 Nov;331(2-3):146–151. doi: 10.1007/BF00634231. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A., Lundberg J. M., Hökfelt T. Somatostatin: an inhibitory parasympathetic transmitter in the human heart? Eur J Pharmacol. 1986 Dec 2;132(1):101–102. doi: 10.1016/0014-2999(86)90019-1. [DOI] [PubMed] [Google Scholar]
- Gerstheimer F. P., Metz J. Distribution of calcitonin gene-related peptide-like immunoreactivity in the guinea pig heart. Anat Embryol (Berl) 1986;175(2):255–260. doi: 10.1007/BF00389603. [DOI] [PubMed] [Google Scholar]
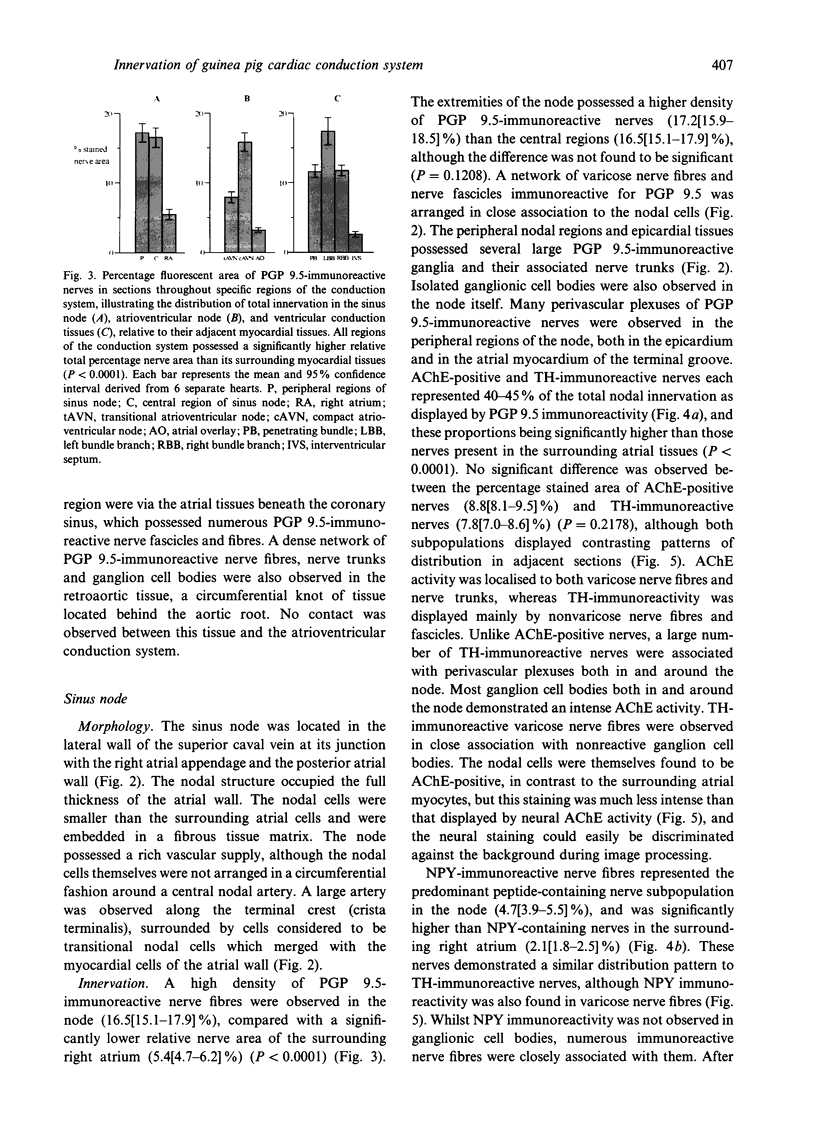
- Gerstheimer F. P., Simon T., Kölb J., Höpker W., Metz J. Computer-assisted morphometric study of the innervation of the guinea pig heart. Histochemistry. 1988;88(3-6):545–551. doi: 10.1007/BF00570322. [DOI] [PubMed] [Google Scholar]
- Gibbins I. L., Furness J. B., Costa M., MacIntyre I., Hillyard C. J., Girgis S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci Lett. 1985 Jun 12;57(2):125–130. doi: 10.1016/0304-3940(85)90050-3. [DOI] [PubMed] [Google Scholar]
- Gordon L., Polak J. M., Moscoso G. J., Smith A., Kuhn D. M., Wharton J. Development of the peptidergic innervation of human heart. J Anat. 1993 Aug;183(Pt 1):131–140. [PMC free article] [PubMed] [Google Scholar]
- Gulbenkian S., Wharton J., Polak J. M. The visualisation of cardiovascular innervation in the guinea pig using an antiserum to protein gene product 9.5 (PGP 9.5). J Auton Nerv Syst. 1987 Mar;18(3):235–247. doi: 10.1016/0165-1838(87)90122-6. [DOI] [PubMed] [Google Scholar]
- Hassall C. J., Burnstock G. Intrinsic neurones and associated cells of the guinea-pig heart in culture. Brain Res. 1986 Jan 29;364(1):102–113. doi: 10.1016/0006-8993(86)90991-1. [DOI] [PubMed] [Google Scholar]
- Ho S. Y., McComb J. M., Scott C. D., Anderson R. H. Morphology of the cardiac conduction system in patients with electrophysiologically proven dual atrioventricular nodal pathways. J Cardiovasc Electrophysiol. 1993 Oct;4(5):504–512. doi: 10.1111/j.1540-8167.1993.tb01239.x. [DOI] [PubMed] [Google Scholar]
- Hoffman B. F. Autonomic control of cardiac rhythm. Bull N Y Acad Med. 1967 Dec;43(12):1087–1096. [PMC free article] [PubMed] [Google Scholar]
- Kent K. M., Epstein S. E., Cooper T., Jacobowitz D. M. Cholinergic innervation of the canine and human ventricular conducting system. Anatomic and electrophysiologic correlations. Circulation. 1974 Nov;50(5):948–955. doi: 10.1161/01.cir.50.5.948. [DOI] [PubMed] [Google Scholar]
- Konishi S., Otsuka M. Blockade of slow excitatory post-synaptic potential by substance P antagonists in guinea-pig sympathetic ganglia. J Physiol. 1985 Apr;361:115–130. doi: 10.1113/jphysiol.1985.sp015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers W. H., Wessels A., Verbeek F. J., Moorman A. F., Virágh S., Wenink A. C., Gittenberger-de Groot A. C., Anderson R. H. New findings concerning ventricular septation in the human heart. Implications for maldevelopment. Circulation. 1992 Oct;86(4):1194–1205. doi: 10.1161/01.cir.86.4.1194. [DOI] [PubMed] [Google Scholar]
- Lund D. D., Schmid P. G., Roskoski R., Jr Choline acetyltransferase activity in rat and guinea pig heart following vagotomy. Am J Physiol. 1979 Apr;236(4):H620–H623. doi: 10.1152/ajpheart.1979.236.4.H620. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Franco-Cereceda A., Hua X., Hökfelt T., Fischer J. A. Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur J Pharmacol. 1985 Feb 5;108(3):315–319. doi: 10.1016/0014-2999(85)90456-x. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Hua X. Y., Franco-Cereceda A. Effects of neuropeptide Y (NPY) on mechanical activity and neurotransmission in the heart, vas deferens and urinary bladder of the guinea-pig. Acta Physiol Scand. 1984 Aug;121(4):325–332. doi: 10.1111/j.1748-1716.1984.tb07463.x. [DOI] [PubMed] [Google Scholar]
- Meijler F. L., Janse M. J. Morphology and electrophysiology of the mammalian atrioventricular node. Physiol Rev. 1988 Apr;68(2):608–647. doi: 10.1152/physrev.1988.68.2.608. [DOI] [PubMed] [Google Scholar]
- Mendez C., Moe G. K. Demonstration of a dual A-V nodal conduction system in the isolated rabbit heart. Circ Res. 1966 Aug;19(2):378–393. doi: 10.1161/01.res.19.2.378. [DOI] [PubMed] [Google Scholar]
- Metz J., Gerstheimer F. P., Herbst M. Distribution of synaptophysin immunoreactivity in guinea pig heart. Histochemistry. 1986;86(2):221–224. doi: 10.1007/BF00493392. [DOI] [PubMed] [Google Scholar]
- Quirion R., Regoli D., Rioux F., St-Pierre S. An analysis of the negative inotropic action of somatostatin. Br J Pharmacol. 1979 Jun;66(2):251–257. doi: 10.1111/j.1476-5381.1979.tb13673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARNOFF S. J., GILMORE J. P., MITCHELL J. H. Influence of atrial contraction and relaxation on closure of mitral valve. Observations on effects of autonomic nerve activity. Circ Res. 1962 Jul;11:26–35. [PubMed] [Google Scholar]
- Schmid P. G., Greif B. J., Lund D. D., Roskoski R., Jr Regional choline acetyltransferase activity in the guinea pig heart. Circ Res. 1978 May;42(5):657–660. doi: 10.1161/01.res.42.5.657. [DOI] [PubMed] [Google Scholar]
- Shavit S., Gassner S., Korczyn A. D. Cardiac denervation supersensitivity produced in guinea pigs by 6-hydroxy-dopamine. Pharmacol Res Commun. 1984 Feb;16(2):171–179. doi: 10.1016/s0031-6989(84)80092-2. [DOI] [PubMed] [Google Scholar]
- Sternini C., Brecha N. Distribution and colocalization of neuropeptide Y- and tyrosine hydroxylase-like immunoreactivity in the guinea-pig heart. Cell Tissue Res. 1985;241(1):93–102. doi: 10.1007/BF00214630. [DOI] [PubMed] [Google Scholar]
- Tago H., Kimura H., Maeda T. Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical procedure. J Histochem Cytochem. 1986 Nov;34(11):1431–1438. doi: 10.1177/34.11.2430009. [DOI] [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D. Mechanism of presynaptic actions of adenosine and acetylcholine on noradrenaline release in the guinea-pig heart. Neuroscience. 1982;7(9):2267–2276. doi: 10.1016/0306-4522(82)90137-3. [DOI] [PubMed] [Google Scholar]
- Weihe E., Reinecke M., Forssmann W. G. Distribution of vasoactive intestinal polypeptide-like immunoreactivity in the mammalian heart. Interrelation with neurotensin- and substance P-like immunoreactive nerves. Cell Tissue Res. 1984;236(3):527–540. doi: 10.1007/BF00217219. [DOI] [PubMed] [Google Scholar]
- Wessels A., Vermeulen J. L., Verbeek F. J., Virágh S., Kálmán F., Lamers W. H., Moorman A. F. Spatial distribution of "tissue-specific" antigens in the developing human heart and skeletal muscle. III. An immunohistochemical analysis of the distribution of the neural tissue antigen G1N2 in the embryonic heart; implications for the development of the atrioventricular conduction system. Anat Rec. 1992 Jan;232(1):97–111. doi: 10.1002/ar.1092320111. [DOI] [PubMed] [Google Scholar]
- Wharton J., Gulbenkian S. Peptides in the mammalian cardiovascular system. Experientia. 1987 Jul 15;43(7):821–832. doi: 10.1007/BF01945360. [DOI] [PubMed] [Google Scholar]
- Wharton J., Polak J. M., Gordon L., Banner N. R., Springall D. R., Rose M., Khagani A., Wallwork J., Yacoub M. H. Immunohistochemical demonstration of human cardiac innervation before and after transplantation. Circ Res. 1990 Apr;66(4):900–912. doi: 10.1161/01.res.66.4.900. [DOI] [PubMed] [Google Scholar]
- Wharton J., Polak J. M., McGregor G. P., Bishop A. E., Bloom S. R. The distribution of substrate P-like immunoreactive nerves in the guinea-pig heart. Neuroscience. 1981;6(11):2193–2204. doi: 10.1016/0306-4522(81)90007-5. [DOI] [PubMed] [Google Scholar]
- Wu D., Yeh S. J., Wang C. C., Wen M. S., Chang H. J., Lin F. C. Nature of dual atrioventricular node pathways and the tachycardia circuit as defined by radiofrequency ablation technique. J Am Coll Cardiol. 1992 Oct;20(4):884–895. doi: 10.1016/0735-1097(92)90189-t. [DOI] [PubMed] [Google Scholar]