Abstract
Background/Objectives: Gastric cancer is one of the leading malignancies worldwide. B vitamins play important roles in DNA synthesis and methylation because they are considered co-enzymes in one-carbon metabolism. There is inconclusive evidence regarding the associations between dietary vitamins B1, B2, and B6 with the risk of gastric cancer in different epidemiologic studies. We, therefore, investigated such associations in a hospital-based case-control study comprising 1182 incident cases of gastric cancer and 2995 controls in Vietnam. Methods: Dietary vitamins B1, B2, and B6 were derived from a semi-quantitative validated food frequency questionnaire. An unconditional logistic regression model was used to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for the risk of gastric cancer in relation to dietary intake of vitamins B1, B2, and B6. Results: Overall, dietary vitamins B1 (ORper-SD increment = 0.83; 95% CI: 0.78–0.89; Ptrend < 0.001) and B6 (ORper-SD increment = 0.88; 95% CI: 0.81–0.94; Ptrend < 0.001) were associated with a reduced risk of gastric cancer. Compared with the lowest quintile, the ORs (95% CIs) of gastric cancer for quintiles 2, 3, 4, and 5 of the vitamin B1 intake were 0.64 (0.51–0.79), 0.54 (0.43–0.69), 0.57 (0.44–0.74), and 0.42 (0.31–0.55), respectively; for vitamin B6 intake, quintiles 2, 3, 4, and 5 were 0.53 (0.42–0.66), 0.54 (0.42–0.70), 0.61 (0.46–0.81), and 0.46 (0.33–0.63), respectively. This inverse association was not different across sex, BMI, and smoking statuses. No association was found between dietary vitamin B2 and gastric cancer risk. Conclusions: Dietary vitamins B1 and B6 were associated with a reduced risk of gastric cancer in the Vietnamese population. Future studies are warranted to replicate our findings, which also have great implications for gastric cancer prevention and control programs in low- and middle-income countries.
Keywords: vitamins B1, vitamin B2, vitamin B6, dietary intake, gastric cancer, risk factor, case-control study, Vietnam
1. Introduction
Gastric cancer is considered one of the leading malignancies worldwide, with an annual 1,100,000 new cases and 770,000 deaths [1]. While gastric cancer makes up a small proportion of total cancer diagnoses in North American or European countries, it is more common in Asian countries [2,3]. More than two-thirds of total gastric cancer cases in 2020 occurred in Eastern and South-Eastern Asia countries [1]. The incidence rate of gastric cancer is highest in Eastern Asia (32.5/100,000 men and 13.2/100,000 women). In South-Eastern Asia, which Vietnam is part of, the incidence rate of gastric cancer is 7.3/100,000 men and 4.0/100,000 women. In Vietnam, gastric cancer is one of the leading cancers, ranking fourth in incidence and third in mortality [4]. Patients with an advanced stage have a poor prognosis, with a 5-year survival rate of 4.7%, despite many advancements in early detection and treatment regimens [5]. Established factors, either protective or risk, in relation to gastric cancer include non-modifiable factors (i.e., age, sex, and genetics) and modifiable factors (i.e., smoking, alcohol consumption, H. pylori infection status, and diet) [5,6].
One factor that is considerably different from one population to another is diet, and prior studies suggest that, while the dietary intake of vegetables or fruits or vitamin C that provides anti-oxidant nutrients, as well as selected carotenoids, may be associated with a reduced risk of gastric cancer, other foods such as salt or salt-preserved foods might be associated with an increased risk of gastric cancer [5,6,7,8]. B vitamins, including thiamine (or vitamin B1), riboflavin (or vitamin B2), pyridoxal phosphate (PLP or vitamin B6), folate (or vitamin B9), and vitamin B12, play an important role in DNA synthesis and methylation because they are considered co-enzymes in one-carbon metabolism [9]. A deficiency or imbalance of B vitamins may cause the disruption of DNA synthesis or methylation, resulting in the interference or aberration of DNA methylation, repair, and replication, each of which could contribute to carcinogenesis [10]. Such B vitamins are water-soluble, necessary, and sourced via dietary intakes.
Prior studies on the associations between dietary vitamins B1, B2, and B6 and the risk of gastric cancer across existing epidemiologic studies have provided conflicting results. Accordingly, all three prospective cohort studies, one from Australia (the Melbourne Collaborative Cohort Study, consisting of 41,513 study participants) [11], one from the U.S. (the NIH-AARP Cohort Study, consisting of 492,293 study participants) [12], and one from China (the Shanghai Women’s Health Study, consisting of 73,009 Chinese women) [13] reported null associations between such vitamins (i.e., B1, B2, and B6) and gastric cancer risk. However, several case-control studies found a protective effect against gastric cancer with vitamin B intake. For instance, in a case-control study comprising 687 patients with gastric cancer and 1595 controls from several U.S. states (i.e., New Jersey, Connecticut, and Washington), Mayne et al. [14] reported that dietary vitamin B6 was associated with a reduced risk of gastric cancer (OR = 0.59; 95% CI: 0.45–0.79). In another case-control study in Belgium, including 301 patients with gastric cancer and 2851 controls, Kaaks et al. [15] found both vitamins B1 and B6 to be associated with a decreased risk of gastric cancer, whereas a higher level of vitamin B2 was associated with an increased risk of gastric cancer. In a case-control study nested in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort Study, consisting of 235 patients and 601 controls, inverse associations were found between vitamin B2 (OR = 0.85; 95% CI: 0.72–1.01) and B6 (OR = 0.78; 95% CI: 0.65–0.98) intake and gastric cancer [16]. However, in a case-control study in Italy, including 200 patients with gastric cancer and 547 controls, null associations were shown between these three vitamins (i.e., B1, B2, and B6) and the risk of gastric cancer [17]. To our knowledge, there are no studies examining the relationship between these vitamins and the risk of gastric cancer. Because dietary composition is different across populations and races/ethnicities, it is important to have a better understanding of the effects of these vitamins on gastric cancer in diverse populations and/or racial/ethnic backgrounds.
Therefore, we conducted an analysis to determine the association between vitamins B1, B2, and B6 and the risk of gastric cancer in a case-control study that included 1182 patients with gastric cancer and 2995 controls in Hanoi, Vietnam.
2. Materials and Methods
2.1. Study Population
For the current analysis, we used data generated from a hospital-based case-control study in Vietnam. The methods, study design, and initial results of this study were described previously [18,19,20]. In brief terms, eligible study participants for this study were recruited during 2003–2019 period from four leading hospitals in Hanoi, Vietnam, including Bach Mai Hospital, Viet Duc University Hospital, National Cancer Hospital, and Hanoi Medical University Hospital. The enrollment of study participants in the present study was conducted during four durations, including (1) 2003–2006 (n = 625 study participants), (2) 2006–2007 (n = 1342 study participants), (3) 2008 (n = 407 study participants), and (4) 2018–2019 (n = 4902 study participants). Long enrollment in this study was due to resource allocation. All study participants agreed to provide written informed consent before participating in our study. The present study was approved by the participating Institutional Review Boards (IRBs) of Hanoi Medical University (#3918/HMUIRB) and the International University of Health and Welfare, Japan (#19-Ig-17).
2.2. Recruitment of Patients with Gastric Cancer
The details of our recruitment of patients with gastric cancer have been published [18,19,20]. Briefly, gastric cancer patients were enrolled a few days or a week before surgery. Potential gastric cancer cases were identified by reviewing the list of patients who were scheduled to undergo surgery and who met the inclusion criteria for our study, including (1) those who were able to undergo surgery physically, (2) those who were able to provide information via a research questionnaire, including exposure information, (3) those who were confirmed by pathologic diagnosis to have gastric cancer, and (4) those who consented/agreed to participate in the study. The exclusion criteria for the present study were (1) those who refused to participate in the study, (2) those who were unable to provide exposure data, and (3) those who had changed their diet during their illness.
2.3. Recruitment of Controls
Controls recruited in the present study were those who received different surgeries at the same hospital and at the same time of recruitment as patients with gastric cancer and who did not have a cancer diagnosis or history of any cancer. We recruited them if they met the following criteria: (1) individuals who were cancer-free at the time of enrollment and without a history of cancer; (2) individuals who were able to provide exposure and related information; and (3) individuals who agreed to participate in the study. We excluded individuals if they met one of the following exclusion criteria: (1) those who refuse to participate in the study and (2) those who changed their diets due to health conditions or illness [18,19,21].
The reason for selecting cases and controls before surgery was that they had newly diagnosed disease outcomes and had not changed their diet habits or lifestyles yet. The number of gastric cancer cases (n = 1182) was not similar to the number of controls (n = 2995) in the present study because (1) more cancer patients were admitted than non-cancer patients and because of (2) prior matching by sex and age [18,19,21].
2.4. Information from Structured Questionnaire
On the day before participating patients’ surgery dates, a trained interviewer used a structured questionnaire to obtain the following information from study participants: (1) sociodemographic factors, (2) body weight and height, (3) lifetime tobacco and alcohol use, (4) occupational exposure, (5) family history of cancer, (6) medical history, and (7) dietary information (see the dietary assessment below). Information from medical records was extracted by trained extractors, including infection status for hepatitis B, hepatitis C, or HIV viruses and/or H. pylori (if any).
2.5. Dietary Assessment
Dietary information from study participants was obtained using a semi-quantitative food frequency questionnaire (FFQ), which consisted of 85 food items commonly consumed by Vietnamese people and contributed up to 90% or higher essential nutrition. To develop the FFQ for the present study, we conducted household surveys with a general population using 24 h food records, one in 2009 and the other in 2017. In the FFQ, study participants were asked how frequently they had consumed food and food groups during the past 12 months. Six groups of frequent answers were listed as “6–11 times/year”, “1–3 times/month”, “1–2 times/week”, “3–4 times/week”, “5–6 times/week”, and “1–3 times/day”. Following such questions, study participants were also asked about portion sizes by indicating the amount of food consumed. Portion sizes were divided into three categories (a) small, (b) medium, and (c) large. We used the Vietnamese Food Composition Database to calculate the average daily intakes of 95 nutrients and compounds, including B vitamins [22]. The FFQ was validated during the October–November 2017 period by conducting a validation study in 1327 participants, using two 24 h dietary recalls (24-HDRs). This validation study was conducted once every weekday and once every three consecutive days. The Pearson correlation coefficients (R2) between the FFQ and 24-HDR were between 0.38 (protein) and 0.53 (energy); and the R2 for vitamins B1, B2, and B6 was 0.20, 0.18, and 0.11, respectively [23].
2.6. Assessment of Other Covariates
The following information was collected using the structured questionnaire and included in the multivariable analysis. Accordingly, body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared and was then categorized into four groups: <18.5, 18.5–22.9, 23–24.9, and ≥25 kg/m2. Overweight or obesity was defined if an individual had a BMI ≥ 23 kg/m2, following the recommendation from the World Health Organization (WHO) for the Asian population [24,25]. Age was categorized into six categories, including 15–39, 40–49, 50–59, 60–69, 70–79, and ≥80. Education level was grouped as primary, secondary, and high school or higher. Smoking status was grouped into never-smokers and ever-smokers, whereas alcohol drinking status was divided into never-drinkers and ever-drinkers, as was coffee drinking status, with never-drinkers and ever-drinkers. In addition, the history of type 2 diabetes was dichotomized as yes versus no.
2.7. Statistical Analysis
We calculated means and standard deviations (SDs) continuous variables and count and proportions for categorical variables. We performed a t-test (or ANOVA) and an χ2 test for continuous and categorical variables, respectively, to compare the differences in the distribution of study characteristics between cases and controls. The odds ratios (ORs) and 95% confidence intervals (CIs) were generated from unconditional logistic regression to examine the association between vitamin B1, B2, and B6 and the risk of gastric cancer. The following covariates were adjusted for in the multivariable regression models: (1) age (15–39, 40–49, 50–59, ≥60), (2) sex (male vs. female), (3) education level (primary school/secondary school/high school or higher), (4) BMI (<18.5, 18.5–22.9, 23–24.9, ≥25 kg/m2), (5) alcohol consumption (never- vs. ever-drinker), (6) family history of cancer (yes vs. no), (7) smoking status (never- vs. ever-smoker), (8) history of diabetes (yes vs. no), (9) coffee drinking (never- vs. ever-drinker), (10) total energy intake (nine quantiles, kcal/day), (11) enrollment periods (2003–2006, 2006–2007, 2008, and 2018–2019) to control for temporal variations in diet or healthcare access, (12) blood groups (A, AB, B, O), and (13) H. pylori status.
We conducted stratified analyses by sex (male versus female), histologic type (non-cardia versus cardia), BMI (<23 kg/m2 versus ≥23 mg/m2), smoking status (never versus ever-smoker), alcohol drinking (never- versus ever-drinker), history of type 2 diabetes (yes versus no), H. pylori status (negative versus positive), and blood groups (A, B, AB, and O). Linear trends were tested for the association between the intakes of vitamin B1, B2, and B6 and gastric cancer risk using ordinal values of the quintiles of intakes of vitamins B1, B2, and B6. The test for interactions between sex, BMI, smoking status, alcohol drinking status, history of type 2 diabetes, H. pylori status, and blood groups with vitamins B1, B2, and B6 intakes were performed by including product terms between such variables and vitamins B1, B2, and B6 intakes in multivariable regression models.
Stata statistical package (version 14.0; Stata Corp., College Station, TX, USA) was used in the present. All tests were two-sided, and p < 0.05 was considered a statistically significant level.
3. Results
The current study included 1182 gastric cancer patients and 2995 controls. Compared to patients with cancer, the control subjects were more likely to be male, have a younger age, have higher education levels, be less likely to have a family history of cancer, have a higher BMI, be less likely to be smokers and alcohol drinkers, be more likely to be coffee drinkers, be less likely to have a history of type 2 diabetes, be more likely to have blood groups B and/or O, have higher intakes of vitamins B1, B2, and B6, and have a higher level of energy intake (all p’s <0.05). No difference was observed between cases and controls with respect to H. pylori infection status (p = 0.88) (Table 1). The correlation between vitamins ranged from 0.04 (vitamins B6 vs. B12) to 0.71 vitamins B5 vs. B6) (Table S1).
Table 1.
Characteristics of study participants in the current case-control study.
| Characteristics | Total | Cancer (n = 1182) | Controls (n = 2995) | p-Value |
|---|---|---|---|---|
| Age, mean (SD) | 55.36 (12.13) | 57.6 (11.5) | 54.5 (12.2) | |
| Sex | ||||
| Men | 2580 | 823 (69.6) | 1757 (58.7) | <0.001 |
| Women | 1597 | 359 (30.4) | 1238 (41.3) | |
| Highest level of education | ||||
| Primary school | 650 | 208 (17.6) | 442 (14.8) | <0.001 |
| Secondary school | 1890 | 568 (48.1) | 1322 (44.1) | |
| High school or higher | 1637 | 406 (34.3) | 1231 (41.1) | |
| Fridge use a | ||||
| Yes | 3130 | 764 (68.0) | 2366 (82.5) | <0.001 |
| No | 1047 | 359 (32.0) | 503 (17.5) | |
| BMI, mean (SD) a | 21.3 (3.05) | 19.4 (2.8) | 21.3 (3) | |
| <18.5 | 960 | 469 (41.5) | 491 (16.8) | <0.001 |
| 18.5–22.9 | 2171 | 541 (47.8) | 1630 (55.7) | |
| 23.0–24.9 | 595 | 86 (7.6) | 509 (17.4) | |
| ≥25 | 334 | 35 (3.1) | 299 (10.2) | |
| Family history of cancer | ||||
| No | 3830 | 1066 (90.2) | 2764 (92.3) | 0.03 |
| Yes | 347 | 116 (9.8) | 231 (7.7) | |
| Smoking status | ||||
| Never smoker | 2423 | 601 (50.8) | 1822 (60.8) | <0.001 |
| Ever-smoker | 1754 | 581 (49.2) | 1173 (39.2) | |
| Alcohol consumption | ||||
| Never-drinkers | 2318 | 612 (51.8) | 1706 (57.0) | <0.001 |
| Ever-drinkers | 1859 | 570 (48.2) | 1289 (43.0) | |
| Coffee drinking status | ||||
| Never drinker | 3211 | 922 (78.0) | 2289 (76.4) | <0.001 |
| Ever-drinker | 966 | 260 (22.0) | 706 (23.6) | |
| History of diabetes | ||||
| Yes | 200 | 29 (2.9) | 171 (6.5) | <0.001 |
| No | 3457 | 878 (97.1) | 2479 (93.5) | |
| Total energy intake (Kcal/day), mean (SD) | 1688.63 (445.86) | 1650.6 (455) | 1703.6 (441.4) | |
| Quintile 1 | 838 | 281 (23.8) | 557 (18.6) | 0.006 |
| Quintile 2 | 834 | 223 (18.9) | 611 (20.4) | |
| Quintile 3 | 836 | 231 (19.5) | 605 (20.2) | |
| Quintile 4 | 835 | 221 (18.7) | 614 (20.5) | |
| Quintile 5 | 834 | 226 (19.1) | 608 (20.3) | |
| Total vegetable intake (gr/week), mean (SD) | 1004.3 (729.8) | 1042.8 (723.3) | 1033.92 (724.81) | <0.001 |
| Total red meat intake (gr/week), mean (SD) | 1042.3 (763.1) | 1331.4 (762.6) | 1271.48 (771.5) | <0.001 |
| Blood group a | ||||
| A | 735 | 258 (26.6) | 477 (20.8) | 0.001 |
| AB | 166 | 55 (5.7) | 111 (4.8) | |
| B | 949 | 278 (28.5) | 671 (29.2) | |
| O | 1417 | 380 (39.1) | 1037 (45.2) | |
| H. pylori infection a | ||||
| Negative | 827 | 265 (39.8) | 562 (40.2) | 0.88 |
| Positive | 1236 | 400 (60.2) | 836 (59.8) | |
| Vitamin B1 intake, mg/day mean (range) | 1.04 (±0.38) | 1.17 (±0.40) | ||
| Quintile 1 [0.7 (0.3–0.8)] | 846 | 326 (27.6) | 520 (17.4) | <0.001 |
| Quintile 2 [0.9 (0.8–1.0)] | 847 | 248 (21.0) | 599 (20.0) | |
| Quintile 3 [1.1 (1.0–1.2)] | 848 | 224 (19.0) | 624 (20.8) | |
| Quintile 4 [1.3 (1.2–1.4)] | 807 | 222 (18.8) | 585 (19.5) | |
| Quintile 5 [1.7 (1.4–4.8)] | 829 | 162 (13.7) | 667 (22.3) | |
| Vitamin B2 intake, mg/day mean (range) | 0.57 (±0.23) | 0.61 (±0.21) | ||
| Quintile 1 [0.4 (0.1–0.4)] | 881 | 316 (26.7) | 565 (18.9) | <0.001 |
| Quintile 2 [0.5 (0.4–0.5)] | 798 | 220 (18.6) | 578 (19.3) | |
| Quintile 3 [0.6 (0.5–0.6)] | 890 | 228 (19.3) | 662 (22.1) | |
| Quintile 4 [0.7 (0.6–0.8)] | 804 | 214 (18.1) | 590 (19.7) | |
| Quintile 5 [0.9 (0.8–2.1)] | 804 | 204 (17.3) | 600 (20.0) | |
| Vitamin B6 intake, mg/day mean (range) | 1.13 (±0.35) | 1.20 (±0.33) | ||
| Quintile 1 [0.8 (0.4–0.9)] | 861 | 319 (27.0) | 542 (18.1) | <0.001 |
| Quintile 2 [1.0 (0.9–1.1)] | 845 | 214 (18.1) | 631 (21.1) | |
| Quintile 3 [1.2 (1.1–1.2)] | 829 | 217 (18.4) | 612 (20.4) | |
| Quintile 4 [1.3 (1.2–1.4)] | 822 | 244 (20.6) | 578 (19.3) | |
| Quintile 5 [1.7 (1.4–4.0)] | 820 | 188 (15.9) | 632 (21.1) |
a Based on available data, SD is standard deviation, and BMI is body mass index (Asian category, kg/m2).
Overall, vitamins B1 (ORper-SD increment = 0.83, 95% CI: 0.78–0.89 Ptrend < 0.001) and B6 (ORper-SD increment = 0.88, 95% CI: 0.81–0.94; Ptrend < 0.001) were associated with a reduced risk of gastric cancer. Compared with the lowest quintile, the ORs (95% CIs) of gastric cancer for quintiles 2, 3, 4, and 5 of vitamin B1 intake were 0.64 (0.51–0.79), 0.54 (0.43–0.69), 0.57 (0.44–0.74), and 0.42 (0.31–0.55), respectively. Similarly, the ORs (95% CIs) of gastric cancer for quintiles 2, 3, 4, and 5 of the vitamin B6 intake were 0.53 (0.42–0.66), 0.54 (0.42–0.70), 0.61 (0.46–0.81), and 0.46 (0.33–0.63), respectively, compared with the lowest quintile. This inverse association was present in both sexes for vitamin B1, but only in men (ORper-SD increment = 0.86, 95% CI: 0.77–0.98 Ptrend = 0.008) and not in women (ORper-SD increment = 0.93, 95% CI: 0.81–1.06; Ptrend = 0.26, Pheterogeneity = 0.44) for vitamin B6. A null association between vitamin B2 intake and gastric cancer risk was observed (Table 2).
Table 2.
Association between vitamins B1, B2, and B6 with the risk of gastric cancer, overall and stratified by sex, in the current study.
| Entire Study | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Vitamin Intake | Control | Case | OR (95% CI) * | Control | Case | OR (95% CI) * | Control | Case | OR (95% CI) * |
| Vitamin B1 intake | |||||||||
| Quintile 1 | 520 | 326 | 1.00 | 281 | 221 | 1.00 | 239 | 105 | 1.00 |
| Quintile 2 | 599 | 248 | 0.64 (0.51–0.79) | 341 | 165 | 0.59 (0.45–0.78) | 258 | 83 | 0.71 (0.49–1.03) |
| Quintile 3 | 624 | 224 | 0.54 (0.43–0.69) | 374 | 162 | 0.51 (0.38–0.68) | 250 | 62 | 0.60 (0.40–0.89) |
| Quintile 4 | 585 | 222 | 0.57 (0.44–0.74) | 363 | 160 | 0.52 (0.38–0.72) | 222 | 62 | 0.69 (0.44–1.06) |
| Quintile 5 | 667 | 162 | 0.42 (0.31–0.55) | 398 | 115 | 0.39 (0.27–0.55) | 269 | 47 | 0.48 (0.29–0.78) |
| Continuous (per SD increment) | 2995 | 1182 | 0.83 (0.78–0.89) | 1757 | 823 | 0.82 (0.76–0.89) | 1238 | 359 | 0.86 (0.77–0.96) |
| Ptrend | <0.001 | <0.001 | 0.008 | ||||||
| Pheterogeneity | 0.24 | ||||||||
| Vitamin B2 intake | |||||||||
| Quintile 1 | 565 | 316 | 1.00 | 301 | 210 | 1.00 | 264 | 106 | 1.00 |
| Quintile 2 | 578 | 220 | 0.72 (0.57–0.89) | 334 | 147 | 0.68 (0.52–0.90) | 244 | 73 | 0.77 (0.53–1.10) |
| Quintile 3 | 662 | 228 | 0.71 (0.57–0.90) | 384 | 152 | 0.66 (0.50–0.88) | 278 | 76 | 0.83 (0.57–1.21) |
| Quintile 4 | 590 | 214 | 0.76 (0.60–0.98) | 384 | 163 | 0.72 (0.54–0.98) | 206 | 51 | 0.86 (0.55–1.33) |
| Quintile 5 | 600 | 204 | 0.85 (0.65–1.11) | 354 | 151 | 0.86 (0.62–1.19) | 246 | 53 | 0.83 (0.52–1.32) |
| Continuous (per SD increment) | 2995 | 1182 | 0.97 (0.91–1.03) | 1757 | 823 | 0.97 (0.90–1.05) | 1238 | 359 | 0.97 (0.87, 1.08) |
| Ptrend | 0.32 | 0.47 | 0.54 | ||||||
| Pheterogeneity | 0.15 | ||||||||
| Vitamin B6 intake | |||||||||
| Quintile 1 | 542 | 319 | 1.00 | 279 | 206 | 1.00 | 263 | 113 | 1.00 |
| Quintile 2 | 631 | 214 | 0.53 (0.42–0.66) | 0:00 | 148 | 0.53 (0.39–0.71) | 293 | 66 | 0.52 (0.35–0.76) |
| Quintile 3 | 612 | 217 | 0.54 (0.42–0.70) | 346 | 145 | 0.50 (0.36–0.69) | 266 | 72 | 0.63 (0.41–0.96) |
| Quintile 4 | 578 | 244 | 0.61 (0.46–0.81) | 373 | 179 | 0.55 (0.39–0.79) | 205 | 65 | 0.74 (0.45–1.21) |
| Quintile 5 | 632 | 188 | 0.46 (0.33–0.63) | 421 | 145 | 0.42 (0.28–0.62) | 211 | 43 | 0.57 (0.32–1.00) |
| Continuous (per SD increment) | 2995 | 1182 | 0.88 (0.81–0.94) | 1757 | 823 | 0.85 (0.78–0.94) | 1238 | 359 | 0.93 (0.81–1.06) |
| Ptrend | <0.001 | 0.001 | 0.26 | ||||||
| Pheterogeneity | 0.44 | ||||||||
* Model adjusted for age (15–29, 30–39, 40–49, 50–59, 60–69, 70+) (if applicable), sex, education level (primary, secondary, and high school or higher), BMI (kg/m2, <18.5, 18.5–22.9, 23–24.9, ≥25), alcohol consumption (yes/no), family history of cancer (yes/no), smoking status (ever/never), history of type 2 diabetes (yes/no), coffee drinking (yes/no), total energy intake (kcal/day, tertile), fridge at home, blood group (A, AB, B, and O), four periods of data collection, and H. pylori status. Abbreviations: CI: confidence interval; OR: odds ratio; bold font: statistical significance (p < 0.05).
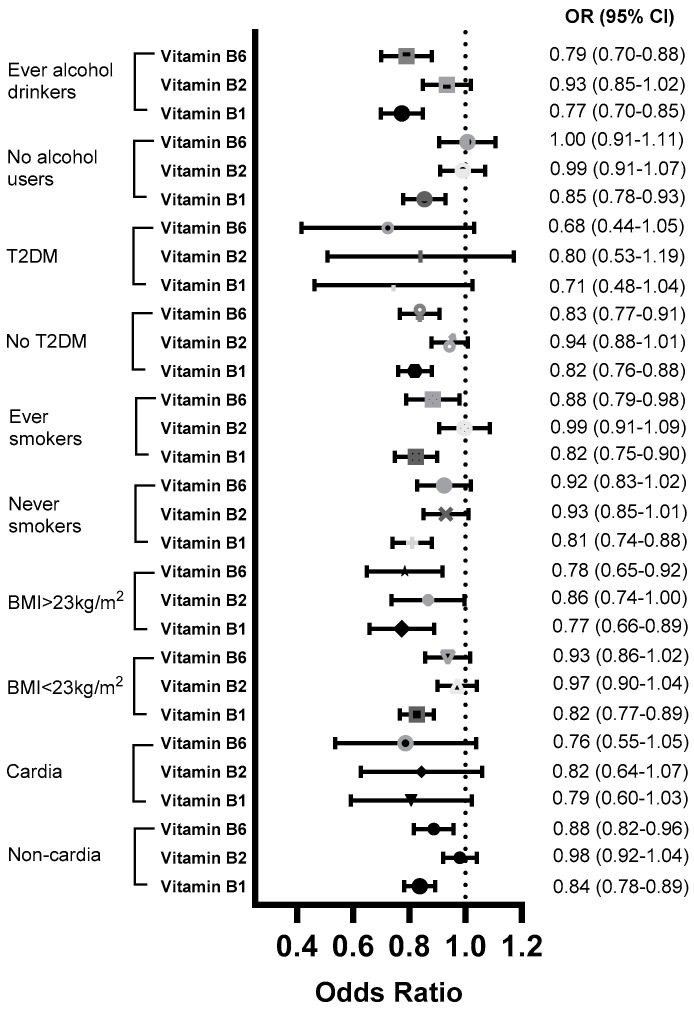
In stratified analysis, an inverse association between vitamin B1 intake and gastric cancer risk was found in individuals with BMI < 23 kg/m2 and BMI ≥ 23 kg/m2 (Pheterogeneity = 0.003), never-smokers and ever-smokers (Pheterogeneity = 0.15), alcohol non-users and habitual alcohol drinkers (Pheterogeneity = 0.02), patients with a non-cardia histologic type (ORper-SD increment = 0.84, 95% CI: 0.78–0.89; Ptrend < 0.001), and individuals without a history of type 2 diabetes (ORper-SD increment = 0.82, 95% CI: 0.76–0.88; Ptrend < 0.001; Pheterogeneity = 0.13). A similar pattern was also found for vitamin B6 intake, except the inverse association was only found for ever-alcohol drinkers (ORper-SD increment = 0.79, 95%; CI: 0.70–0.88; Ptrend < 0.001) and alcohol non-users (ORper-SD increment = 1.00; 95% CI: 0.91–1.11; Ptrend = 0.97; Pheterogeneity < 0.001) (Figure 1 and Table 3).
Figure 1.
Association between vitamins B1, B2, and B6 and the risk of gastric cancer, stratified by BMI, smoking status, alcohol drinking status, and history of type 2 diabetes, in the current study (estimates per SD increment). CI, confidence interval; OR, odds ratio; T2DM, type 2 diabetes mellitus.
Table 3.
Association between vitamins B1, B2, and B6 with the risk of gastric cancer, stratified by BMI, smoking status, alcohol drinking status, and history of type 2 diabetes, in the current study.
| Vitamin B1 | Vitamin B2 | Vitamin B6 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Vitamin Intake | Control | Case | OR (95% CI) * | Control | Case | OR (95% CI) * | Control | Case | OR (95% CI) * |
| Non-cardia | |||||||||
| Quintile 1 | 520 | 314 | 1.00 | 565 | 304 | 1.00 | 542 | 310 | 1.00 |
| Quintile 2 | 599 | 242 | 0.65 (0.52–0.82) | 578 | 214 | 0.73 (0.59–0.92) | 631 | 206 | 0.53 (0.42–0.68) |
| Quintile 3 | 624 | 217 | 0.55 (0.44–0.70) | 662 | 220 | 0.73 (0.58–0.92) | 612 | 208 | 0.55 (0.42–0.71) |
| Quintile 4 | 585 | 215 | 0.59 (0.45–0.76) | 590 | 208 | 0.78 (0.61–1.00) | 578 | 237 | 0.63 (0.47–0.84) |
| Quintile 5 | 667 | 157 | 0.43 (0.32–0.57) | 600 | 199 | 0.88 (0.67–1.15) | 632 | 184 | 0.48 (0.35–0.67) |
| Continuous (per SD increment) | 0.84 (0.78–0.89) | 0.98 (0.92–1.04) | 0.88 (0.82–0.96) | ||||||
| Ptrend | <0.001 | 0.44 | 0.002 | ||||||
| Cardia | |||||||||
| Tertile 1 | 520 | 12 | 1.00 | 565 | 12 | 1.00 | 542 | 9 | 1.00 |
| Tertile 2 | 1223 | 13 | 0.44 (0.18–1.06) | 1240 | 14 | 0.57 (0.25–1.31) | 1243 | 17 | 0.62 (0.23–1.61) |
| Tertile 3 | 1252 | 12 | 0.38 (0.13–1.10) | 1190 | 11 | 0.47 (0.17–1.29) | 1210 | 11 | 0.33 (0.09–1.23) |
| Continuous (per SD increment) | 0.79 (0.60–1.03) | 0.82 (0.64–1.07) | 0.76 (0.55–1.05) | ||||||
| Ptrend | 0.08 | 0.14 | 0.09 | ||||||
| BMI < 23 kg/m2 | |||||||||
| Quintile 1 | 368 | 263 | 1.00 | 412 | 265 | 1.00 | 378 | 260 | 1.00 |
| Quintile 2 | 437 | 226 | 0.70 (0.55–0.89) | 420 | 188 | 0.73 (0.57–0.93) | 478 | 190 | 0.57 (0.44–0.73) |
| Quintile 3 | 443 | 191 | 0.56 (0.43–0.72) | 449 | 192 | 0.74 (0.57–0.94) | 429 | 189 | 0.65 (0.49–0.87) |
| Quintile 4 | 427 | 195 | 0.61 (0.46–0.80) | 426 | 188 | 0.79 (0.60–1.03) | 408 | 206 | 0.74 (0.54–1.02) |
| Quintile 5 | 446 | 135 | 0.40 (0.29–0.55) | 414 | 177 | 0.84 (0.63–1.12) | 428 | 165 | 0.59 (0.41–0.84) |
| Continuous (per SD increment) | 2121 | 1010 | 0.82 (0.77–0.89) | 2121 | 1010 | 0.97 (0.90–1.04) | 2121 | 1010 | 0.93 (0.86–1.02) |
| Ptrend | <0.001 | 0.34 | 0.09 | ||||||
| BMI ≥ 23 kg/m2 | |||||||||
| Quintile 1 | 152 | 63 | 1.00 | 153 | 51 | 1.00 | 164 | 59 | 1.00 |
| Quintile 2 | 162 | 22 | 0.30 (0.17–0.53) | 158 | 32 | 0.60 (0.35–1.01) | 153 | 24 | 0.40 (0.23–0.69) |
| Quintile 3 | 181 | 33 | 0.41 (0.24–0.70) | 213 | 36 | 0.55 (0.33–0.93) | 183 | 28 | 0.36 (0.20–0.65) |
| Quintile 4 | 158 | 27 | 0.37 (0.20–0.68) | 164 | 26 | 0.53 (0.28–1.00) | 170 | 38 | 0.51 (0.27–0.95) |
| Quintile 5 | 221 | 27 | 0.28 (0.15–0.52) | 186 | 27 | 0.52 (0.27–0.99) | 204 | 23 | 0.26 (0.12–0.54) |
| Continuous (per SD increment) | 874 | 172 | 0.77 (0.66–0.89) | 874 | 172 | 0.86 (0.74–1.00) | 874 | 172 | 0.78 (0.65–0.92) |
| Ptrend | 0.001 | 0.16 | 0.004 | ||||||
| Pheterogeneity | 0.003 | 0.007 | 0.008 | ||||||
| Never-Smokers | |||||||||
| Quintile 1 | 323 | 171 | 1.00 | 364 | 170 | 1.00 | 341 | 169 | 1.00 |
| Quintile 2 | 371 | 124 | 0.64 (0.48–0.86) | 348 | 121 | 0.80 (0.60–1.07) | 411 | 103 | 0.49 (0.36–0.67) |
| Quintile 3 | 375 | 117 | 0.59 (0.43–0.80) | 406 | 116 | 0.69 (0.51–0.93) | 367 | 124 | 0.68 (0.49–0.95) |
| Quintile 4 | 340 | 115 | 0.62 (0.45–0.88) | 335 | 102 | 0.81 (0.58–1.13) | 349 | 118 | 0.67 (0.46–0.99) |
| Quintile 5 | 413 | 74 | 0.34 (0.23–0.50) | 369 | 92 | 0.71 (0.49–1.02) | 354 | 87 | 0.54 (0.35–0.84) |
| Continuous (per SD increment) | 1822 | 601 | 0.81 (0.74–0.88) | 1822 | 601 | 0.93 (0.85–1.01) | 1822 | 601 | 0.92 (0.83–1.02) |
| Ptrend | <0.001 | 0.08 | 0.10 | ||||||
| Ever-Smokers | |||||||||
| Quintile 1 | 197 | 155 | 1.00 | 201 | 146 | 1.00 | 201 | 150 | 1.00 |
| Quintile 2 | 228 | 124 | 0.67 (0.48–0.93) | 230 | 99 | 0.63 (0.45–0.88) | 220 | 111 | 0.68 (0.48–0.95) |
| Quintile 3 | 249 | 107 | 0.50 (0.35–0.71) | 256 | 112 | 0.69 (0.49–0.97) | 245 | 93 | 0.50 (0.34–0.73) |
| Quintile 4 | 245 | 107 | 0.53 (0.36–0.78) | 255 | 112 | 0.73 (0.51–1.06) | 229 | 126 | 0.72 (0.47–1.09) |
| Quintile 5 | 254 | 88 | 0.41 (0.27–0.62) | 231 | 112 | 0.90 (0.61–1.33) | 278 | 101 | 0.48 (0.30–0.76) |
| Continuous (per SD increment) | 1173 | 581 | 0.82 (0.75–0.90) | 1173 | 581 | 0.99 (0.91–1.09) | 1173 | 581 | 0.88 (0.79–0.98) |
| Ptrend | <0.001 | 0.90 | 0.02 | ||||||
| Pheterogeneity | 0.15 | 0.08 | 0.54 | ||||||
| No History of Type 2 Diabetes | |||||||||
| Quintile 1 | 455 | 294 | 1.00 | 495 | 286 | 1.00 | 480 | 296 | 1.00 |
| Quintile 2 | 504 | 211 | 0.66 (0.52–0.84) | 487 | 182 | 0.71 (0.56–0.90) | 545 | 190 | 0.54 (0.42–0.69) |
| Quintile 3 | 522 | 175 | 0.53 (0.41–0.68) | 565 | 187 | 0.69 (0.54–0.88) | 520 | 183 | 0.53 (0.41–0.70) |
| Quintile 4 | 460 | 172 | 0.60 (0.46–0.80) | 458 | 163 | 0.75 (0.57–0.98) | 461 | 176 | 0.54 (0.40–0.74) |
| Quintile 5 | 538 | 126 | 0.39 (0.29–0.53) | 474 | 160 | 0.77 (0.57–1.03) | 473 | 133 | 0.40 (0.28–0.57) |
| Continuous (per SD increment) | 0.82 (0.76–0.88) | 0.94 (0.88–1.01) | 0.83 (0.77–0.91) | ||||||
| Ptrend | <0.001 | 0.10 | <0.001 | ||||||
| History of Type 2 Diabetes | |||||||||
| Tertile 1 | 39 | 13 | 1.00 | 41 | 10 | 1.00 | 50 | 15 | 1.00 |
| Tertile 2 | 70 | 9 | 0.31 (0.09–1.03) | 75 | 13 | 0.69 (0.22–2.21) | 68 | 8 | 0.25 (0.07–0.92) |
| Tertile 3 | 62 | 7 | 0.26 (0.06–1.19) | 55 | 6 | 0.40 (0.08–2.00) | 53 | 6 | 0.22 (0.04–1.32) |
| Continuous (per SD increment) | 0.71 (0.48–1.04) | 0.80 (0.53–1.19) | 0.68 (0.44–1.05) | ||||||
| Ptrend | 0.08 | 0.27 | 0.08 | ||||||
| Pheterogeneity | 0.13 | 0.85 | 0.35 | ||||||
| Alcohol Non-Users | |||||||||
| Quintile 1 | 323 | 149 | 1.00 | 358 | 157 | 1.00 | 360 | 153 | 1.00 |
| Quintile 2 | 360 | 133 | 0.76 (0.57–1.03) | 353 | 116 | 0.74 (0.55–0.99) | 391 | 110 | 0.66 (0.48–0.90) |
| Quintile 3 | 352 | 128 | 0.71 (0.52–0.97) | 380 | 132 | 0.83 (0.62–1.12) | 346 | 122 | 0.83 (0.60–1.16) |
| Quintile 4 | 307 | 111 | 0.68 (0.48–0.96) | 294 | 99 | 0.81 (0.57–1.14) | 286 | 123 | 1.01 (0.69–1.47) |
| Quintile 5 | 364 | 91 | 0.48 (0.32–0.70) | 321 | 108 | 0.91 (0.64–1.30) | 323 | 104 | 0.80 (0.52–1.22) |
| Continuous (per SD increment) | 1706 | 612 | 0.85 (0.78–0.93) | 1706 | 612 | 0.99 (0.91–1.07) | 1706 | 612 | 1.00 (0.91–1.11) |
| Ptrend | <0.001 | 0.74 | 0.97 | ||||||
| Ever-Alcohol Drinkers | |||||||||
| Quintile 1 | 197 | 177 | 1.00 | 207 | 159 | 1.00 | 182 | 166 | 1.00 |
| Quintile 2 | 239 | 115 | 0.54 (0.39–0.75) | 225 | 104 | 0.71 (0.51–0.98) | 240 | 104 | 0.46 (0.33–0.65) |
| Quintile 3 | 272 | 96 | 0.40 (0.28–0.57) | 282 | 96 | 0.55 (0.39–0.78) | 266 | 95 | 0.37 (0.25–0.55) |
| Quintile 4 | 278 | 111 | 0.50 (0.34–0.72) | 296 | 115 | 0.73 (0.51–1.03) | 292 | 121 | 0.43 (0.28–0.66) |
| Quintile 5 | 303 | 71 | 0.28 (0.19–0.43) | 279 | 96 | 0.70 (0.47–1.04) | 309 | 84 | 0.29 (0.18–0.47) |
| Continuous (per SD increment) | 1289 | 570 | 0.77 (0.70–0.85) | 1289 | 570 | 0.93 (0.85–1.02) | 1289 | 570 | 0.79 (0.70–0.88) |
| Ptrend | <0.001 | 0.12 | <0.001 | ||||||
| Pheterogeneity | 0.02 | 0.05 | <0.001 | ||||||
* Model adjusted for age (15–29, 30–39, 40–49, 50–59, 60–69, and 70+) (if applicable), sex, education level (primary, secondary, and high school or higher), BMI (kg/m2, <18.5, 18.5–22.9, 23–24.9, ≥25), alcohol consumption (yes/no), family history of cancer (yes/no), smoking status (ever/never), history of type 2 diabetes (yes/no), coffee drinking (yes/no), total energy intake (kcal/day, tertile), fridge at home, blood group (A, AB, B, and O), four periods of data collection, and H. pylori status. Abbreviations: CI: confidence interval; OR: odds ratio; bold font: statistical significance (p < 0.05).
Further stratified analysis found null associations between vitamin B1, B2, and B6 with the risk of gastric cancer in individuals, regardless their H. pylori test results. The inverse association pattern between vitamin B1 and gastric cancer risk was identified in individuals with blood groups A ((ORper-SD increment = 0.85; 95% CI: 0.74–0.98; Ptrend = 0.03) and O (ORper-SD increment = 0.75; 95% CI: 0.67–0.84; Ptrend < 0.001) but not in individuals with blood group B (ORper-SD increment = 0.84; 95% CI: 0.74–0.96; Ptrend = 0.11) or AB (ORper-SD increment = 0.79; 95% CI: 0.56–1.13; Ptrend < 0.20). Since the p-heterogeneity was not significant, such an association appeared not to cause a difference between the stratified groups. For vitamin B6, this reduced risk pattern with gastric cancer was only found in individuals with blood group O (ORper-SD increment = 0.86; 95% CI: 0.75–0.98; Ptrend < 0.02) (Table S2).
4. Discussion
In a case-control study of 1182 patients with gastric cancer and 2995 controls, we found a dose–response inverse association between vitamin B1 and B6 intake and the risk of gastric cancer in the Vietnamese population. This inverse association was not different across sex, BMI, and smoking statuses. A stratified analysis did not find a significant association between vitamin intake (i.e., B1, B2, and B6) and gastric cancer risk among individuals with a history of type 2 diabetes, those with or without H. pylori infection status, or individuals with blood groups O and AB.
Our main findings on the protective effects of vitamin B1 and B6 intake against the risk of gastric cancer are in line with two prior case-control studies. Accordingly, in a case-control study in Belgium (i.e., 301 gastric cancer cases and 2851 controls), Kaaks et al. [15] reported that both vitamins B1 and B6 were associated with a decreased risk of gastric cancer. Another case-control study in the U.S., recruiting 687 patients with gastric cancer and 1595 controls from three states, including New Jersey, Connecticut, and Washington, also showed that dietary vitamin B6 was associated with a more than 40% risk reduction for gastric cancer [14]. Our finding on a null association between vitamin B2 intake and gastric cancer risk was inconsistent with a finding from a recent nested case-control study within the EPIC Prospective Cohort Study (i.e., 235 cases and 601 controls), in which they reported inverse associations between the intake of vitamins B2 (OR = 0.85; 95% CI: 0.72–1.01) and B6 (OR = 0.78; 95% CI: 0.65–0.98) and gastric cancer [16]. The null association between vitamin B2 intake and gastric cancer risk was, however, consistent with a case-control study in Italy (i.e., 200 patients with gastric cancer vs. 547 controls), showing no association between vitamins B1, B2, and B6 and the risk of gastric cancer [17]. On the other hand, our findings are inconsistent with those from three prospective cohort studies in Australia, the Melbourne Collaborative Cohort Study (n = 41,513) [11], in the U.S., the NIH-AARP Cohort Study (n = 492,293) [12], and in China, the Shanghai Women’s Health Study (n = 73,009 Chinese women) [13], all of which reported that there was no association between these vitamins and the risk of gastric cancer. Different study designs and study populations may partly explain such a discrepancy in the results.
Rich sources of vitamin B1 are foods with less refined cereal products, peas, nuts, wholegrain breads, or some fresh fruits (bananas and oranges), whereas foods with major sources of vitamin B6 are pork, poultry, fish, peanuts, soybeans, oats, or milk. In research using data from the NIH-AARP Diet and Health Study, a prospective cohort study that recruited 566,407 individuals who were 50–71 years of age at the baseline (1995–1996 period), Hashmenian et al. [26] reported that participants in the highest category of nut consumption had a lower risk of developing gastric non-cardia adenocarcinoma compared with those who did not consume nuts or peanut butter; a result that was consistent with our current finding that both vitamin B1 and B6 reduced gastric cancer risk. The consumption of nuts, including tree nuts, peanuts, and peanut butter, was also found to be associated with a decreased risk of gastric cancer in another large cohort study, the Netherland Cohort Study, that included 120,852 males and females, aged 55–69 years [27]. A recent meta-analysis of 183 studies, conducted by Bouras et al. [28], also found that fruit and citrus fruit consumption provided a protective effect against the development of gastric cancer (pooled relative risk = 0.93; 95% CI: 0.89–0.98; and 0.62; 95% CI: 0.39–0.99; respectively). Also, in a study using data from the Health Examinees-Gem (HEXA-G) Study, a prospective cohort study that included a total of 139,267 participants aged 40–69 years between 2004 and 2013 in South Korea, Shin et al. [29] found that individuals who consumed two servings of tofu per week had a 37% lower risk of gastric cancer compared with those who almost never consumed tofu (HR = 0.63; 95% CI: 0.45–0.89). Similarly, a meta-analysis of 43 studies, including 11 cohort studies and 32 case-control studies, conducted by Kim et al. [30] observed an inverse association between the consumption of white meat and gastric cancer risk (pooled RR = 0.80; 95% CI: 0.69–0.92). Taken together, these prior epidemiologic studies support the findings obtained in our study suggesting that dietary vitamins B1 and B6 were associated with a reduced risk of gastric cancer in the Vietnamese population.
Experimental studies identified different genes that link thiamine (or vitamin B1) to cancer, including the solute carrier transporter (SLC19) gene, the poly (ADP-ribose) polymerase-1 gene, transcription factor p53, or the reduced form of nicotinamide adenine dinucleotide phosphate. Vitamin B1 has effects on the matrix metalloproteinases (MMPs), cyclooxygenase-2 (COX-2), nitric oxide synthase (NOS), reactive oxygen species (ROS), or prostaglandins, thus implicating cancer. Alternatively, vitamin B1 supplementation might have resulted in a high rate of tumor cell survival, proliferation, or chemotherapy resistance (reviews by Luong et al. [31]). Also, a long-term deficiency of vitamin B6 increases homocysteine levels, which subsequently results in increased oxidative stress. A study using a mouse model showed that the immune system was affected due to a deficiency of vitamin B6 via three mechanisms: (1) inhibiting the expression of T-bet, (2) upregulating the expression of the immune cell activator T-bet, and (3) downregulating the expression of cytokine signaling 1 protein inhibitors [32]. However, the exact biological mechanisms of protective effects of vitamins B1 and B6 in gastric cancer are unclear. Future studies are thus warranted to better understand such mechanisms.
Our study has several limitations. First, because this is a hospital-based study, selection bias is inescapable. This particularly involves the selection of hospital-based control subjects who were not representative of the general healthy population. Next, the current study recruited patients and control subjects from Northern Vietnam; our findings could not be generalizable to other parts of Vietnam or Asian countries. Also, even though control subjects recruited in our study were cancer-free patients, they still had medical issues and were undergoing surgery; therefore, they were not considered healthy controls like those in population-based case-control studies. Additionally, recall bias might have occurred because diet information was obtained in the FFQ by asking about their diet 12 months prior to the interview date; thus, study participants might not have answered correctly or might have changed their diets or eating patterns due to precancerous symptoms. We were also not able to perform a stratified analysis of vitamins B1, B2, and B6 by food sources because they are not available in the Vietnam Food Composition Database. Also, long-term enrollment (2003–2019 period) might have resulted in a temporal variation in diet and lifestyle. However, we tried to minimize this influence in the multivariable regression model by including this variable as one of the potential confounding factors. Finally, residual confounding from unmeasured factors, including dietary factors, might have occurred even though we adjusted for the multivariable model using a comprehensive set of covariates.
Our study also has several strengths. This might be the first study, to our knowledge, to determine the association between vitamins B1, B2, and B6 and gastric cancer risk in Vietnam using a relatively large sample size. We used a semi-quantitative FFQ to obtain diet information from study participants and generated macro-and micronutrients from the Vietnamese Food Composition Database, which is tailored to local Vietnamese foods with an emphasis on a high intake of vegetables and fruits, consequently providing more accuracy in nutrition intake calculations. Finally, a comprehensive set of covariates used in the multivariable models allowed us to minimize the potential confounding effects.
5. Conclusions
In conclusion, in a large, hospital-based case-control study of 1182 patients with gastric cancer and 2995 controls, we observed a dose-response inverse association between vitamin B1 and B6 intakes and the risk of gastric cancer in the Vietnamese population, but such an association was not observed for B2. This inverse association was not consistent across sex, BMI, and smoking statuses. Future studies are warranted to replicate our findings, which also have great potential for prevention strategies in gastric cancer control programs in low-and middle-income settings. Future research should also be conducted using a longitudinal study design to confirm causality, explore underlying mechanisms, and investigate other dietary patterns or micronutrients that could influence gastric cancer risk.
Acknowledgments
The authors thank all study participants in the three northern provinces of Vietnam for their participation. We also thank Felicia Steele for editing this manuscript.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu16244370/s1: Table S1: Spearman correlation coefficients between B vitamins in the current study; Table S2: Association between vitamins B1, B2, and B6 and the risk of gastric cancer, stratified by blood group and H. pylori infection status, in the current study.
Author Contributions
Conceptualization, N.T.L., Y.T.-H.P. and H.N.L.; methodology, N.T.L., Y.T.-H.P., H.T.D., L.T.L., J.C. and H.N.L.; software, N.T.L., Y.T.-H.P. and H.N.L.; validation, N.T.L., Y.T.-H.P. and H.N.L.; formal analysis, N.T.L., Y.T.-H.P. and H.N.L.; investigation, all authors; resources, N.T.L. and H.N.L.; data curation, N.T.L., H.T.D. and N.Y.-N.H.; writing—original draft preparation, N.T.L., Y.T.-H.P. and H.N.L.; writing—review and editing, all authors; visualization, N.T.L., Y.T.-H.P. and H.N.L.; supervision, N.T.L. and H.N.L.; project administration, N.T.L.; funding acquisition, N.T.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Boards (IRBs) of the Hanoi Medical University (approval number NCS33/HMU-IRB dated 29 March 2019) and the International University of Health and Welfare, Japan (approval number 21-Ig-92 dated 21 August 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author due to ethical reasons.
Conflicts of Interest
The authors declare no conflicts of interest. The funders played no role in the design of this study, in the collection, analyses, or interpretation of data, in the writing of this manuscript, or in the decision to publish the results.
Funding Statement
This research was funded by the Vietnam Ministry of Science and Technology, grant number 18/FIRST/1a/HMU (2017–2019, PI: N.T.L.). Y.T.-H.P. was supported by an NIH Training Grant, number T32CA186873 (PI: J-M Yuan), for her work at the University of Pittsburgh. The APC was funded by the University of Pittsburgh Medical Center, Hillman Cancer Center start-up grant (PI. Luu). This work was also supported by a UICC 2013 American Cancer Society Beginning Investigators Fellowship funded by the American Cancer Society, a UICC 2015 Yamagiwa-Yoshida Memorial International Cancer Study Grant.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Morgan E., Arnold M., Camargo M.C., Gini A., Kunzmann A.T., Matsuda T., Meheus F., Verhoeven R.H.A., Vignat J., Laversanne M., et al. The Current and Future Incidence and Mortality of Gastric Cancer in 185 Countries, 2020-40: A Population-Based Modelling Study. eClinicalMedicine. 2022;47:101404. doi: 10.1016/j.eclinm.2022.101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Q., Xu D., Yang Y., Lu S., Wang D., Wang L. Global, Regional, and National Burden of Gastric Cancer in Adolescents and Young Adults, 1990-2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Am. J. Gastroenterol. 2024;119:454–467. doi: 10.14309/ajg.0000000000002551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferlay J., Ervik M., Lam F., Laversanne M., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. Global Cancer Observatory: Cancer Today. Global Cancer Observatory; Lyon, France: 2024. [Google Scholar]
- 5.Thrift A.P., Wenker T.N., El-Serag H.B. Global Burden of Gastric Cancer: Epidemiological Trends, Risk Factors, Screening and Prevention. Nat. Rev. Clin. Oncol. 2023;20:338–349. doi: 10.1038/s41571-023-00747-0. [DOI] [PubMed] [Google Scholar]
- 6.Lyons K., Le L.C., Pham Y.T.-H., Borron C., Park J.Y., Tran C.T.D., Tran T.V., Tran H.T.-T., Vu K.T., Do C.D., et al. Gastric Cancer: Epidemiology, Biology, and Prevention: A Mini Review. Eur. J. Cancer Prev. 2019;28:397–412. doi: 10.1097/CEJ.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 7.Tsugane S., Sasazuki S. Diet and the Risk of Gastric Cancer: Review of Epidemiological Evidence. Gastric Cancer. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 8.Liu C., Russell R.M. Nutrition and Gastric Cancer Risk: An Update. Nutr. Rev. 2008;66:237–249. doi: 10.1111/j.1753-4887.2008.00029.x. [DOI] [PubMed] [Google Scholar]
- 9.Johansson M., Relton C., Ueland P.M., Vollset S.E., Midttun Ø., Nygård O., Slimani N., Boffetta P., Jenab M., Clavel-Chapelon F., et al. Serum B Vitamin Levels and Risk of Lung Cancer. JAMA. 2010;303:2377–2385. doi: 10.1001/jama.2010.808. [DOI] [PubMed] [Google Scholar]
- 10.Das P.M., Singal R. DNA Methylation and Cancer. J. Clin. Oncol. 2004;22:4632–4642. doi: 10.1200/JCO.2004.07.151. [DOI] [PubMed] [Google Scholar]
- 11.Dugué P.-A., Bassett J.K., Brinkman M.T., Southey M.C., Joo J.E., Wong E.M., Milne R.L., English D.R., Giles G.G., Boussioutas A., et al. Dietary Intake of Nutrients Involved in One-Carbon Metabolism and Risk of Gastric Cancer: A Prospective Study. Nutr. Cancer. 2019;71:605–614. doi: 10.1080/01635581.2019.1577982. [DOI] [PubMed] [Google Scholar]
- 12.Xiao Q., Freedman N.D., Ren J., Hollenbeck A.R., Abnet C.C., Park Y. Intakes of Folate, Methionine, Vitamin B6, and Vitamin B12 with Risk of Esophageal and Gastric Cancer in a Large Cohort Study. Br. J. Cancer. 2014;110:1328–1333. doi: 10.1038/bjc.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kweon S.-S., Shu X.-O., Xiang Y., Yang G., Ji B.-T., Li H., Gao Y.-T., Zheng W., Shrubsole M.J. One-Carbon Metabolism Dietary Factors and Distal Gastric Cancer Risk in Chinese Women. Cancer Epidemiol. Biomark. Prev. 2014;23:1374–1382. doi: 10.1158/1055-9965.EPI-14-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayne S.T., Risch H.A., Dubrow R., Chow W.H., Gammon M.D., Vaughan T.L., Farrow D.C., Schoenberg J.B., Stanford J.L., Ahsan H., et al. Nutrient Intake and Risk of Subtypes of Esophageal and Gastric Cancer. Cancer Epidemiol. Biomark. Prev. 2001;10:1055–1062. [PubMed] [Google Scholar]
- 15.Kaaks R., Tuyns A.J., Haelterman M., Riboli E. Nutrient Intake Patterns and Gastric Cancer Risk: A Case-Control Study in Belgium. Int. J. Cancer. 1998;78:415–420. doi: 10.1002/(SICI)1097-0215(19981109)78:4<415::AID-IJC4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 16.Eussen S.J.P.M., Vollset S.E., Hustad S., Midttun Ø., Meyer K., Fredriksen A., Ueland P.M., Jenab M., Slimani N., Boffetta P., et al. Plasma Vitamins B2, B6, and B12, and Related Genetic Variants as Predictors of Colorectal Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2010;19:2549–2561. doi: 10.1158/1055-9965.EPI-10-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelucchi C., Tramacere I., Bertuccio P., Tavani A., Negri E., La Vecchia C. Dietary Intake of Selected Micronutrients and Gastric Cancer Risk: An Italian Case-Control Study. Ann. Oncol. 2009;20:160–165. doi: 10.1093/annonc/mdn536. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen M.T., Huynh N.N.Y., Nguyen D.D., Ta N.H., Van Nguyen T., Dang H.T., Le N.T. Vitamin D Intake and Gastric Cancer in Viet Nam: A Case-Control Study. BMC Cancer. 2022;22:838. doi: 10.1186/s12885-022-09933-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sengngam K., Hoc T.H., Hang D.V., Tran Ngoan L. Trans-Lycopene and β-Cryptoxanthin Intake and Stomach Cancer in Vietnamese Men: A Pilot Case-Control Study. Asian Pac. J. Cancer Prev. 2022;23:861–865. doi: 10.31557/APJCP.2022.23.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran H.H., Sengngam K., Pham P.V., Le N.T. Case-Control Study of Alcohol Usage and Fruit Intake and Stomach Cancer in the North Viet Nam. Asian Pac. J. Cancer Prev. 2021;22:2903–2908. doi: 10.31557/APJCP.2021.22.9.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen T.G., Truong D.T.T., Le P.H., Kim Vo T.C., Ikeda S., Tran Le N. Calcium Intake Contributed by Whole Foods and Gastric Cancer in Viet Nam: A Case-Control Study. Nutr. Cancer. 2023;75:1243–1253. doi: 10.1080/01635581.2023.2187721. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen K.C., Nguyen L.T., Ha D.T.A., Le D.H., Le M.B., Nguyen S.V., Ha K.H., Bui D.M., Nguyen L.T. Vietnamese Food Composition Table. FAO; Rome, Italy: 2007. [Google Scholar]
- 23.Le N.T., Le H.X., Pham P.V., Nguyen D.Q., Tran H.H., Pham O.T., Nguyen B.T., Dinh M.T., Nguyen H.T., Pham P.T., et al. Reproducibility of a Designed Semi-Quantitative Food Frequency Questionnaire in General Populations in the North Vietnam. Southeast Asia J. Sci. 2018;6:188–197. [Google Scholar]
- 24.WHO Expert Consultation Appropriate Body-Mass Index for Asian Populations and Its Implications for Policy and Intervention Strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 25.Pan W.-H., Yeh W.-T. How to Define Obesity? Evidence-Based Multiple Action Points for Public Awareness, Screening, and Treatment: An Extension of Asian-Pacific Recommendations. Asia Pac. J. Clin. Nutr. 2008;17:370–374. [PubMed] [Google Scholar]
- 26.Hashemian M., Murphy G., Etemadi A., Dawsey S.M., Liao L.M., Abnet C.C. Nut and Peanut Butter Consumption and the Risk of Esophageal and Gastric Cancer Subtypes. Am. J. Clin. Nutr. 2017;106:858–864. doi: 10.3945/ajcn.117.159467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieuwenhuis L., van den Brandt P.A. Tree Nut, Peanut, and Peanut Butter Consumption and the Risk of Gastric and Esophageal Cancer Subtypes: The Netherlands Cohort Study. Gastric Cancer. 2018;21:900–912. doi: 10.1007/s10120-018-0821-2. [DOI] [PubMed] [Google Scholar]
- 28.Bouras E., Tsilidis K.K., Triggi M., Siargkas A., Chourdakis M., Haidich A.-B. Diet and Risk of Gastric Cancer: An Umbrella Review. Nutrients. 2022;14:1764. doi: 10.3390/nu14091764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin W.-K., Lee H.-W., Huang D., De la Torre K., Min S., Shin A., Lee J.-K., Lee J.E., Kang D. Soybean Product Consumption Decreases Risk of Gastric Cancer: Results from the Health Examinees Study. Eur. J. Nutr. 2023;62:1743–1753. doi: 10.1007/s00394-023-03115-x. [DOI] [PubMed] [Google Scholar]
- 30.Kim S.R., Kim K., Lee S.A., Kwon S.O., Lee J.-K., Keum N., Park S.M. Effect of Red, Processed, and White Meat Consumption on the Risk of Gastric Cancer: An Overall and Dose-Response Meta-Analysis. Nutrients. 2019;11:826. doi: 10.3390/nu11040826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lúóng K.V.Q., Nguyễn L.T.H. The Role of Thiamine in Cancer: Possible Genetic and Cellular Signaling Mechanisms. Cancer Genom. Proteom. 2013;10:169–185. [PubMed] [Google Scholar]
- 32.Qian B., Shen S., Zhang J., Jing P. Effects of Vitamin B6 Deficiency on the Composition and Functional Potential of T Cell Populations. J. Immunol. Res. 2017;2017:2197975. doi: 10.1155/2017/2197975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available upon request from the corresponding author due to ethical reasons.