Abstract
Background:
Fine-needle aspiration cytology (FNAC) of the lymph nodes is the first-line evaluation of lymphadenopathy of unknown etiology. For better diagnostic clarity and proper communication to clinicians, the Sydney System was proposed in 2020 for the performance, classification, and reporting of lymph node cytopathology. The present study was conducted to analyze the diagnostic performance and risk of malignancy (ROM) associated with each of the diagnostic categories of the proposed Sydney System.
Materials and Methods:
This retrospective study was conducted over 2 years. All patients with lymphadenopathy undergoing FNAC during the study period for which subsequent histopathological examination (HPE) reports or clinical follow-up data were available were included in the study. The original diagnoses were reviewed, and each case was assessed according to the first diagnostic level of the Sydney System classification. The diagnostic accuracy and ROM were correlated with FNAC diagnoses. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy of lymph node FNAC were calculated using SPSS version 20.0.
Results:
A total of 456 out of 753 cases were selected in the study as they had subsequent histopathological correlation and/or clinical follow-up data. The majority were females n = 294 (64.4%). The most common lymph node was the cervical group (n = 274, 60%).
On Statistical Analysis:
sensitivity 82.8%, specificity 97.5%, PPV 95.3%, NPV 90.1%, and diagnostic accuracy 92%.
Conclusion:
The sydney system, which introduces a uniform categorization, may increase the lymph node FNAC diagnostic accuracy.
Keywords: FNAC, lymph node, Sydney system
INTRODUCTION
Fine-needle aspiration cytology (FNAC) of the lymph nodes is a safe, quick, cost-effective, reliable, and minimally invasive tool in the first-line evaluation of lymphadenopathy of unknown etiology.[1] It has been widely used in the diagnosis and staging of non-lymphoid malignancies, diagnosis of lymphoma, and evaluation of causes for reactive and benign lymphadenopathy.[2] Moreover, lymph node FNAC is excellent in the diagnosis of metastatic malignancy, reducing the need for diagnostic excision biopsy in many patients.[3] It facilitates a wide range of ancillary tests, including microbiological cultures, cell-block immunocytochemistry (ICC), flow cytometric (FC) immunophenotyping, real-time polymerase chain reaction, fluorescence in situ hybridization, and targeted next-generation sequencing, in addition to providing materials for cytomorphologic evaluation.[4] However, lymph node FNAC poses a difficult situation. A cytopathologist’s understanding of the clinical history, physical examination, and radiological features, as well as the use of a standardized categorization and communication to clinicians, is necessary for better outcomes of the patients with lymphadenopathy.[5,6] In order to meet this requirement, an expert panel proposed the Sydney System for the performance, classification, and reporting of lymph node cytopathology in 2020. This system uses five diagnostic categories, and it also includes a second diagnostic level that is intended to identify specific diagnostic entities.[7] The reporting system includes two diagnostic levels. The first should provide basic diagnostic information and includes five categories: inadequate/insufficient, benign, atypical lymphoid cells of undetermined/uncertain significance, suspicious, and malignant. For each category, specific recommendations are provided. The second diagnostic level, when achievable, identifies specific benign or malignant entities and additional information by utilizing ancillary testing.[7] The Sydney System, however, is still underutilized, and there are very few literature works available currently. In order to close this knowledge gap, the current study was undertaken to evaluate the Sydney System’s applicability to lymph node FNAC, as well as the diagnostic accuracy and risk of malignancy (ROM) for each diagnostic category.
Aim of the study
The present study was conducted to analyze the diagnostic performance and assess the risk of malignancy associated with each of the diagnostic categories of the proposed Sydney System.
MATERIALS AND METHODS
The study was conducted in the Department of Pathology, Assam Medical College and Hospital, Dibrugarh. It was a retrospective hospital-based study conducted over a period of 2 years, from August 2021 to July 2023. The study was approved by the Institutional Ethics Committee (H), Assam Medical College.
Inclusion criteria
All patients with lymphadenopathy undergoing FNAC during the study period for which subsequent histopathological examination (HPE) reports or clinical follow-up data were available were included in the study.
Exclusion criteria
Cases without corresponding histopathological correlation and those who were lost to follow-up for subsequent clinical data were excluded from the study.
The original diagnoses were reviewed, and each case was assessed according to the first diagnostic level of the Sydney System classification (L1-inadequate/non-diagnostic; L2-benign; L3-atypical cells of undetermined significance/atypical lymphoid cells of uncertain significance (AUS/ALUS); L4-suspicious; L5- malignant). The diagnostic accuracy and the ROM for each diagnostic category were assessed by correlating histopathologic diagnoses with FNAC diagnoses. Clinical follow-up was taken into account when no HPE reports were available. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall diagnostic accuracy of lymph node FNAC were calculated using SPSS Windows, version 20.0. ROM was calculated by dividing the number of cases with confirmed malignant lesions by the total number of cases with a histological or clinical follow-up within each diagnostic category.
RESULTS
Out of 753 cases, a total of 456 cases were enrolled in the study as they had subsequent histopathological correlation and/or clinical follow-up data. FNACs were performed on patients of all age groups, ranging from 2 to 77 years. It was observed that the majority (31.1%) of the study population belonged to the age group of 21–30 years. The major study population comprised females n = 294 (64.4%), with n = 162 (35.6%) being males. The lymph node locations included cervical group (n = 274, 60%), axillary (n = 56, 12.2), submandibular (n = 51, 11.1%), inguinal (n = 20, 4.4%), supraclavicular (n = 41, 9.0%), and sub-mental (n = 14, 3%).
Diagnostic categories
In the present study, n = 30 (7%) were re-categorized as L1 (inadequate/non-diagnostic); n = 274 (60%) as L2 (benign); n = 15 (3%) as L3 (AUS/ALUS); n = 32 (7%) as L4 (suspicious), and n = 105 (23%) as L5 (malignant) [Table 1].
Table 1.
Distribution of cases as per the Sydney System
Histopathological correlation and/or clinical follow-up
The correlation between the categorization of cytological smears and final diagnosis based on HPE and/or clinical follow-up data is shown in Table 2.
Table 2.
Correlation of the cases categorized under each category with final diagnosis based on the histopathological correlation/clinical follow-up
| Sydney system categories | Final diagnosis based on the histopathological correlation/clinical follow-up | |
|---|---|---|
| Non – malignant | Malignant | |
| L1 (n = 30) |
N = 12 tubercular lymphadenitis – 8 Reactive lymphadenitis – 4 |
N = 18 metastatic carcinoma – 18 • SCC – 10 • Invasive breast carcinoma – 8 |
| L2 (n = 274) |
N = 262 tubercular lymphadenitis – 187 Reactive lymphadenitis – 49 Granulomatous lymphadenitis – 21 Suppurative lymphadenitis – 3 Sinus histiocytosis – 2 |
N = 12 metastatic carcinoma – 10 • SCC – 6 • Invasive breast carcinoma- 4 Hodgkin’s lymphoma –2 |
| L3 (n = 15) | N = 4 reactive lymphadenitis – 4 |
N = 11 metastatic carcinoma – 9 • SCC – 7 • Poorly differentiated carcinoma – 2 NHL – 2 |
| L4 (n = 32) | N = 3 reactive lymphadenitis – 3 |
N = 29 Non-Hodgkin’s lymphoma – 16 Hodgkin’s lymphoma – 13 |
| L5 (n = 105) | N = 0 |
N = 105 metastatic carcinoma – 60 • SCC – 34 • Invasive breast carcinoma – 25 • Secretory carcinoma of the salivary gland – 1 Non-Hodgkin’s lymphoma – 24 Hodgkin’s lymphoma – 19 Malignant melanoma – 2 |
Statistical analysis
When suspicious (L4) and malignant (L5) cases were considered positive, the statistical analysis showed the following results: sensitivity 76.5%, specificity 98.9%, PPV 97.8%, NPV 87.1%, and diagnostic accuracy 90.3%. When atypical lymphoid cells of undetermined/uncertain significance (L3), suspicious (L4), and malignant (L5) cases were considered positive, the statistical analysis showed the following results: sensitivity 82.8%, specificity 97.5%, PPV 95.3%, NPV 90.1%, and diagnostic accuracy 92% [Tables 4 and 5]. The ROM was calculated for each diagnostic category, when the histopathologic correlation or clinical follow-up was available. Categories L4 and L5 had higher ROM values of 90.6% and 100%, respectively; the lower value of ROM (4.3%) was observed in category L2. Intermediate ROM values were associated with categories L1 (60%) and L3 (73.3%) [Table 3].
Table 4.
Calculation of sensitivity, specificity, PPV, NPV, and diagnostic accuracy (considering L4 and L5 cases to be true positive)
| Indicators | Percentage |
|---|---|
| Sensitivity = TP/(TP + FN) | 134/(134 + 41) = 76.5% |
| Specificity = TN/(TN + FP) | 278/(278 + 3) = 98.9% |
| PPV = TP/(TP + FP) | 134/(134 + 3) = 97.8% |
| NPV = TN/(TN + FN) | 278/(278 + 41) = 87.1% |
| Diagnostic accuracy = (TP + TN)/(TP + TN + FP + FN) | (134 + 278)/(134 + 278 + 3 + 41) = 90.3% |
Table 5.
Calculation of sensitivity, specificity, PPV, NPV, and diagnostic accuracy (considering L3, L4, and L5 cases to be true positive)
| Indicators | Percentage |
|---|---|
| Sensitivity = TP/(TP + FN) | 145/(145 + 30) = 82.8% |
| Specificity = TN/(TN + FP) | 274/(274 + 7) = 97.5% |
| PPV = TP/(TP + FP) | 145/(145 + 7) = 95.3% |
| NPV = TN/(TN + FN) | 274/(274 + 30) = 90.1% |
| Diagnostic accuracy = (TP + TN)/(TP + TN + FP + FN) | (145 + 274)/(145 + 274 + 7 + 30) = 92% |
Table 3.
Stratification of ROM in the Sydney system diagnostic categories
| Sydney system categories | Histological or clinical follow-up | Confirmed malignant cases | ROM (%) |
|---|---|---|---|
| L1 | 30 | 18 | 60 |
| L2 | 274 | 12 | 4.3 |
| L3 | 15 | 11 | 73.3 |
| L4 | 32 | 29 | 90.6 |
| L5 | 105 | 105 | 100 |
DISCUSSION
Cytological evaluation of lymphadenopathies can be very difficult; however, a growing body of data demonstrates that the correct handling of the diagnostic material to perform ancillary techniques, combined with clinical data, ensures satisfactory diagnostic accuracy. However, due to lack of standardized reporting systems, the use of lymph node FNAC is still not universally embraced by clinicians. As observed in other areas of cytopathology, the use of standardized reporting methods allows for the communication of clinically pertinent information in a consistent manner, while limiting interobserver variability.[5,6] Additionally, by employing management suggestions tailored to each diagnostic category, the rate of clinician misinterpretation of cytological results may be decreased. To do this, risk stratification and the identification of ROM values that are shared by various entities are essential.
In the present study, n = 30 (7%) were categorized as L1 (inadequate/non-diagnostic); n = 274 (60%) as L2 (benign); n = 15 (3%) as L3 (AUS/ALUS); n = 32 (7%) as L4 (suspicious); and n = 105 (23%) as L5 (malignant) [Figures 1 and 2], and we showed the ability of the Sydney System to stratify lymph node FNACs into categories with increasing ROMs. Interestingly, the ROM of L1 category was remarkably high (60%) owing to their deep-seated locations, and in maximum cases, only the necrotic material was aspirated. Also, FNACs were performed in four patients on subcentrimetric lymph nodes, and the material aspirated was less in amount. This is more than the 50% found by Vigliar et al.,[8] and much more to 27.5% found by Gupta et al.[4] and comparable to 66.7% found by Caputo et al.[1]
Figure 1.
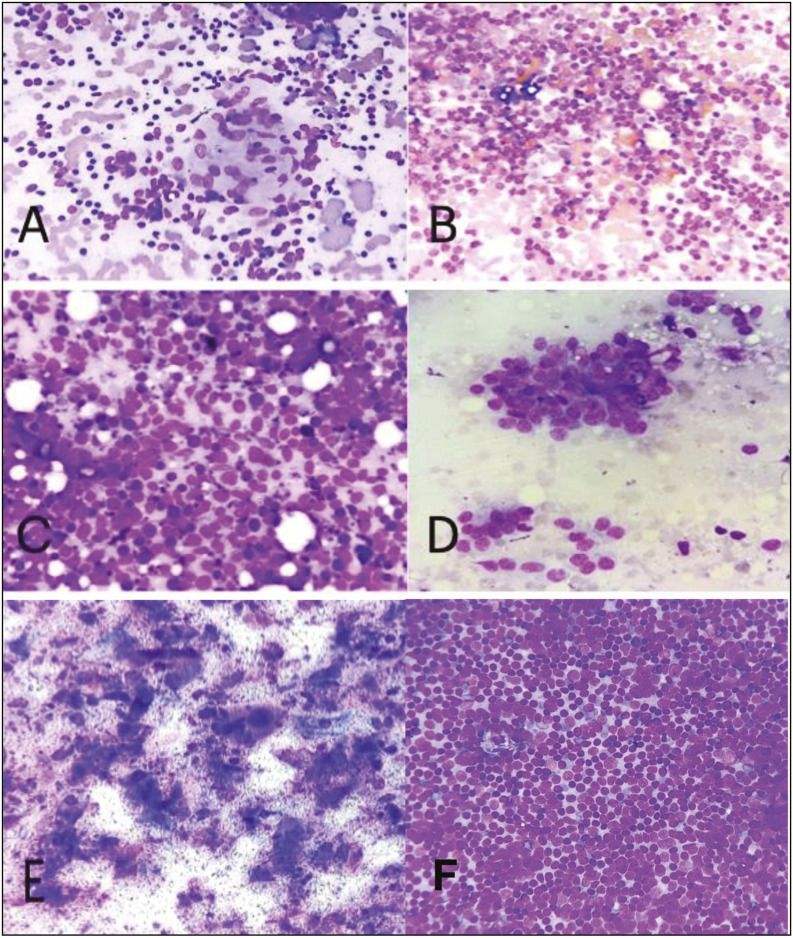
A [L2 category] Granulomatous lymphadenitis. B [L2 category] Reactive lymphadenitis. C [L3 Category] heterogenous population of lymphoid cells suggesting a reactive picture along with presence of atypical large cells. D [L4 Category] suspicious of lymphoma. E [L5 Category] Metastatic Squamous Cell Carcinoma. F [L5 Category] Non Hodgkin Lymphoma (Small Lymphocytic Lymphoma) (MGG stain, 40x)
Figure 2.
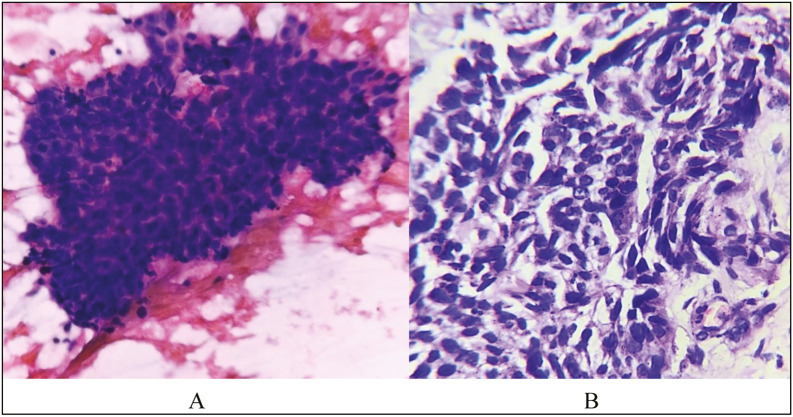
Microphotograph showing cells arranged in irregular solid cohesive fragments. Individual cells have high N:C ratio, elongated nuclei, coarse chromatin and scant cytoplasm in a background of lymphoglandular bodies (PAP, 40x) – Metastatic Squamous Cell Carcinoma (FNAC). Metastatic Squamous Cell Carcinoma on HPE (H & E stain, 40x)
As expected, the L2 category showed the lowest ROM (4.3%). This is slightly higher than that found by Vigliar et al.[8] (1.92%) but lower than that found by Gupta et al.[4] (11.5%) and Caputo et al.[1] (9.38%). Out of the 12 malignant cases included in this category, 10 cases were of metastatic carcinomatous deposits in lymph nodes. This similar problem was faced by Vigliar et al.[8] and Garg et al.[9] in their respective studies, where partial involvement of the lymph nodes by metastatic carcinoma did not yield representative malignant cells on cytologic analysis. On histopathological follow-up, two cases of Hodgkin’s lymphoma placed under this category were found to be of the lymphocyte predominant type, and so predominantly small lymphocytes appeared on the FNAC smears, leading to misclassification.
The L3 category showed an intermediate ROM (73.3%). The four cases diagnosed as benign on histological follow-up in the L3 category were diagnosed as reactive lymphadenitis. Due to inter-follicular expansion, the FNAC smears showed large cells with irregular nuclei, prominent nucleoli, and scant cytoplasm, leading to their placement in this category. Vigliar et al.[8] similarly encountered the highest number of conflicting cases in this category due to large cells from inter-follicular expansion of reactive lymph nodes being represented on cytology slides. Other studies also experienced similar pitfalls in cytology smears of reactive lymph nodes due to inter-follicular expansion and viral etiologies.[10,11]
L4 and L5 categories had a very high ROM of 90.6% and 100%, respectively. The ROM calculated for these categories by Gupta et al.[4] was 88% and 99.6%, respectively. Vigliar et al.[8] calculated it to be 100% for both the categories, which is owing to the extensive use of ancillary procedures and flow cytometry. The benign cases categorized under the L4 category showed cytomorphological features of parafollicular hyperplasia containing large immunoblast-like cells, histiocytes along with numerous tingible body macrophages, which on FNAC smears resembled Reed–Sternberg (RS) like cells. Immunoblasts are often confused with RS cells on cytologic analysis, which is a commonly reported finding.[12,13]
The maximum number of cases were categorized under the benign category (60%), and only 23% of cases were categorized as malignant. Studies conducted by Vigliar et al.,[8] Caputo et al.,[1] and Gupta et al.[4] had more cases categorized under the malignant category.
Similar studies
In the study done by Vigliar et al.,[8] out of 300 samples, n = 20 cases (6.7%) were categorized as L1-inadequate/non-diagnostic; n = 104 (34.7%) as benign (L2); n = 25 (8.3%) as atypical (L3); n = 13 (4.3%) as suspicious (L4), and n = 138 (46%) as malignant (L5). Statistical analysis showed the following results: sensitivity 98.47%, specificity 95.33%, PPV 96.27%, NPV 98.08%, and accuracy 97.06%. The ROM was 50% for the category L1, 1.92% for L2, 58.3% for L3, and 100% for L4 and L5.
Caputo et al.,[1] in their study of 1458 LN-FNACs, reformulated the diagnoses according to the Sydney System and compared them to the histological control where available (n = 551, 37.8%). The risk of malignancy for each of the five categories was 66.7% for inadequate/insufficient, 9.38% for benign, 28.6% for atypical, 100% for suspicious, and 99.8% for malignant. LN-FNAC showed a sensitivity of 97.94%, a specificity of 96.92%, a positive predictive value of 99.58%, and a negative predictive value of 86.30%.
Gupta et al.[4] reported that among 6983 lymph node FNAs, 289 (4.1%) cases were reported as non-diagnostic/ inadequate (L1); 3397 (48.6%) were reported as benign (L2); 33(0.5%) as atypical cells of undetermined significance (L3), 96 (1.4%) as suspicious for malignancy (L4), and 3168 (45.4%) as malignant (L5). The overall sensitivity, specificity, positive and negative predictive values, and diagnostic accuracy of LN-FNA were 79.9%, 98.7%, 98.4%, 83.1%, and 89.3%, respectively. The risk of malignancy was 27.5% for the non-diagnostic category, 11.5% for the benign, 66.7% for the atypical, 88% for the suspicious, and 99.6% for the malignant categories.
Novelty of the present study
Due to the low availability of resources during the study period, the present study was carried out using the 1st level of Sydney System, and ancillary techniques were used only in a few cases, whereas similar studies done by Vigliar et al.[8] and Gupta et al.[4] focused on both the diagnostic levels.
In our study, the maximum number of cases were categorized under the benign category (60%), and only 23% of cases were categorized as malignant. Studies conducted by Vigliar et al.,[8] Caputo et al.,[1] and Gupta et al.[4] had a greater number of cases categorized under the malignant category.
In the present study, the sensitivity, specificity, PPV, NPV, and diagnostic accuracy were found to be 82.8%, 97.5%, 95.3%, 90.1%, and 92%, respectively, whereas statistical analysis showed the following results: sensitivity 98.47%, specificity 95.33%, PPV 96.27%, NPV 98.08%, and accuracy 97.06% in the study by Vigliar et al.[8] The overall sensitivity, specificity, positive and negative predictive values, and diagnostic accuracy of LN-FNA were 79.9%, 98.7%, 98.4%, 83.1%, and 89.3%, respectively, in the study done by Gupta et al.[4]
The Sydney System also proposes a second diagnostic level wherever feasible, aiming on the identification of specific benign or malignant entities. In cases of malignancy, ancillary techniques such as FC and ICC in combination with cytological features are essential to provide a second diagnostic level.[8] In our study due to the low availability of resources during the study period, we mainly focused on the first diagnostic level of the Sydney System, and ancillary techniques were used only in a few cases. However, we plan to carry forward the study implementing the 2nd diagnostic level of the Sydney System in the near future.
CONCLUSION
The Sydney System, which introduces a uniform categorization, may increase the lymph node FNAC diagnostic accuracy. Additionally, clinicians would benefit from management suggestions tailored to each diagnostic category with increasing ROMs, improving patient outcomes. The limitations of our study were the single-institutional concept, retrospective nature, and limited use of ancillary techniques.
Conflicts of interest
There are no conflicts of interest.
Funding Statement
Nil
REFERENCES
- 1.Caputo A, Ciliberti V, D’Antonio A, D’Ardia A, Fumo R, Giudice V, et al. Real-world experience with the Sydney System on 1458 cases of lymph node fine needle aspiration cytology. Cytopathology. 2022;33:166–75. doi: 10.1111/cyt.13079. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Li F, Meng L, Hao F, Liu X, Zhao C, et al. Fine needle aspiration cytology for lymph nodes: A three-year study. Br J Biomed Sci. 2016;73:28–31. doi: 10.1080/09674845.2016.1144947. [DOI] [PubMed] [Google Scholar]
- 3.Attard J, Galea J, Betts A. The efficay of lymph node fine needle aspiration cytology. Malta Med J. 2015;27:16–21. [Google Scholar]
- 4.Gupta P, Gupta N, Kumar P, Bhardwaj S, Srinivasan R, Dey P, et al. Assessment of risk of malignancy by application of the proposed Sydney system for classification and reporting lymph node cytopathology. Cancer Cytopathol. 2021;129:701–18. doi: 10.1002/cncy.22432. [DOI] [PubMed] [Google Scholar]
- 5.Sundling KE, Kurtycz DFI. Standardized terminology systems in cytopathology. Diagn Cytopathol. 2019;47:53–63. doi: 10.1002/dc.24103. [DOI] [PubMed] [Google Scholar]
- 6.Pitman MB, Black-Schaffer WS. Post-fine-needle aspiration biopsy communication and the integrated and standardized cytopathology report. Cancer Cytopathol. 2017;125:486–93. doi: 10.1002/cncy.21821. [DOI] [PubMed] [Google Scholar]
- 7.Al-Abbadi MA, Barroca H, Bode-Lesniewska B, Calaminici M, Caraway NP, Chhieng DF, et al. A proposal for the sperformance, classification, and reporting of lymph node fine-needle aspiration cytopathology: The Sydney system. Acta Cytol. 2020;64:306–22. doi: 10.1159/000506497. [DOI] [PubMed] [Google Scholar]
- 8.Vigliar E, Acanfora G, Iaccarino A, Mascolo M, Russo D, Scalia G, et al. A novel approach to classification and reporting of lymph node fine-needle cytology: Application of the proposed Sydney system. Diagnostics (Basel) 2021;11:1314. doi: 10.3390/diagnostics11081314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg S, Rohilla M, Srinivasan R, Bal A, Das A, Dey P, et al. Fine-needle aspiration diagnosis of lymphoma based on cytomorphology alone: How accurate is it? - A Cyto-Histopathology correlative study. J Cytol. 2021;38:164–70. doi: 10.4103/JOC.JOC_217_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendon ME. Fine needle aspiration cytology of lymph nodes. Prog Diagn Cytol. 1999;32:453–6. [Google Scholar]
- 11.Nasuti JF, Yu G, Boudousquie A, Gupta P. Diagnostic value of lymph node fine needle aspiration cytology: An institutional experience of 387 cases observed over a 5-year period. Cytopathology. 2000;11:18–31. doi: 10.1046/j.1365-2303.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Yu X, Zheng Y, Yang Y, Xie J, Zhou X. Value of fine needle aspiration cell blocks in the diagnosis and classification of lymphoma. Int J Clin Exp Pathol. 2014;7:7717–25. [PMC free article] [PubMed] [Google Scholar]
- 13.Malakar D, Swarup K. A clinical evaluation of fine needle aspiration of cytology in the diagnosis of lymphadenopathy. Ind J Tuberc. 1991;38:8–17. [Google Scholar]