Abstract
Wine lees, the second most significant by-product of winemaking after grape pomace, have received relatively little attention regarding their potential for valorization. Despite their rich content in bioactive components such as β-glucans, industrial utilization faces challenges, particularly due to variability in their composition. This inconsistency impacts the reliability and standardization of final products, limiting broader adoption in industrial applications. β-Glucans are dietary fibers or polysaccharides renowned for their diverse bioactive properties, including immunomodulatory, antioxidant, anti-inflammatory, antitumor, and cholesterol- and glucose-lowering effects. They modulate the immune system by activating Dectin-1 and TLR receptors on immune cells, enhancing phagocytosis, cytokine production, and adaptive immune responses. Their antioxidant activity arises from neutralizing free radicals and reducing oxidative stress, thereby protecting cells and tissues. β-Glucans also exhibit antitumor effects by inhibiting cancer cell growth, inducing apoptosis, and preventing angiogenesis, the formation of new blood vessels essential for tumor development. Additionally, they lower cholesterol and glucose levels by forming a viscous gel in the intestine, which reduces lipid and carbohydrate absorption, improving metabolic health. The biological activity of β-glucans varies with their molecular weight and source, further highlighting their versatility and functional potential. This study investigates how grape variety, vinification technology and extraction methods affect the yield and properties of β-glucans extracted from wine lees. The physico-chemical and mineral composition of different wine lees were analyzed, and two extraction methods of β-glucans from wine lees were tested: acid-base extraction and autolysis. These two methods were also tested under ultrasound-assisted conditions at different frequencies, as well as without the use of ultrasound. The β-glucan yield and properties were evaluated under different conditions. FTIR spectroscopy was used to assess the functional groups and structural characteristics of the β-glucans extracted from the wine lees, helping to confirm their composition and quality. Rheological behavior of the extracted β-glucans was also assessed to understand the impact of extraction method and raw material origin. The findings highlight that vinification technology significantly affects the composition of wine lees, while both the extraction method and yeast origin influence the yield and type of β-glucans obtained. The autolysis method provided higher β-glucan yields (18.95 ± 0.49% to 39.36 ± 0.19%) compared to the acid–base method (3.47 ± 0.66% to 19.76 ± 0.58%). FTIR spectroscopy revealed that the β-glucan extracts contain a variety of glucan and polysaccharide types, with distinct β-glucans (β-1,4, β-1,3, and β-1,6) identified through specific absorption peaks. The rheological behavior of suspensions exhibited pseudoplastic or shear-thinning behavior, where viscosity decreased significantly as shear rate increased. This behavior, observed across all β-glucan extracts, is typical of polymer-containing suspensions. These insights are critical for optimizing β-glucan extraction processes, supporting sustainability efforts and waste valorization in the wine industry. Efficient extraction of β-glucans from natural sources like wine lees offers a promising path toward their industrial application as valuable functional compounds.
Keywords: winery yeast lees, β-glucans, ultrasound, autolysis, alkali–acidic extraction
1. Introduction
In an era where we produce a larger amount of waste products, their valorization, in the context of the circular economy, is not only an opportunity but a pressing necessity [1,2,3]. This study investigates the physicochemical and mineral composition of winery yeast lees, compares two distinct methods for extracting β-glucans, and evaluates the quality of the resulting extracts through FTIR spectroscopy. Furthermore, the study analyzes the rheological properties of β-glucan suspensions, providing insights into their potential applications in various industries, including food, pharmaceuticals, and cosmetics. These findings aim to contribute to the development of sustainable solutions for waste valorization, supporting the transition to a circular economy. Winemaking is a sector of the economy that generates by-products containing numerous valuable compounds, many of which are still understudied and underutilized [4,5]. According to the report of the International Organization of Vine and Wine from April 2024 [6], worldwide in 2023, 237 mhL of wine were produced. Of the total amount of wine produced, approximately 2–6% represents wine lees [7], which would be approximately 4,740,000–14,220,000 hL produced worldwide in 2023. One of the valuable compounds of this by-product are β-glucans, a polysaccharide with interesting physical-chemical, functional and therapeutic properties. Obtaining β-glucans from yeasts has been quite studied in recent years, yeasts being a rich source of β-glucans, which represent 55–65% of their cell wall [8]. Many authors have tested different methods of extracting β-glucans from dry or grown yeast Saccharomyces cerevisiae (β 1-3 and β 1-6 glucans) [9,10,11,12]. In recent years, residual yeasts have increasingly been studied as a source of β-glucans, especially brewer’s yeasts [13,14,15,16]. The extraction of β-glucans from residual winemaking yeasts is much more difficult because they contain tartaric salts, pectin, and phenolic compounds [17]. These have already been studied [8,18].
The winemaking industry constitutes one of the pillars of the economy of the Republic of Moldova, providing contributions to the generation of employment in rural areas and to the country’s exports with a production scale of approximately 17.1 mdaL in 2021, despite the global and regional challenge [19]. Taking into account that 2–6% of the amount of wine produced will be residual yeasts, then, in 2021, approximately 342–1026 thousand daL was produced in the Republic of Moldova [7,20]. The sustainable management of by-products from winemaking, particularly wine lees, is gaining increasing attention. Despite their potential, wine lees remain an underutilized resource, often discarded or employed in limited applications such as ethanol production and soil fertilizers. However, these applications are more a way to reduce disposal costs than a real by-product valorization strategy [21].
In this study, we compared two methods for extracting β-glucans from winery yeast lees derived from four wine types produced using different technologies. The first method involves alkaline–acid extraction with ultrasound, using NaOH and acetic acid, while the second employs ultrasound-assisted autolysis followed by acid extraction with acetic acid. These two methods were selected due to their distinct advantages. The acid–base method effectively solubilizes the structural components of the yeast cell wall and breaks the bonds between polysaccharides and proteins, thereby releasing β-glucans. This method is also cost-efficient, utilizes readily available reagents, and can be easily scaled up for industrial applications. The autolysis extraction method promotes the degradation of the yeast cell wall through natural enzymatic mechanisms, making it eco-friendly and sustainable. Each approach has benefits and limitations. For instance, other types of extractions, like solvent extraction techniques, can produce great efficiency but present safety and environmental issues; enzymatic techniques are cost-effective and ecologically friendly; and supercritical fluid extraction is novel but necessitates expensive equipment. Most yeast samples studied originated from wine storage, where yeast cells undergo autolysis or enter inactive phases, leading to thinner cell walls and potentially increasing nutrient availability for active cells [22]. The aim of this study is to explore innovative methods for transforming waste into valuable resources, focusing on the winery yeast lees biomass generated in industrial processes.
2. Materials and Methods
2.1. Winery Yeast Lees
In this study, winery yeast lees from four types of wines from three Moldavian wineries were used. The four types of wine lees from three winemakers in Moldova have been summarized in Table 1.
Table 1.
Winery yeast lees and their provenance.
| Winery Yeast Lees | Abbreviation | Grape Variety | Harvest Year | Manufacturer | Recovery Stage |
|---|---|---|---|---|---|
| Semi-dry white wine | SVAM | Muscat | 2023 | Chateau Vartely winery | Collected after the wine was stored |
| Dry red wine | SVRS | Shiraz | 2023 | Purcari winery | Collected after the wine was stored |
| Sweet white wine (Ice wine) | SVR | Traminer and Muscat | 2023 | Poiana winery | Collected after fermentation |
| White sparkling wine | SVS | Chardonnay, Pinot Blanc and Pinot Noir | 2022 | Purcari winery | Collected after disgorging the sparkling wine |
2.2. Analysis of Winery Yeast Lees
2.2.1. Extraction and Analysis of β-Glucans
Ultrasound-assisted alkali–acidic extraction of β-glucans
The extraction method is the one described by Karslioglu et al., 2021 [9] with some modifications. A 25 mL volume of residual yeast biomass, of each type, was taken for the extraction. In order to remove the remaining alcohol present in the yeast biomass, it was centrifuged at 5000 rpm for 15 min. The supernatant was removed. To the solid biomass of residual yeast was added 1 M, 1.5 M and 2 M sodium hydroxide solution, in the volume ratio of 1:4. The mixture was treated with ultrasound in an ultrasound water bath at 80 °C, applying ultrasonic waves at 40 kHz for 15 min. It was placed in the water bath for 1 h at a temperature of 90 °C. After cooling, the suspension was centrifuged at 5000 rpm, 4 °C for 15 min. The precipitate was washed with distilled water and centrifuged at 5000 rpm, for 5 min. The supernatant was removed, and 3% acetic acid was added in the volume ratio of 1:4. The suspension was heated to 85 °C and left for 1 h in the water bath. It was left to cool and centrifuged at 5000 rpm, 4 °C for 15 min. The precipitate was washed with distilled water and centrifuged at 5000 rpm for 5 min. The supernatant was removed, and the precipitate was washed with ethanol and centrifuged at 5000 rpm, 4 °C for 15 min. Then, once again, the precipitate was washed with distilled water and centrifuged at 5000 rpm for 5 min. The obtained β-glucan compounds were dried in an air oven at 55 °C for 6 h. All chemicals used in this paper were of analytical grade and were purchased from Sigma Aldrich (St. Louis, MO, USA) and Merck, Darmstadt, Germany.
-
B.
Ultrasound-assisted autolysis extraction of β-glucans
The same amount of yeast biomass of 25 mL was taken from each type, and it was centrifuged at 5000 rpm for 15 min. Sodium phosphate buffer solution (30%, pH = 7.3) was added to the yeast biomass. The mixture was incubated for 24 h at 55 °C. Then, it was centrifuged at 5000 rpm, 4 °C for 15 min. The precipitate was washed with distilled water and centrifuged at 5000 rpm for 5 min. The supernatant was removed, and 3% acetic acid was added in the volume ratio of 1:4. The suspension was heated to 85 °C and left for 1 h in the water bath. It was left to cool and centrifuged at 5000 rpm, 4 °C for 15 min. The precipitate was washed with distilled water and centrifuged at 5000 rpm for 5 min. The supernatant was removed, and the precipitate was washed with ethanol and centrifuged at 5000 rpm, 4 °C for 15 min. Then, once again, the precipitate was washed with distilled water and centrifuged at 5000 rpm for 5 min. The obtained β-glucan compounds were dried in an air oven at 55 °C for 6 h.
2.2.2. Physico-Chemical Parameters of Winery Yeast Lees
Total ash was determined in wine lees samples according to the method described by the International Organization of Vine and Wine OIV-MA-VI-07: R2000 [23], and the dry substance was determined by the simple method of removing water by heating to a temperature of 100–110 °C and determining the mass difference between the initial and final sample. The total carbohydrates in wine lees were determined using the Antron method. The absorption was recorded at 620 nm [24]. Lipids were determined according to the Bligh and Dyer method using a mixture of ethanol, chloroform and acetic acid [25]. The total protein content of the sample was determined using the Lowry method, which is a spectrophotometric assay that detects the presence of peptide bonds [26].
2.2.3. Total Phenolic Compounds
The total content of polyphenols in yeast lees samples was determined by the Folin–Ciocalteu colorimetric method described by Makkar et al., 2003 [27]. The sample solution (0.1 mL) was mixed with 0.5 mL of Folin–Ciocalteu reagent and 0.4 mL of 7.5% sodium carbonate, and the absorbance was measured at 765 nm after 10 min at 37 °C. The total polyphenol content was expressed as mg gallic acid equivalents (GAE)/kg wine lees. The calibration curve of the polyphenols was performed by using gallic acid at concentrations of 10–200 mg/L with the regression coefficient R2 = 0.99872 and equation y = 0.00949× + 0.02950.
2.2.4. Anthocyanin Content
The anthocyanin content was measured spectrophotometrically at 535 nm, following extraction with a water–ethanol solution [28]. Spectrophotometric assays were performed on a UV-VIS Shimadzu spectrophotometer (Shimadzu Corporation, Kyoto, Japan).
where 98.2 is the extinction coefficient od cyanidin 3-galactoside.
2.2.5. Mineral Content
The mineral content was determined using inductively coupled plasma optical emission spectrometry (ICP-OES) with a Thermo Scientific iCAP 6200 Duo spectrometer (Thermo Fisher Scientific, Cambridge, UK) controlled by the software iTEVA (Trial version) [29]. Ash obtained from the calcination was dissolved using high-purity reagents (HNO3, Merck, Germany, Suprapur grade).
2.2.6. Microscopic Analyses of Winery Yeast Lees
The yeast sediments from the four types of wine were analyzed using a Zeiss Primostar 3 Stereomicroscope with AxioCam 208 Color Camera (Carl Zeiss IMT Co., Ltd., Shanghai, China) and built-in software, Zen, ver. 3.4.91.00000, at 100× magnification. The wine yeast sediment was subjected to a dilution of 50 relative to the dry substance and stained with methylene blue for the viability test and to allow the observation of cell morphology [30].
2.2.7. Calculation of Extracted β-Glucan Yield
The yield of glucan compounds was determined gravimetrically, as the ratio between the weight of the obtained dry extract and the initial amount of residual yeast reported to the dry matter [30].
2.2.8. Fourier Transform Infrared Spectroscopy (FTIR) of Extracted β-Glucans
The analysis was performed on the raw, undried samples of β-glucans. The determinations were performed using a Nicolet iS10 spectrometer from Thermo Scientific (Karlsruhe, Dieselstraβe, Germany), equipped with a diamond crystal. Measurements were performed in reflective absorbance mode (ATR-FTIR), at 4 cm−1 resolution in the range of a mid-infrared region of 650–4000 cm−1, with 32 scans in transmission mode at a resolution of 4 cm−1.
2.2.9. Rheological Properties of β-Glucan Suspensions
To analyze the rheological properties of β-glucan, 2% solutions/suspensions of extracted β-glucan were prepared. The measurements were carried out on the first, second and seventh day of storage of the suspensions at a temperature of 4 °C. Before taking measurements, the suspensions were allowed to warm up to room temperature. For rheological testing, a MARS 40 rheometer (Thermo-HAAKE Co., Ltd., Karlsruhe, Germany) with parallel plate geometry (40 mm diameter) was used. The temperature was controlled at 20 °C. The shear rate was linearly increased from 0 to 1400 1/s [31].
2.2.10. Statistical Analysis
All measurements were performed in triplicate. Data were analyzed by ANOVA and Tukey’s test (n = 3, confidence level 95%) by using the SPSS 26.0 (trial version) software (IBM, New York, NY, USA).
3. Results and Discussion
In this study, we used two extraction methods (alkali–acid and autolysis combined with ultrasound) and compared whether the extraction method had a greater influence on the yield and quality of β-glucans or the type of residual yeasts used. The macromolecular structures of the yeast cell wall (proteins, polysaccharides, lipids) present potential binding sites for various organic and inorganic molecules, present in must and wine, which makes the extraction of β-glucans difficult [18,32].
All four types of residual yeasts, in addition to the fact that they were obtained from wines of different varieties, also underwent different technological processes. The first type of yeast biomass came from a semi-dry white wine, produced from the Muscat grape variety. This type of wine contains sugars after the end of fermentation, of approximately 4 to 12 g/L of residual sugar. The activity of the winery yeast lees in this wine was stopped by the anaerobic conditions, the temperature and the presence of sulfites. The second type of residual yeast biomass was from a red wine, obtained from the Shiraz grape variety. It is recognized as one of the varieties with the highest polyphenol, tannins and antioxidant content [33]. The third type of residual yeast biomass contained yeasts collected after fermentation, but kept for half a year under refrigerated conditions. These residual yeasts were from the sweet wine produced from frozen grapes Muscat and Traminer, with a residual sugar content of over 45 g/L. The fourth type of residual yeast biomass was from the production of sparkling wine obtained by the classic fermentation method from Chardonnay, Pinot Noir and Pinot Blanc wine varieties. The specificity of these residual yeasts is that they are collected after disgorging stages, i.e., the yeast residue from the bottle is frozen and removed from the bottle.
Compared to the residual yeasts after alcoholic fermentation, all four types of these yeasts were in the inactive phase. In this phase, the Saccharomyces cerevisiae yeast cell undergoes a series of changes that allow it to survive in unfavorable conditions. This phase is essential for yeast survival in environments where there are insufficient nutrients or where conditions are otherwise hostile to active metabolic activity. One of the important changes that takes place is the thickening of the cell wall.
3.1. Physico-Chemical Characteristics and Mineral Content of Wine Lees
In order to understand the subsequent behavior and properties of yeast sediments, we first analyzed their physico-chemical properties. Table 2 shows the results of the physico-chemical analyses performed on the four types of wine lees.
Table 2.
Physico-chemical characteristics and mineral content of wine lees.
| Nr. | Parameters | Wine Lees | |||
|---|---|---|---|---|---|
| Semi-Dry White Wine (SVAM) | Sweet White Wine (SVR) | Dry Red Wine (SVRS) | White Sparkling Wine (SVS) | ||
| Physico-chemical characteristics | |||||
| 1 | Ash, % | 0.04 ± 0.11 a | 0.03 ± 0.26 b | 0.04 ± 0.26 a | 0.04 ± 0.14 a |
| 2 | Dry matter, % | 24.16 ± 0.14 a | 21.09 ± 0.12 b | 26.09 ± 0.17 b | 12.22 ± 0.04 c |
| 3 | Carbohydrates, % SU | 21.16 ± 0.65 b | 24.61 ± 0.66 c | 19.15 ± 0.09 a | 20.31 ± 0.65 a |
| 4 | Lipids, % SU | 8.11 ± 0.49 a | 11.09 ± 0.95 b | 4.71 ± 0.21 c | 8.13 ± 0.96 a |
| 5 | Proteins, % SU | 41.12 ± 0.87 a | 32.62 ± 0.19 c | 45.98 ± 0.23 b | 42.42 ± 0.43 a |
| 6 | Total phenolic substances, mg GAE/kg | 162.12 ± 0.84 b | 192.59 ± 1.04 a | 3350.17 ± 2.42 c | 142.19 ± 0.77 b |
| 7 | Total anthocyanins, mg GAE/kg fw | 227.48 ± 1.14 a | 277.12 ± 0.94 a | 1311.08 ± 0.92 c | 269.98 ± 0.49 b |
| Mineral content, mg/g | |||||
| 1 | K | 59.1 ± 0.14 c | 67.9 ± 0.11 b | 99.7 ± 0.10 a | 93.2 ± 0.04 a |
| 2 | Na | 0.1 ± 0.41 b | 0.3 ± 0.04 b | 0.8 ± 0.24 c | 0.4 ± 0.11 a |
| 3 | Mg | 0.4 ± 0.16 b | 0.6 ± 0.23 c | 1.4 ± 0.04 a | 1.1 ± 0.21 a |
| 4 | P | 3.2 ± 0.04 b | 4.6 ± 0.29 a | 15.1 ± 0.07 c | 4.1 ± 0.43 a |
| 5 | Ca | 2.6 ± 0.11 a | 1.9 ± 0.19 b | 6.9 ± 0.17 c | 2.7 ± 0.07 a |
| 6 | S | 1.1 ± 0.21 a | 2.5 ± 0.41 b | 3.9 ± 031 c | 1.9 ± 0,15 a |
Mean values with different letters in the same column are significantly different (p < 0.05).
The results of the analyses show that the winery yeast lees were rich in proteins (from 41.12 ± 0.87 to 45.98 ± 0.23%) and phenolic substances (from 142.19 ± 0.77 to 3350.17 ± 2.42 mg GAE/kg for red wine). Total phenolic content and total anthocyanin content in grape skins vary among grape varieties, and wine grape varieties have been observed to have higher phenolic and anthocyanin content compared to table grape varieties [34,35]. Research has shown that large amounts of beneficial polyphenols are present in pomace extracts and that the type of grape used, agronomic practices and winemaking method all influence the quantity and quality of the extracts. At the same time, 28.3915 ± 7.0 mg/kg of polyphenols was identified from Vermentino pomace and 11.3163 ± 6.5 mg/kg from Malvasia pomace obtained from Italian wines [36].
We obtained practically similar values for dry matter, ash and carbohydrates for all types of yeast lees. It is known that total lipids and the composition of fatty acids vary at different ripening stages and depending on the grape variety. At the same time, the lipid content increases significantly due to prolonged skin/pomace contact. Furthermore, the extraction of lipids and fatty acids increases linearly with the concentration of ethanol and the contact time with pomace [37]. In our case, we obtained lipid values between 4.71 ± 0.21% and 11.09 ± 0.95%. According to the obtained results, the yeast sediments from winemaking could serve as a source of proteins, whose nutritional value is expressed by the high content of essential amino acids [38,39].
The results of the analyses showed that the yeast sediments contained macroelements: K from 59.1 ± 0.14 to 99.7 ± 0.10 mg/g dry weight; Na from 0.1 ± 0.41 to 0.8 ± 0.24 mg/g dry weight; Mg from 0.4 ± 0.16 to 1.4 ± 0.04 mg/g dry weight; P from 3.2 ± 0.04 to 15.1 ± 0.07 mg/g dry weight; Ca from 1.9 ± 0.19 to 6.9 ± 0.17 mg/g dry weight; and S from 1.1 ± 0.21 to 3.9 ± 0.31 mg/g dry weight. We observed that red wine stood out with the maximum amounts of the six elements. At the same time, minerals present in the soil and transferred to the grapes of Vitis vinifera L. “Cabernet Sauvignon” harvested in three Mexican vineyards influenced the amounts and types of bioactive compounds present in the wine. The phenolic content and, therefore, the organoleptic characteristics of the wine are related to the mineral composition and the origin of the viticultural soil [40]. Most often, the mineral content is correlated with the temperature of the growing season and the hours of sunshine, as well as with the appearance of the vineyard. Studies of Chablis wine from the wine region in northern Burgundy, France, suggest that the soils and geology are not a primary source of minerality in the wine. On the other hand, the heat and sunlight of the growing season are relevant for the mineral content of the wine [41]. Mineral elements, along with volatile components and metabolites, are the basis of the geographical origin of the wine and are of great importance, because falsification of the origin is quite common in the wine industry [42,43]. Research studies based on characteristic variables (mineral elements, volatile components and metabolites) have very good performance. This demonstrates the effectiveness of a multi-source data fusion strategy for validating the authenticity of Chinese wine [44,45].
3.2. Microscopy of Winery Yeast Lees
Since three of the four wine yeast sediment samples were collected after the wine had been stored, we also analyzed these sediments with the help of a microscope to observe if the yeasts were still active and if there were other types of microorganisms that had developed in the meantime. Because yeast lees are a semi-solid product with a paste-like consistency, the 1:50 dilution ratio was chosen to achieve an optimal concentration of yeast cells that allowed clear visualization and accurate analysis under the microscope.
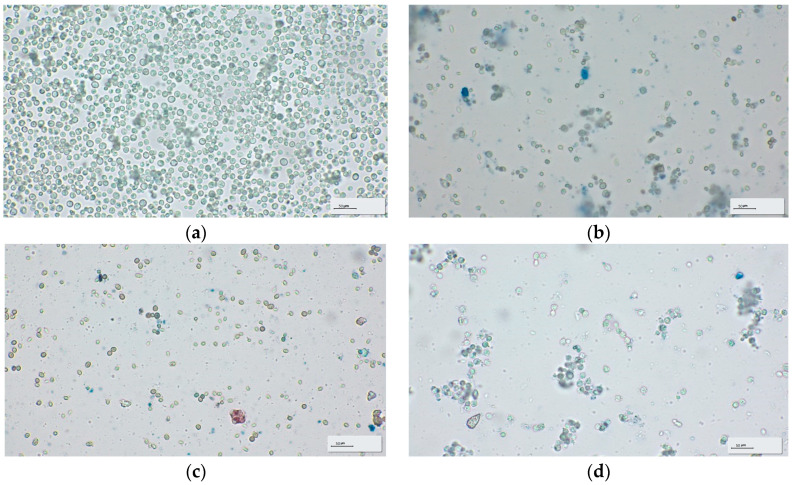
The microscopic image of the semi-dry white wine (Figure 1a) shows a high concentration of yeast cells, most of which are intact, suggesting an incomplete or ongoing fermentation. This is typical of a semi-dry wine, where residual sugar levels are higher, supporting yeast activity. The image of the sweet white wine (Figure 1b) presents a smaller number of yeasts, many of them in degradation stages. Sweet wines are generally higher in alcohol and sugar, which can inhibit yeast activity and lead to cell death. The microscopic examination of dry red wine (Figure 1c) reveals that the number of yeast cells is even lower, many of them being fragmented or lysed. This is typical of dry wines, where fermentation is complete and the yeast is no longer active. In the case of white sparkling wine (Figure 1d), the image shows a significant presence of yeast cells, some intact, some decayed, suggesting yeast autolysis, a crucial process in the maturation of sparkling wines, where dead yeast contributes to flavor and texture development. Semi-dry white wine, having more residual sugar, keeps the yeast active for a longer period (Figure 1a). This results in a higher concentration of intact yeasts in the sediment, unlike dry or sparkling wines, where fermentation is complete and most of the yeasts have already been eliminated or autolyzed.
Figure 1.
Microscopic images of winery yeast lees (100× magnification objective). (a) Semi-dry white wine (SVAM), (b) sweet white wine (SVR), (c) dry red wine (SVRS), (d) sparkling white wine (SVS).
In the context of the above, we note that yeast sediments have a different microbiological characteristic that is primarily influenced by the technological process underlying the production of the four types of wines. More than that, the microbiological characteristic of the sediments (Figure 1a–d) confirms that the exploitation of microbial resources is important for improving the sustainability of the winemaking process. This is a fairly recent approach, and it seems that quite a few studies are being conducted on it so far. Reviewing the potential of microorganisms and their interactions as a natural, environmentally friendly tool, improving sustainability aspects throughout the production chain, including waste treatment, becomes imperative to ensure sustainability in viticulture [46,47].
At the same time, it is known that the microbiota of grapes depends on factors such as environmental characteristics, geographical location, cultivation mode and others [48,49,50]. However, there are three fundamental microorganisms in the winemaking process: Saccharomyces cerevisiae yeasts, non-Saccharomyces yeasts and lactic acid bacteria [49]. In fermentation, microorganisms metabolize the sugar present in the grapes and produce several metabolites responsible for the wine’s quality, including flavor and hue, among others [51,52]. In relation to wine production, it is known that during industrial fermentation, it is possible to inoculate microorganisms in order to improve the wine’s characteristics. On the other hand, the process of fermentation in artisanal conditions takes place naturally, and the microorganisms present come from the microbiota specific to the grapes [53,54,55,56].
3.3. Yield of Extracted β-Glucans
Table 3 shows the yield of β-glucans extracted by two methods. In both methods, ultrasound treatment was used to determine if it has an influence on β-glucan extraction. For both methods, ultrasound was used at frequencies of 25 kHz and 45 kHz. In the case of the acid–base method, three different concentrations of the NaOH solution (1, 1.5, 2 M) were tested.
Table 3.
Yield of the extracted β-glucan compounds, %.
| Sample | Yield of β-Glucan Compounds, % | Sample | Yield of β-Glucan Compounds, % | |||
|---|---|---|---|---|---|---|
| Acid–base method assisted with ultrasound | ||||||
| Semi-dry white wine | 1 M NaOH, 25 kHz | SVAM 01AA | 9.65 ± 0.30 a | 1 M NaOH, 45 kHz | SVAM 11AA | 3.47 ± 0.66 b |
| Dry red wine | SVRS 01AA | 14.38 ± 0.45 a | SVRS 11AA | 16.39 ± 0.39 a | ||
| Sweet white wine | SVR 01AA | 5.99 ± 0.16 a | SVR 11AA | 5.17 ± 0.19 a | ||
| Sparkling white wine | SVS 01AA | 14.33 ± 0.22 b | SVS 11AA | 5.27 ± 0.25 a | ||
| Semi-dry white wine | 1.5 M NaOH, 25 kHz | SVAM 02AA | 19.76 ± 0.58 a | 1.5 M NaOH, 45 kHz | SVAM 11AA | 5.72 ± 0.44 b |
| Dry red wine | SVRS 02AA | 18.98 ± 0.29 b | SVRS 11AA | 9.49 ± 0.29 a | ||
| Sweet white wine | SVR 02AA | 9.99 ± 0.30 a | SVR 11AA | 6.70 ± 0.10 a | ||
| Sparkling white wine | SVS 02AA | 12.68 ± 0.12 b | SVS 11AA | 5.11 ± 0.17 c | ||
| Semi-dry white wine | 2 M NaOH, 25 kHz | SVAM 03AA | 5.89 ± 0.56 a | 2 M NaOH, 45 kHz | SVAM 12AA | 5.89 ± 0.33 a |
| Dry red wine | SVRS 03AA | 10.64 ± 0.37 a | SVRS 12AA | 11.36 ± 0.16 b | ||
| Sweet white wine | SVR 03AA | 9.65 ± 0.21 a | SVR 12AA | 6.23 ± 0.24 b | ||
| Sparkling white wine | SVS 03AA | 14.38 ± 0.15 a | SVS 12AA | 8.07 ± 0.08 c | ||
| Autolysis assisted with ultrasound method | ||||||
| Semi-dry white wine | Autolysis, 25 kHz | SVAM 01A | 37.28 ± 0.29 a | Autolysis, 45 kHz | SVAM 11A | 39.36 ± 0.19 b |
| Dry red wine | SVRS 01A | 26.59 ± 0.0.6 a | SVRS 11A | 30.91 ± 0.07 b | ||
| Sweet white wine | SVR 01A | 21.97 ± 0.07 a | SVR 11A | 21.03 ± 0.20 a | ||
| Sparkling white wine | SVS 01A | 18.95 ± 0.49 a | SVS 11A | 37.06 ± 0.06 c | ||
| Extraction without the use of ultrasound | ||||||
| Semi-dry white wine | SVAM AA | 2.77 ± 0.14 c | SVAM 01A | 37.62 ± 0.20 a | ||
| Dry red wine | SVRS AA | 13.80 ± 0.05 b | SVRS 01A | 26.59 ± 0.04 c | ||
| Sweet white wine | 2 M NaOH | SVR AA | 6.46 ± 0.22 c | Autolysis | SVR 01A | 21.90 ± 0.17 a |
| Sparkling white wine | SVS AA | 17.30 ± 0.30 c | SVS 01A | 41.34 ± 0.31 a | ||
Mean values with different letters in the same column are significantly different (p < 0.05).
The data obtained show that there are differences between the yield of β-glucans obtained by these two methods. The extraction of β-glucans using autolysis is much more efficient in terms of yield. For example, in semi-dry white wine, the yield with the autolysis-assisted method was 37.28–39.36%, while with the acid–base method, it was in the range of 2.77–19.76%. For sparkling white wine, the yield obtained by autolysis varied between 18.95 ± 0.49−41.34 ± 0.31% and that by acid–base extraction, between 5.11 ± 0.17 and 17.30 ± 0.30%. The action of cell enzymes was much stronger than the effect using hot NaOH, but the factor that remained to be elucidated was the purity of the β-glucans obtained by these two methods. At the same time, comparing application of the methods with and without ultrasound, there were no significant differences. The observed differences can be explained as being due to the bonds between mannoproteins and β-1,3 and β-1,6 structures at the cell wall level that have not been fully cleaved. Of course, these glucan-mannan structures have high potential for use as alternatives to antibiotics, and the future prospects underline the idea of using the two compounds. For example, for dry red wine, the extraction yield by autolysis varied with ultrasound, between 26.59 ± 0.0.6% and 30.91 ± 0.07%, and without it, measured 26.59 ± 0.04%. At the same time, for the same wine lees, the yield with acid–base extraction with ultrasound varied between 9.49 ± 0.29 and 18.98 ± 0.29, and without ultrasound, measured 13.80 ± 0.05%. We can say that the application of ultrasound in the case of these two methods in the given conditions did not have a significant influence. Also, the ultrasound frequency used did not influence the extraction yield in the case of both methods (e.g., for sweet white wine using the acid–base method, at 25 kHz the yield was between 5.99 ± 0.16% and 9.99 ± 0.30%, and at the frequency of 45 kHz, between 5.17 ± 0.19% and 6.70 ± 0.10%). The type of yeast used has the greatest influence on the yield of extracted β-glucans. The highest β-glucan yield using autolysis was observed in sparkling white wine (41.34 ± 0.31% without ultrasound), indicating that certain wine residues may respond more effectively to enzymatic degradation. The technological processes, the subsequent storage conditions of the wine and the way of keeping the winery yeast lees directly influence the extraction yield. The percentage of β-glucan obtained by the acid–base method was very close to that obtained by Karslioglu et al., 2021 [9], 3.47 ± 0.66% and 19.76 ± 0.58%, even though the author used yeast grown on culture medium. However, the percentage obtained by autolysis was much higher than the one reported by the same author. The yield obtained after extraction by autolysis was close to that obtained by Varelas et al., 2016 [8], 18.95 ± 0.49% and 39.36 ± 0.19%.
3.4. FTIR Spectroscopy
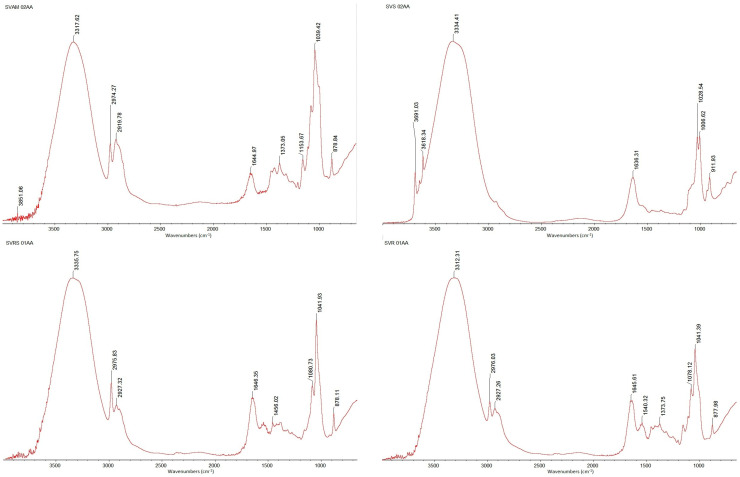
The recorded FTIR spectra are shown in Figure 2. All samples have a few peaks that are repeated in each sample, namely in the range of 1040–1030 cm−1. The presence of absorption peaks in the 900–1200 cm−1 region confirms the C–C and C–O stretching vibrations, indicating that polysaccharides are a major component [11]. According to Binati et al. 2024, the peaks near 995, 1040 and 1025 cm−1 are typical for β-glucans (the peaks at 1025 are characteristic for β-1,4 glucans) [57]. In the sample SVS 02AA (sparkling wine yeast lees), there is also a peak near 1008 cm−1 that indicates the presence of β-1,6 glucans [58]. In the SVAM 02AA (semi-dry white wine) sample, we also observed a peak at 1153 cm−1, which corresponds to α-linked glucans. Another peak that could be found in practically all samples was at 1042, 1041 cm−1, indicating the presence of β-1,3-glucan [32]. Different peaks around 1636 cm−1 were mainly associated with the C=O stretching vibration and are characteristic for amide I, absorption of proteins [59]. In the spectra for samples SVAM 02AA and SVRS 01AA were found peaks at 878 cm−1; the absorption bands near 890 cm−1 are characteristic for β-anomeric configurations [60,61,62]. Another characteristic peak for a majority of the samples was found in the region 2950–2850 cm− 1, corresponding to C–H groups commonly found in polysaccharide [63,64]. A strong and broad band in the 3600–3000 cm−1 region (in our case, in all samples, it was a peak around 3300 cm−1) corresponded to the stretching vibration of abundant OH in polysaccharide [24,25,26,65,66,67,68].
Figure 2.
FT−IR−ATR spectra of the most representative samples.
Another peak that was found in several samples was near 2975 cm−1. According to author Hong et al., 2021, a well-resolved group of moderate bands located in the 3000–2500 cm−1 region can be assigned to the symmetric and asymmetric stretching vibrations of skeletal CH and CH2 in polysaccharides [67].
Infrared Fourier transform spectroscopy revealed that the examined samples contained a variety of glucan and polysaccharide types. The polysaccharide composition was confirmed by the primary absorption areas (900–1200 cm−1) that correspond to the C–C and C–O stretching vibrations. Furthermore, the diversity of glucan molecules was shown by the distinct existence of β-1,4, β-1,3, and β-1,6 glucans, as indicated by the various spectral peaks (particularly at 1000–1150 cm−1). A polysaccharide–protein-rich composition was suggested by specific protein-associated C=O vibrations (1636 cm−1) and broad bands in the 3300 cm−1 area, which are related to polysaccharide OH groups. The molecular fingerprints of the various glucan and polysaccharide types found in each sample, as observed in the specific spectral peaks, provided detailed insights into the structural diversity and composition of the samples. For instance, peaks associated with β-1,4, β-1,3, and β-1,6 glucans (1000–1150 cm−1) highlighted the variety of glucan linkages, while the C=O stretching vibration at 1636 cm−1 suggested the presence of protein–polysaccharide interactions. These spectral features confirmed the polysaccharide–protein-rich nature of the samples and underscored the complexity of their molecular composition [68].
3.5. Rheological Properties of β-Glucan Suspensions
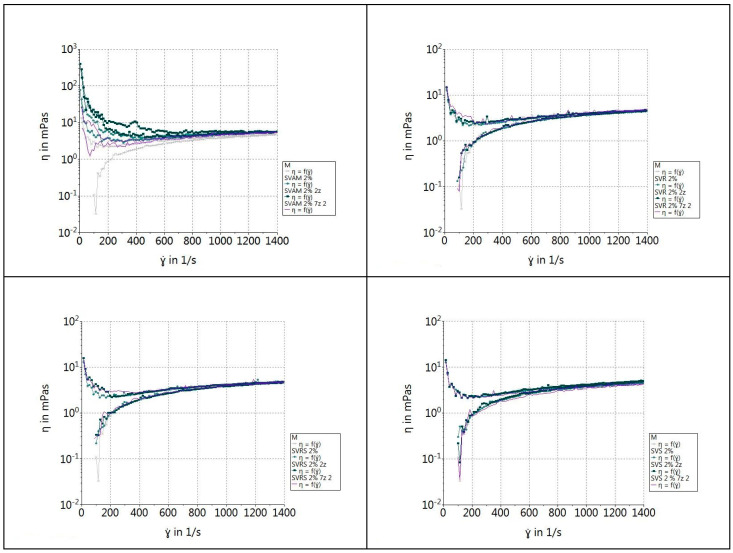
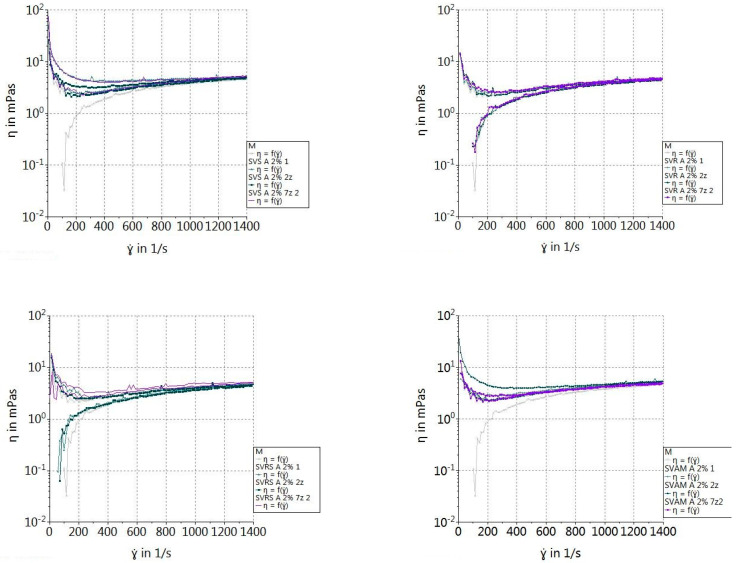
The results of these tests are presented in Figure 3 and Figure 4. In all the samples, we observed that the initial viscosity was increased, after which it stabilized with the increase in shear rates; this happened around the value of 200 1/s. This showed that the selected shear rate range was too large for this concentration. However, we could observe different behaviors of the four types of suspensions of β-glucan extracts. As the shear rate increased, the viscosity decreased significantly, indicating pseudoplastic or shear-thinning behavior. This phenomenon is common for suspensions containing polymers or supramolecular structures, where the applied forces break down the internal structure, thus reducing the viscosity. This is also mentioned by Lante et al. 2023 for solutions that have a concentration greater than 1% [69].
Figure 3.
Shear rate dependence of viscosity for 2% suspension of β-glucans extracted by the acid–base method.
Figure 4.
Shear rate dependence of viscosity for 2% suspension of β-glucans extracted by autolysis.
For both extraction methods, we could observe that the samples obtained from the yeast sediment of dry red wine (SVRS), sweet white wine (SVR) and sparkling white wine (SVS) had the same behavior and approached the control sample. However, the suspension obtained from yeasts from semi-dry white wine (SVAM) had a higher viscosity compared to the control sample in the entire time interval. The presence of a yield stress, which is often attributed to the presence of particles in the macromolecular medium, was indicated by an increase in apparent viscosity at low shear rate as the shear rate dropped. However, weak intermolecular interactions may also be responsible for this phenomenon. However, low-frequency viscoelastic measurements showed that the system had a propensity to exhibit gel-like characteristics. Physical cross-links created by intermolecular interactions could be the cause of this.
We can also state that there were no significant differences in viscosity between the suspensions obtained by autolysis and the acid-base method for the samples SVR and SVRS, and small differences for the samples SVAM and SVS. According to Petravi-Tominac et al. 2011, the differences in the β-1,3/1,6 ratio affect the functional properties of β-glucans [31]. The removal of β-1,6-glucan, which happens in harsh acidic conditions, as in the case of the two methods we used [12], leads to changes in the composition and chemical structure of β-glucan molecules and therefore induces changes in rheological properties [31].
4. Conclusions
The winery yeast lees represent a by-product rich in nutrients such as proteins, lipids, carbohydrates, mineral substances, and bioactive compounds such as polyphenols and β-glucans. The winery yeast lees analyzed had a protein content between 41.12 ± 0.87% and 45.98 ± 0.23%, lipid content between 4.71 ± 0.21% and 11.09 ± 0.95%, total phenolic content between 142.19 ± 0.77 mg GAE/kg and 3350.17±2.42 mg GAE/kg, and β-glucan content that varied between 2.77 ± 0.14% and 39.36 ± 0.19%. These results were due to the variability between the yeasts lees obtained by different winemaking technologies. Thus, the yield and the types of β-glucans and their properties are influenced by several factors, such as winemaking technology, the type of yeast used and the extraction method. In terms of yield, the extraction by autolysis was more efficient (between 18.95 ± 0.49% and 41.34 ± 0.31%) than the acid–base method (yield between 2.77 ± 0.14% and 17.30 ± 0.30%). Also, the type of yeast or winemaking technology has an important influence on the extraction of β-glucans from winery yeasts lees. Fourier transform infrared spectroscopy showed the presence of β-glucans in all extracted samples. The rheological properties of the 2% suspension also showed that both the extraction method and the winemaking technology have an influence on the viscosity of the β-glucans. Further studies on different types of yeast lees are necessary to improve the efficiency of utilizing these valuable compounds. At the same time, the obtained results on the extraction and properties of β-glucans contribute to a better understanding of how these compounds can be integrated into various functional foods, providing health benefits (β-glucans extracted from winery yeasts lees can be used to improve the texture and nutritional value of bread, fermented dairy products and natural juices).
Acknowledgments
This work was supported by a grant of the Ministry of Research, Innovation and Digitization, CNCS-UEFISCDI, project number PN-IV-P8-8.3-ROMD-2023-0121, within PNCDI and Institutional Project, subprogram 020405 “Optimizing food processing technologies in the context of the circular bioeconomy and climate change”, Bio-OpTehPAS, being implemented at the Technical University of Moldova.
Author Contributions
Conceptualization, A.C. (Ana Chioru), I.A. and A.D.; Methodology, A.C. (Aurica Chirsanova) and A.C. (Ancuța Chetrariu); Formal Analysis, A.C. (Ana Chioru) and A.B.; Investigation, A.C. (Ana Chioru); Resources, A.D. and I.A.; Writing—Original Draft Preparation, A.C. (Ana Chioru) and I.A.; Writing—Review and Editing, A.C. (Ancuța Chetrariu), A.B. and A.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by “Stefan cel Mare” University of Suceava.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gherghel A., Teodosiu C., De Gisi S. A Review on Wastewater Sludge Valorisation and Its Challenges in the Context of Circular Economy. J. Clean. Prod. 2019;228:244–263. doi: 10.1016/j.jclepro.2019.04.240. [DOI] [Google Scholar]
- 2.Borta A.-M., Sturza R. Ways of application of the circular bioeconomy in the wine industry. J. Eng. Sci. 2024;30:124–146. doi: 10.52326/jes.utm.2023.30(4).10. [DOI] [Google Scholar]
- 3.Leder N., Kumar M., Rodrigues V.S. Influential Factors for Value Creation within the Circular Economy: Framework for Waste Valorisation. Resour. Conserv. Recycl. 2020;158:104804. doi: 10.1016/j.resconrec.2020.104804. [DOI] [Google Scholar]
- 4.Constantin O.E., Stoica F., Rațu R.N., Stănciuc N., Bahrim G.E., Râpeanu G. Bioactive Components, Applications, Extractions, and Health Benefits of Winery By-Products from a Circular Bioeconomy Perspective: A Review. Antioxidants. 2024;13:100. doi: 10.3390/antiox13010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kokkinomagoulos E., Kandylis P. Sustainable Exploitation of By-Products of Vitivinicultural Origin in Winemaking. Proceedings. 2020;67:5. doi: 10.3390/ASEC2020-07521. [DOI] [Google Scholar]
- 6.International Organization of Vine and Wine STATE OF THE WORLD VINE AND WINE SECTOR IN 2023. [(accessed on 31 October 2024)]. Available online: https://www.oiv.int/sites/default/files/2024-04/OIV_STATE_OF_THE_WORLD_VINE_AND_WINE_SECTOR_IN_2023.pdf.
- 7.Sancho-Galán P., Amores-Arrocha A., Jiménez-Cantizano A., Palacios V. Physicochemical and Nutritional Characterization of Winemaking Lees: A New Food Ingredient. Agronomy. 2020;10:996. doi: 10.3390/agronomy10070996. [DOI] [Google Scholar]
- 8.Varelas V., Tataridis P., Liouni M., Nerantzis E.T. Valorization of Winery Spent Yeast Waste Biomass as a New Source for the Production of β-Glucan. Waste Biomass Valor. 2016;7:807–817. doi: 10.1007/s12649-016-9530-4. [DOI] [Google Scholar]
- 9.Karslioğlu F., Ertunç S., Yilmazer Hitit Z., Akay B. Investigation of Extraction Method Effect on Yeast Beta Glucan Production. Eurasian J. Biol. Chem. Sci. 2021;4:51–55. doi: 10.46239/ejbcs.734046. [DOI] [Google Scholar]
- 10.Magnani M., Calliari C.M., de Macedo F.C., Mori M.P., de Syllos Cólus I.M., Castro-Gomez R.J.H. Optimized Methodology for Extraction of (1 → 3)(1 → 6)-β-d-Glucan from Saccharomyces cerevisiae and in Vitro Evaluation of the Cytotoxicity and Genotoxicity of the Corresponding Carboxymethyl Derivative. Carbohydr. Polym. 2009;78:658–665. doi: 10.1016/j.carbpol.2009.05.023. [DOI] [Google Scholar]
- 11.Mahmoud Amer E., Saber S.H., Abo Markeb A., Elkhawaga A.A., Mekhemer I.M.A., Zohri A.-N.A., Abujamel T.S., Harakeh S., Abd-Allah E.A. Enhancement of β-Glucan Biological Activity Using a Modified Acid-Base Extraction Method from Saccharomyces cerevisiae. Molecules. 2021;26:2113. doi: 10.3390/molecules26082113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pham K.N., Nhut N.D., Cuong N.M. New Method for Preparing Purity β-D-Glucans (Beta-Glucan) from Baker’s Yeast (Saccharomyces cerevisiae) Sci. Technol. Dev. J. 2020;23:678–683. doi: 10.32508/stdj.v23i3.2051. [DOI] [Google Scholar]
- 13.Avramia I., Amariei S. Spent Brewer’s Yeast as a Source of Insoluble β-Glucans. Int. J. Mol. Sci. 2021;22:825. doi: 10.3390/ijms22020825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian X., Yang P., Jiang W. Effect of Alkali Treatment Combined with High Pressure on Extraction Efficiency of β-d-Glucan from Spent Brewer’s Yeast. Waste Biomass Valor. 2019;10:1131–1140. doi: 10.1007/s12649-017-0130-8. [DOI] [Google Scholar]
- 15.Thammakiti S., Suphantharika M., Phaesuwan T., Verduyn C. Preparation of Spent Brewer’s Yeast Β-glucans for Potential Applications in the Food Industry. Int. J. Food Sci. Technol. 2004;39:21–29. doi: 10.1111/j.1365-2621.2004.00742.x. [DOI] [Google Scholar]
- 16.Suphantharika M. Preparation of Spent Brewer’s Yeast β-Glucans with a Potential Application as an Immunostimulant for Black Tiger Shrimp, Penaeus monodon. Bioresour. Technol. 2003;88:55–60. doi: 10.1016/S0960-8524(02)00257-2. [DOI] [PubMed] [Google Scholar]
- 17.Rozmierska J., Stecka K.M., Kotyrba D., Piasecka-Jóźwiak K. Preparation of Sedimented Wine Yeast Derived Products for Potential Application in Food and Feed Industry. Waste Biomass Valor. 2019;10:455–463. doi: 10.1007/s12649-017-0068-x. [DOI] [Google Scholar]
- 18.Chiselița N., Chiselița O., Efremova N., Beșliu A. Valorization of the Red Wine Yeast Waste. Pol. J. Environ. Stud. 2024;33:1623–1630. doi: 10.15244/pjoes/173436. [DOI] [Google Scholar]
- 19.Vacarciuc L., Breahna E., Bogatii E. Filiera vitivinicolă a Republicii Moldova în contextul alinierii la cerințele UE; Proceedings of the International Scientific Conference “European Integration Through the Straightening of Education, Re-search, Innovations in Eastern Partnership Countries”; Chisinau, Moldova. 16–17 May 2022. [Google Scholar]
- 20.Bai Z., Jin B., Li Y., Chen J., Li Z. Utilization of Winery Wastes for Trichoderma Viride Biocontrol Agent Production by Solid State Fermentation. J. Environ. Sci. 2008;20:353–358. doi: 10.1016/S1001-0742(08)60055-8. [DOI] [PubMed] [Google Scholar]
- 21.De Iseppi A., Lomolino G., Marangon M., Curioni A. Current and Future Strategies for Wine Yeast Lees Valorization. Food Res. Int. 2020;137:109352. doi: 10.1016/j.foodres.2020.109352. [DOI] [PubMed] [Google Scholar]
- 22.Porras-Agüera J.A., Moreno-García J., Mauricio J.C., Moreno J., García-Martínez T. First Proteomic Approach to Identify Cell Death Biomarkers in Wine Yeasts during Sparkling Wine Production. Microorganisms. 2019;7:542. doi: 10.3390/microorganisms7110542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.2-11-2022. Method OIV-MA-VI-07: R2000 “Determination of Ash Content in Vinegars”. [(accessed on 29 July 2024)]. Available online: https://www.oiv.int/standards/compendium-of-international-methods-of-analysis-for-vinegars/wine-vinegars/methods-of-analysis-for-vinegars/determination-of-ash-content-%28type-ii%29.
- 24.Dey P.M., Harborne J.B. Carbohydrats. Volume 2. Academic Press; Cambridge, MA, USA: 1993. Methods in Plant Biochemistry. [Google Scholar]
- 25.Breil C., Abert Vian M., Zemb T., Kunz W., Chemat F. “Bligh and Dyer” and Folch Methods for Solid–Liquid–Liquid Extraction of Lipids from Microorganisms. Comprehension of Solvatation Mechanisms and towards Substitution with Alternative Solvents. Int. J. Mol. Sci. 2017;18:708. doi: 10.3390/ijms18040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951;193:265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- 27.Makkar H.P.S. Quantification of Tannins in Tree and Shrub Foliage. Springer; Dordrecht, The Netherlands: 2003. Measurement of Total Phenolics and Tannins Using Folin-Ciocalteu Method; pp. 49–51. [Google Scholar]
- 28.Lima A.D.J.B., Corrêa A.D., Saczk A.A., Martins M.P., Castilho R.O. Anthocyanins, Pigment Stability and Antioxidant Activity in Jabuticaba [Myrciaria Cauliflora (Mart.) O. Berg] Rev. Bras. Frutic. 2011;33:877–887. doi: 10.1590/S0100-29452011000300023. [DOI] [Google Scholar]
- 29.SW-846 Test Method 3015A: Microwave Assisted Acid Digestion of Aqueous Samples and Extracts; 2007. [(accessed on 28 July 2024)]; Available online: https://www.epa.gov/sites/default/files/2015-12/documents/3015a.pdf.
- 30.Avramia I. Cercetari Privind Extratia de B-Glucani Din Drojdia de Bere Reziduala Si Valorificarea Lor in Filme Bioactive. Performatica; Iasi, Romania: 2022. [Google Scholar]
- 31.Petravic-Tominac V., Zechner-Krpan V., Berkovi K., Galovi P., Herceg Z. Rheological Properties, Water-Holding and Oil-Binding Capacities of Particulate b-Glucans Isolated from Spent Brewer’s Yeast by Three Different Procedures. Food Technol. Biotechnol. 2011;49:56–64. [Google Scholar]
- 32.Pengkumsri N., Sivamaruthi B.S., Sirilun S., Peerajan S., Kesika P., Chaiyasut K., Chaiyasut C. Extraction of β-Glucan from Saccharomyces cerevisiae: Comparison of Different Extraction Methods and in Vivo Assessment of Immunomodulatory Effect in Mice. Food Sci. Technol. 2016;37:124–130. doi: 10.1590/1678-457x.10716. [DOI] [Google Scholar]
- 33.Meini M.-R., Cabezudo I., Boschetti C.E., Romanini D. Recovery of Phenolic Antioxidants from Syrah Grape Pomace through the Optimization of an Enzymatic Extraction Process. Food Chem. 2019;283:257–264. doi: 10.1016/j.foodchem.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 34.Kok D., Bal E. Compositional Differences in Phenolic Compounds and Anthocyanin Contents of Some Table and Wine Grape (V. vinifera L.) Varieties from Turkey. JO—Oxid. Commun. 2017;40:648. [Google Scholar]
- 35.Živković N., Čakar U., Petrović A. Effects of Spontaneous and Inoculated Fermentation on the Total Phenolic Content and Antioxidant Activity of Cabernet Sauvignon Wines and Fermented Pomace. Food Feed Res. 2024;51:119–129. doi: 10.5937/ffr0-50339. [DOI] [Google Scholar]
- 36.Castangia I., Aroffu M., Fulgheri F., Abi Rached R., Corrias F., Sarais G., Bacchetta G., Argiolas F., Pinna M.B., Murru M., et al. From Field to Waste Valorization: A Preliminary Study Exploring the Impact of the Wine Supply Chain on the Phenolic Profile of Three Sardinian Pomace Extracts. Foods. 2024;13:1414. doi: 10.3390/foods13091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherman E., Yvon M., Grab F., Zarate E., Green S., Bang K.W., Pinu F.R. Total Lipids and Fatty Acids in Major New Zealand Grape Varieties during Ripening, Prolonged Pomace Contacts and Ethanolic Extractions Mimicking Fermentation. Fermentation. 2023;9:357. doi: 10.3390/fermentation9040357. [DOI] [Google Scholar]
- 38.Buzzanca C., Mauro M., Vazzana M., Todaro A., Arizza V., Lucarini M., Durazzo A., Di Stefano V. Analysis of the Amino Acid Profile of Red and White Graphs Winery By-Products from Western Sicily. Meas. Food. 2024;14:100174. doi: 10.1016/j.meafoo.2024.100174. [DOI] [Google Scholar]
- 39.Li X., Wu J., Kang Y., Chen D., Chen G., Zeng X., Wang J. Yeast Mannoproteins Are Expected to Be a Novel Potential Functional Food for Attenuation of Obesity and Modulation of Gut Microbiota. Front. Nutr. 2022;9:1019344. doi: 10.3389/fnut.2022.1019344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Acuña-Avila P.E., Vasquez-Murrieta M.S., Cortés-Camargo S., Hernández-Botello M.T., Ramos-Monroy O., López-Cortéz M.D.S. Wine Polyphenol Content during the Fermentation of Vitis vinifera CS Grapes and Its Relationship with the Presence of Minerals in the Resulting Wine. Appl. Sci. 2023;13:8314. doi: 10.3390/app13148314. [DOI] [Google Scholar]
- 41.Biss A.J., Ellis R.H. Minerality in Wine: Textual Analysis of Chablis Premier Cru Tasting Notes. Aust. J. Grape Wine Res. 2024;2024:4299446. doi: 10.1155/2024/4299446. [DOI] [Google Scholar]
- 42.Shimizu H., Akamatsu F., Kamada A., Koyama K., Iwashita K., Goto-Yamamoto N. Variation in the Mineral Composition of Wine Produced Using Different Winemaking Techniques. J. Biosci. Bioeng. 2020;130:166–172. doi: 10.1016/j.jbiosc.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Núñez L., Serratosa M.P., Godoy A., Fariña L., Dellacassa E., Moyano L. Comparison of Physicochemical Properties, Amino Acids, Mineral Elements, Total Phenolic Compounds, and Antioxidant Capacity of Cuban Fruit and Rice Wines. Food Sci. Nutr. 2021;9:3673–3682. doi: 10.1002/fsn3.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen K., Xue H., Shi Q., Zhang F., Ma Q., Sun J., Liu Y., Tang Y., Wang W. Geographical Identification of Chinese Wine Based on Chemometrics Combined with Mineral Elements, Volatile Components and Untargeted Metabonomics. Food Chem. X. 2024;22:101412. doi: 10.1016/j.fochx.2024.101412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao F., Hao X., Zeng G., Guan L., Wu H., Zhang L., Wei R., Wang H., Li H. Identification of the Geographical Origin of Ecolly (Vitis vinifera L.) Grapes and Wines from Different Chinese Regions by ICP-MS Coupled with Chemometrics. J. Food Compos. Anal. 2022;105:104248. doi: 10.1016/j.jfca.2021.104248. [DOI] [Google Scholar]
- 46.Nardi T. Microbial Resources as a Tool for Enhancing Sustainability in Winemaking. Microorganisms. 2020;8:507. doi: 10.3390/microorganisms8040507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Husain R., Vikram N., Pandey S., Yadav G., Bose S.K., Ahamad A., Shamim M., Kumar K.G., Khan N.A., Zuhaib M., et al. Microorganisms: An Eco-Friendly Tools for the Waste Management and Environmental Safety. In: Arora S., Kumar A., Ogita S., Yau Y.-Y., editors. Biotechnological Innovations for Environmental Bioremediation. Springer Nature; Singapore: 2022. pp. 949–981. [Google Scholar]
- 48.Ayogu P., Martins V., Gerós H. Grape Berry Native Yeast Microbiota: Advancing Trends in the Development of Sustainable Vineyard Pathogen Biocontrol Strategies. OENO One. 2024;58 doi: 10.20870/oeno-one.2024.58.1.7678. [DOI] [Google Scholar]
- 49.Johnston-Monje D., Vergara L.I., Lopez-Mejia J., White J.F. Plant Microbiomes as Contributors to Agricultural Terroir. Front. Agron. 2023;5:1216520. doi: 10.3389/fagro.2023.1216520. [DOI] [Google Scholar]
- 50.Pinto C., Pinho D., Cardoso R., Custódio V., Fernandes J., Sousa S., Pinheiro M., Egas C., Gomes A.C. Wine Fermentation Microbiome: A Landscape from Different Portuguese Wine Appellations. Front. Microbiol. 2015;6:905. doi: 10.3389/fmicb.2015.00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun W., Feng S., Bi P., Han J., Li S., Liu X., Zhang Z., Long F., Guo J. Simultaneous Inoculation of Non-Saccharomyces Yeast and Lactic Acid Bacteria for Aromatic Kiwifruit Wine Production. Food Microbiol. 2024;123:104589. doi: 10.1016/j.fm.2024.104589. [DOI] [PubMed] [Google Scholar]
- 52.Bedoya K., Mas A., Rozès N., Jara C., Portillo M.D.C. The Impact of the Inoculation of Different Pied de Cuve on the Chemical and Organoleptic Profiles of Wines. Microorganisms. 2024;12:1655. doi: 10.3390/microorganisms12081655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su Y., Dong Q., Chen Y., Wang R., Jiang J., Qin Y., Song Y., Liu Y. Impact of Sequential Inoculation Timing on the Quality of Wine Fermented by Indigenous Lachancea thermotolerans and Saccharomyces cerevisiae. LWT. 2024;204:116438. doi: 10.1016/j.lwt.2024.116438. [DOI] [Google Scholar]
- 54.Zhu L.-X., Hui W., Zhang M.-M., Shi Y., Xiang X.-F., Lan Y.-B., Zhang R.-L. Aromatic and Chemical Differences between Msalais Wines Produced at Traditional Craft Workshops and Modern Plants. J. Food Compos. Anal. 2023;116:105029. doi: 10.1016/j.jfca.2022.105029. [DOI] [Google Scholar]
- 55.Choudhary S., Garg V.K. Contribution of Microbial Mechanism and Artificial Intelligence in Wine Production; Proceedings of the 2024 11th International Conference on Computing for Sustainable Global Development (INDIACom); New Delhi, India. 28 February–1 March 2024; Piscataway, NJ, USA: IEEE; 2024. pp. 247–251. [Google Scholar]
- 56.Torres-Guardado R., Esteve-Zarzoso B., Reguant C., Bordons A. Microbial Interactions in Alcoholic Beverages. Int. Microbiol. 2022;25:1–15. doi: 10.1007/s10123-021-00200-1. [DOI] [PubMed] [Google Scholar]
- 57.L. Binati R., Ferremi Leali N., Avesani M., Salvetti E., Felis G.E., Monti F., Torriani S. Application of FTIR Microspectroscopy in Oenology: Shedding Light on Cell Wall Composition of Saccharomyces cerevisiae Strains. Food Bioprocess Technol. 2024;17:1596–1609. doi: 10.1007/s11947-023-03218-7. [DOI] [Google Scholar]
- 58.Novák M., Synytsya A., Gedeon O., Slepička P., Procházka V., Synytsya A., Blahovec J., Hejlová A., Čopíková J. Yeast β(1-3),(1-6)-d-Glucan Films: Preparation and Characterization of Some Structural and Physical Properties. Carbohydr. Polym. 2012;87:2496–2504. doi: 10.1016/j.carbpol.2011.11.031. [DOI] [Google Scholar]
- 59.Nie M., Luo J., Xiao M., Chen J., Bao K., Zhang W., Chen J., Li B. Structural Differences between Fusarium Strains Investigated by FT-IR Spectroscopy. Biochemistry. 2007;72:61–67. doi: 10.1134/S0006297907010075. [DOI] [PubMed] [Google Scholar]
- 60.Chang J., Li W., Liu Q., Zhou Y., Chen X., Lyu Q., Liu G. Preparation, Properties, and Structural Characterization of β-Glucan/Pullulan Blend Films. Int. J. Biol. Macromol. 2019;140:1269–1276. doi: 10.1016/j.ijbiomac.2019.08.208. [DOI] [PubMed] [Google Scholar]
- 61.Mikkelsen M.S., Jespersen B.M., Møller B.L., Lærke H.N., Larsen F.H., Engelsen S.B. Comparative Spectroscopic and Rheological Studies on Crude and Purified Soluble Barley and Oat β-Glucan Preparations. Food Res. Int. 2010;43:2417–2424. doi: 10.1016/j.foodres.2010.09.016. [DOI] [Google Scholar]
- 62.Badshah S.L., Riaz A., Muhammad A., Tel Çayan G., Çayan F., Emin Duru M., Ahmad N., Emwas A.-H., Jaremko M. Isolation, Characterization, and Medicinal Potential of Polysaccharides of Morchella Esculenta. Molecules. 2021;26:1459. doi: 10.3390/molecules26051459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sousa P., Tavares-Valente D., Pereira C.F., Pinto-Ribeiro I., Azevedo-Silva J., Madureira R., Ramos Ó.L., Pintado M., Fernandes J., Amorim M. Circular Economyeast: Saccharomyces cerevisiae as a Sustainable Source of Glucans and Its Safety for Skincare Application. Int. J. Biol. Macromol. 2024;265:130933. doi: 10.1016/j.ijbiomac.2024.130933. [DOI] [PubMed] [Google Scholar]
- 64.Barrientos R.C., Clerigo M.M., Paano A.M.C. Extraction, Isolation and MALDI-QTOF MS/MS Analysis of β- d -Glucan from the Fruiting Bodies of Daedalea Quercina. Int. J. Biol. Macromol. 2016;93:226–234. doi: 10.1016/j.ijbiomac.2016.08.044. [DOI] [PubMed] [Google Scholar]
- 65.Hromádková Z., Ebringerová A., Sasinková V., Šandula J., Hříbalová V., Omelková J. Influence of the Drying Method on the Physical Properties and Immunomodulatory Activity of the Particulate (1→3)-β-d-Glucan from Saccharomyces cerevisiae. Carbohydr. Polym. 2003;51:9–15. doi: 10.1016/S0144-8617(02)00110-8. [DOI] [Google Scholar]
- 66.Alzorqi I., Sudheer S., Lu T.-J., Manickam S. Ultrasonically Extracted β-d-Glucan from Artificially Cultivated Mushroom, Characteristic Properties and Antioxidant Activity. Ultrason. Sonochem. 2017;35:531–540. doi: 10.1016/j.ultsonch.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 67.Hong T., Yin J.-Y., Nie S.-P., Xie M.-Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. X. 2021;12:100168. doi: 10.1016/j.fochx.2021.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pinto M., Coelho E., Nunes A., Brandão T., Coimbra M.A. Valuation of Brewers Spent Yeast Polysaccharides: A Structural Characterization Approach. Carbohydr. Polym. 2015;116:215–222. doi: 10.1016/j.carbpol.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 69.Lante A., Canazza E., Tessari P. Beta-Glucans of Cereals: Functional and Technological Properties. Nutrients. 2023;15:2124. doi: 10.3390/nu15092124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.