Simple Summary
Rhynchophorus ferrugineus is a noxious curculionid species found in date palm and coconut plantations worldwide. Its overall cryptic nature inhibits early detection of infestation symptoms and allows for its rapid expansion. Control methods in plantations usually include single-mode broad-spectrum chemical insecticides to both prevent and mitigate infestations. However, ecological concerns about hazards of both ecosystem and public health, call for safer and more sustainable solutions, including attractants, agronomic approaches, natural enemies and entomopathogenic organisms. This review highlights the published information on the biological traits, host plant spectrum, and management options such as biotechnic and biological control methods like the application of microbial organisms.
Keywords: red palm weevil, life history, distribution, semiochemicals, natural enemies, microbial control agents, host plants
Abstract
Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae), the red palm weevil (RPW), is a concealed voracious pest of different ornamental and economically important palm species, particularly the date palm. It can cause huge losses in ornamental and commercial palm plantations. RPW has spread rapidly from its original distribution in Southeast Asia to date palm-growing countries worldwide. It is now established in more than 50% of date palm-growing countries and 15% of the coconut-growing countries globally. To prevent further expansion of this pest, many countries have implemented strict legislative and quarantine measures surrounding the export of the palms plant genetic resources from RPW-infested countries. This review focuses on the general biology (life history, development), host range, geographical distribution, and management on the basis of biotechnic methods, farming practices, natural enemies, and important microbial control agents.
1. Introduction
Insect invasion is highly damaging to natural, urban, and agricultural areas, causing not only ecological damage but also economic losses of several million dollars (USD) per year related to the cost of control efforts to reduce populations to lower levels [1,2,3]. The red palm weevil (RPW), Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) is a serious invasive pest of high economic importance that invades the tissues of several palm species globally [4,5]. The historical evidence showed that this pest originated from South and Southeast Asia [6] and infested coconut [7], and also date palms in Mesopotamia (Iraq) [8] but was not recognized as having prominent pest status on date palms until the mid-1980s in the Middle Eastern region [9,10]. RPW mainly infested Phoenix canariensis, in the Mediterranean Basin of the Canary Islands, slowly during the mid-1990s and very quickly since 2004; consequently, a mass-eradication campaign was carried out to overcome this pest [11]. Most individuals of this pest are short-distance flyers covering < 500 m, and this might be the reason for the appearance of hot spots [12]. The invasion potential of RPW is due to the increased fecundity of females, which can produce multiple generations per year as a multivoltine species. Its development takes place inside the host palm delaying early detection of this pest, and the high flight capacity of adults can cover long distances [13,14,15,16,17]. However, a major factor in its spread has been the movement of infested palm material into uninfested areas [16].
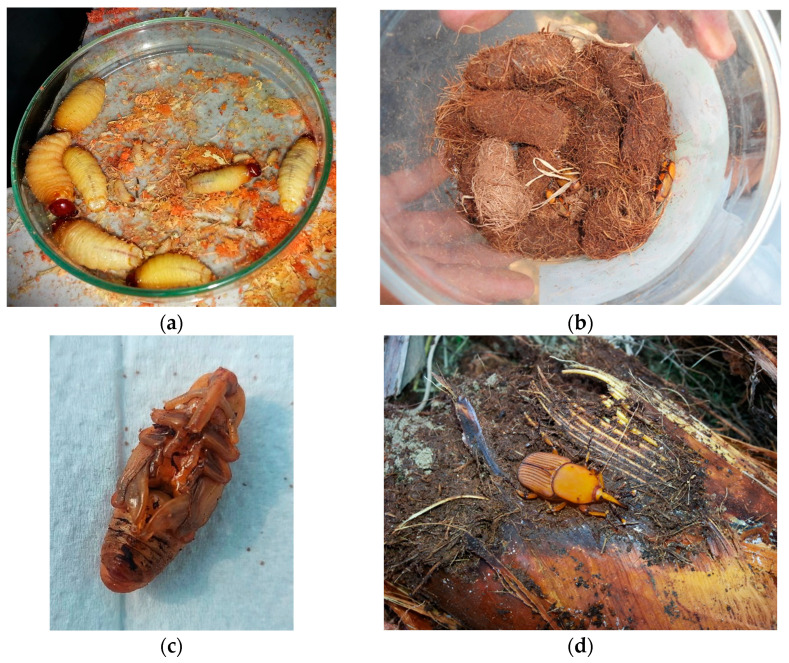
RPW has emerged as the largest weevil within the fauna of Europe and North Africa [18]. The physical dimensions of RPW vary significantly, ranging from 1.5 to 4.0 cm in length (from the tip of the rostrum to the end of the abdomen) and from 0.7 to 1.5 cm in width [18], with males being approximately 10–15% smaller and lighter than females [19]. Typically, the length of individuals in the Mediterranean region is about 3 cm [18]. Adults display variable coloration, often characterized by a reddish-brown thorax with varying degrees of distinct black mottling, as well as black and orange stripes on the elytra (Figure 1d) [20]. Male weevils can be identified from females by the presence of a tuft of reddish-brown hairs along the dorsal side of the snout which is shorter and thicker compared to that of females [18]. Additionally, the front tibiae show sexual dimorphism, with males having a comb-like brush of tightly packed long hairs, while females have only sparse hairs [18,21]. Adults have robust wings that allow them to perform extended flights [11]. Several studies using the flight mill technique have shown the remarkable ability of RPW to fly long distances. For instance, Hoddle et al. [22] recorded that 3 of 192 RPW individuals tested flew a maximum distance of 60–67 km in 24 h, whereas the maximum flight distance of RPW in 6 hours was 23.6 km [13]. The eggs are elongated with a length of 0.25 cm, whitish color, and smooth chorion [18]. The larvae are apodous, yellowish or whitish in color, up to 50 mm long. The head color ranges from russet-red to brown, with strong mandibles (Figure 1a) [23,24]. Full-bodied larvae create a cocoon composed of palm fibers and saliva (Figure 1b,c) where they pupate [25]. Pupae change color from cream to brown, and the average size is approx. 4.5 cm [21].
Figure 1.
Rhynchophorus ferrugineus: (a) larvae, (b) cocoons created by pupating larvae, (c) pupa, (d) adult male on date palm tree.
Palm mortality occurs as a result of larvae feeding internally [14,26,27,28]. The whole life cycle of larvae remains cryptic inside the palm, posing challenges for the early detection of infestations [26,27]. In addition, the larvae are protected from external factors that can cause their death, such as insect pathogens, natural enemies, and insecticides [29]. The feeding activity of larvae over 1 to 2 years can lead to the death of infested palm trees by causing severe damage to apical growing areas and/or resulting in trunk collapse [27,30,31].
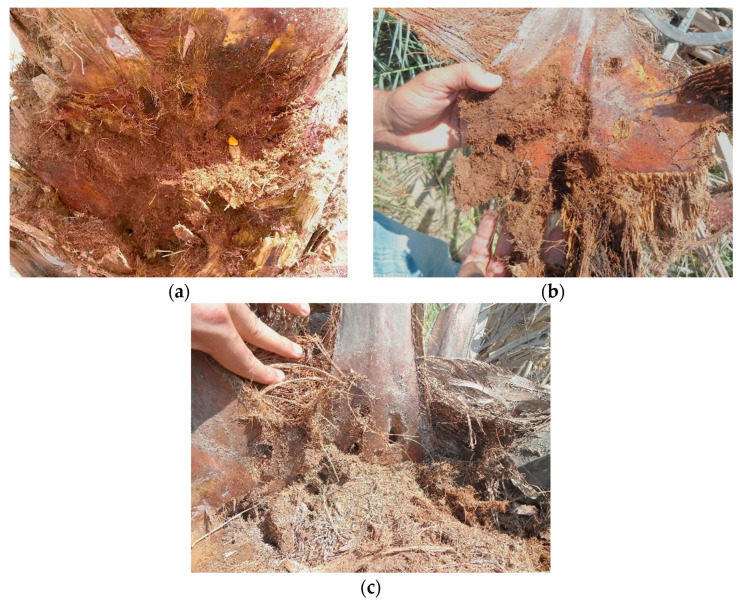
RPW adults are usually attracted to unhealthy and damaged plants from pruning or chainsaw wounds [23]. Fermenting sap of injured trees releases a chemical that attracts RPW to palms [32]. Also, due to the gregarious behavior of the RPW males [33], a pheromone comprising of two compounds, ferrugineol (4-methyl-5-nonanol) and ferrugineone (4-methyl-5-nonanone), induces aggregation [34]. Infestations in Phoenix dactylifera L. (Arecales: Arecaceae) are often recorded in younger palms (<20 years old plants) at approximately 1 m on the trunk, above the soil [18,23,35,36]. Detection of early infestation is challenging as symptoms typically manifest long after the infestation has occurred. Consequently, significant feeding damage inflicted by numerous larvae usually leads to the demise of the palm before symptoms become apparent [37]. Detailed descriptions of symptoms of feeding damage on date palm trees have been provided in the literature [35,37]. Damaged trees include the following symptoms: (i) tunnels at the base of the petiole (Figure 2b,c) and along the trunk (Figure 2a), (ii) audible gnawing sounds caused by grub feeding, (iii) discharge of brownish viscous liquid and chewed fibers emerging from small holes in the stem and crown, (iv) the occurrence of frass (chewed plant tissues), emitting a characteristic fermented odor around the tunnel openings, and (v) the presence of discarded empty pupal cases and dead adults around an infested palm [14,35,37,38,39,40]. Therefore, possessing a comprehensive understanding of the diverse active feeding symptoms exhibited by infested palms is crucial for promptly identifying RPW infestation [40].
Figure 2.
Feeding damage symptoms in date palm trees caused by Rhynchophorus ferrugineus: (a) severe internal damage at the base of the palm trunk, with visible tissue decay and tunneling caused by larval feeding, (b) cross-section showing extensive frass accumulation and damage to the internal fibers of the fronds, and (c) frond base exhibiting boreholes and internal damage, indicating the presence of larval tunnels.
Various methods are available or have been tried for the early detection of RPW: (1) Detection of infested palms by visual inspection is still a common practice [37]. Regular inspection of susceptible palm trees (mostly < 20 years) [14,41] at 1.5-month intervals is a useful tool to restrict the accumulation of RPW in the field since infestations are located prior to adult emergence, which takes approximately 45 days from egg to adult [13]. (2) Training dogs to smell specific chemical signals emitted by RPW-infested date palms is a potential method for early detection [42,43]. For instance, Nakash et al. [44] validated the capability of Golden Retriever dogs to effectively detect the oozing secretion extracted from RPW-infested date palms by sniffing. However, Soroker et al. [43] suggested that the use of dogs for RPW detection purposes could also be appropriate for palm inspection at quarantine facilities, nurseries, and ports of entry. (3) Acoustic methods have proven effective for the detection of both adult and larval stages of RPW within palm trees [45,46,47]. Several researchers have extensively investigated the acoustic activity of RPW, determining that the sound emitted by the weevil can be distinguished and separated from ambient noises and other insect sounds [48,49,50,51]. Pinhas et al. [52] devised a mathematical approach for the automated detection of RPW acoustic activity in plant offshoots. The Laar WD 60 Pro CSC measuring device was regarded as the most optimal and straightforward choice for detecting subtle sound vibrations generated by RPW activity during the initial stages of infestation [32,53]. The detection of RPW during the initial stages of infestation using a bioacoustic sensor developed by Rach et al. [54], proved to be very effective, achieving 90% success, despite the ambient sounds. Martin et al. [51] found that the sound spectrum of RPW larvae remains consistent during biting and chewing actions but varies during the insect’s locomotion. The IoTree Smart acoustic sensor was assessed for its ability to detect sound signals emitted by RPW larvae inside oil palm and coconut trees in Malaysia [55]. This sensor detected the presence of the pest in both plant species. Using data mining, RPW infestations can be predicted with an accuracy up to 93%; however, temperature and tree trunkcircumference are the most important features for this prediction [56].
Since there is a lack of review information dealing with the RPW biology from a biotechnic/biological control point of view, we focused this review on recent information obtained from the available global published literature on the following topics: affected plants, global distribution, life history traits, and different biological management methods (i.e., semiochemicals, agronomic practices, natural enemies, entomopathogens).
2. Host Range
RPW is a polyphagous pest that has been recorded to attack more than 40 palm species worldwide [14,46,47,48,49,50,51,52,53,54,55,57,58,59,60], causing widespread devastation of date palms grown in the Canary Islands, the Mediterranean, Middle East and North Africa [14,27,61]. Table 1 presents the plant species affected by RPW.
Table 1.
List of host plants of Rhynchophorus ferrugineus.
| Host Species | Order | Family | References |
|---|---|---|---|
| Areca catechu L. | Arecales | Arecaceae | [62,63,64] |
| Arenga pinnata (Wurmb) Merr. | Arecales | Arecaceae | [26,37,63,65,66] |
| Bismarckia nobilis Hildebr. & H.Wendl. | Arecales | Arecaceae | [37] |
| Borassus flabellifer L. | Arecales | Arecaceae | [37,65,66] |
| Brahea armata S.Watson | Arecales | Arecaceae | [37,66] |
| Butia capitata (Mart.) Becc. | Arecales | Arecaceae | [37,66] |
| Calamus merrillii Becc. | Arecales | Arecaceae | [37,66] |
| Caryota cumingii Lodd. ex Mart. | Arecales | Arecaceae | [37,63,66] |
| Caryota maxima Blume ex Mart. | Arecales | Arecaceae | [37,63,66] |
| Chamaerops humilis L. | Arecales | Arecaceae | [37,57,66,67,68] |
| Cocos nucifera L. | Arecales | Arecaceae | [27,37,63,66,69,70] |
| Corypha umbraculifera L. | Arecales | Arecaceae | [65] |
| Corypha utan Lamk. | Arecales | Arecaceae | [37,63] |
| Dictyosperma album (Bory) H. Wendl. & Drude ex Scheffer | Arecales | Arecaceae | [37,71] |
| Elaeis guineensis Jacq. | Arecales | Arecaceae | [26,27,65,66,72,73] |
| Howea forsteriana Becc. | Arecales | Arecaceae | [37,66] |
| Jubaea chilensis (Molina) Baill. | Arecales | Arecaceae | [37,66] |
| Livistona chinensis (Jacq.) R.Br. ex Mart. | Arecales | Arecaceae | [26,65,73] |
| Livistona decora (W. Bull) Dowe | Arecales | Arecaceae | [26,37] |
| Livistona saribus (Lour.) Merr. ex A.Chev. | Arecales | Arecaceae | [37] |
| Metroxylon sagu Rottb. | Arecales | Arecaceae | [4,26,37,65,66] |
| Oncosperma horridum (Griff.) Scheff | Arecales | Arecaceae | [37] |
| Oncosperma tigillarium (Jack) Ridl. | Arecales | Arecaceae | [37] |
| Phoenix canariensis Chabaud | Arecales | Arecaceae | [11,18,26,37,66,74,75] |
| Phoenix dactylifera L. | Arecales | Arecaceae | [18,26,37,65,66,70,74,75,76,77] |
| Phoenix sylvestris (L.) Roxb. | Arecales | Arecaceae | [37,65,75,78] |
| Phoenix theophrasti Greuter | Arecales | Arecaceae | [30,37,66] |
| Pritchardia pacifica Seem. & H.Wendl. | Arecales | Arecaceae | [37] |
| Roystonea regia (Kunth) O.F.Cook | Arecales | Arecaceae | [37] |
| Sabal palmetto (Walt.) Lodd. | Arecales | Arecaceae | [37] |
| Saccharum officinarum L. | Poales | Poaceae | [37] |
| Strelitzia nicolai Regel & Körn. | Zingiberales | Strelitziaceae | [37,79] |
| Syagrus romanzoffiana (Cham.) Glassman | Arecales | Arecaceae | [37,80] |
| Trachycarpus fortunei (Hook.) H. Wendl. | Arecales | Arecaceae | [37,66] |
| Washingtonia filifera (L. Lindl) | Arecales | Arecaceae | [37] |
| Washingtonia robusta H.Wendl. | Arecales | Arecaceae | [37] |
3. Geographic Distribution
RPW is a destructive pest of the date palm and has been detected in more than 50% of date-producing countries and 15% of coconut-producing countries [14]. It was first identified outside its native range in Japan in 1975 [81]. Originating from Southeast Asia, during the 1980s, RPW was recognized as an important pest of dates in the Middle East region, showing rapid spread to other countries through the trade of infected ornamental plants [14,82,83]. RPW expanded to the Arabian Peninsula and into the eastern Mediterranean and Spain by the 1990s [18]. It has since been found in more distant regions, including the Canary Islands, Madeira, the Caribbean, Taiwan, and China. By the year 2015, RPW had been reported in 18 of the 21 Mediterranean coastal countries [32,37].
According to the database of the European and Mediterranean Plant Protection Organization [37], in Africa, the RPW is present in Djibouti and widespread in Egypt, and in Tunisia with restricted distribution, while in Libya, Mauritania, and Morocco it is present with few occurrences. The severe outbreak of RPW in the oasis regions of the above-mentioned countries may lead to social issues due to religious and cultural significance [84,85,86]. RPWt is found in Central America, Caribbean, present in the Netherlands and Antilles with few occurrences, and has restricted distribution in Aruba and Guadeloupe. In Asia, it is widespread in India and Saudi Arabia; present in Bahrain, Bangladesh, Cambodia, China, Iran, Kuwait, Lebanon, Myanmar, Oman, Pakistan, Philippines, Qatar, Sri Lanka, Syria, Taiwan, Thailand, United Arab Emirates, and Vietnam. It is also present in Israel, Japan, Malaysia, Yemen with restricted distribution, and Iraq, and Jordan with few occurrences. In Europe, RPW is present in Albania, Georgia, and Malta, is transient in Bulgaria, and has restricted distribution in Bosnia and Herzegovina, Croatia, Cyprus, France, Greece, Italy, Montenegro, Portugal, Russia, Spain, and Turkey. Currently, according to the European and Mediterranean Plant Protection Organization (EPPO), RPW has been a quarantine pest in Israel since 2009, in Tunisia since 2012, Morocco and Mexico since 2018, and China since 2021 [37]. In addition, RPW has been categorized in the EPPO A1 list (pests are absent in the region of EPPO) in Brazil and Georgia since 2018, and United Kingdom since 2020, as well as on the EPPO A2 list (pests are present locally in the region of EPPO) in Bahrain since 2003, Jordan since 2013, Turkey since 2016, and Egypt and Iran since 2018.
4. Life History
Life history studies of RPW have been conducted by several authors in different countries, such as Iran, Philippines, India, Spain, Myanmar, and Indonesia [29,87]. RPW can mate throughout the day, with males and females participating in multiple matings during their lifetime [37,88,89]. RPW undergoes four developmental stages, namely, egg, larva, pupa, and adult to complete its life cycle. The female oviposits separately elongated shiny creamy-white colored eggs (2.5 mm × 1 mm) into excavated holes of a suitable host material which are eventually covered with a dry secretion [86,90]. The daily egg-laying ability of the female decreases with time [75]. A newly hatched larva measures only 5 mm × 2 mm and weighs about 1 mg, while the final larval instar can reach 5 cm × 2 cm and weigh between 4 and 7 g before pupation (Figure 1a). The final larval instar forms a barrel-shaped cocoon made from palm tree fibers measuring 7 cm × 4 cm, while the pupa inside is 3.5 cm × 1.5 cm [86] (Figure 1b,c). Mating duration ranges from 60 to 300 seconds [89], and females use the sperm from their most recent mating to fertilize their eggs [88]. Females begin oviposition within 1 to 11 days after copulation depending on temperature [91], typically selecting tender parts with small scooped holes in the young palm trees (below the age of 20 years [69]), whereas, in mature trees, egg deposition occurs in exposed plant tissues, wounds, petioles, and injuries resulting from another coleopteran pest, Oryctes rhinoceros (L.) (Coleoptera: Scarabaeidae) activity or diseases [14,73]. The number of eggs a female can lay in its lifetime ranges from 3 to 531 (mean = 250) [27,92].
Larval hatch occurs after 4 to 7 days [91]. The newly emerged apodous larvae remain exposed to the external environment for a few hours and then enter the trunk, creating tunnels by chewing the trunk tissues [88] (Figure 2). The larvae’s abdominal muscles occasionally contract, which helps them move further into the trunk [88]. Different studies have revealed considerable variation in larval development periods and number of instars [11]. The duration of the developmental period is influenced by factors such as temperature and feeding host, varying between 24 and 210 days [5,11,88]. Seven larval instars were reported by Esteban-Durán et al. [93] when RPW was reared on sugarcane, while Martín and Cabello, [94] recorded 11–17 instars when reared on the same substrate. As the larvae progress through each subsequent instar, their appetite increases, and they primarily consume the soft tissues that surround the apical meristem. During the final larval instar, they migrate to the periphery to pupate, creating a cocoon composed of chewed fibers [88] (Figure 1). The duration of the pupal period can range from 20 to 25 days [95].
After the adults emerge, they typically stay within the cocoon for a duration of 4 to 17 days, with an average period of 8 days [21], possibly to complete sexual maturation [21]. Regardless of sex, adult individuals live for a period of 2 to 3 months [37]. However, host plants significantly contribute to the wide range of adult lifespan durations, acting as one of the main factors influencing growth periods [96]. Due to overlapping generations of RPW, the pest can be found throughout the year on infested palms. The insect can complete three to four generations within a single palm tree over the course of a year [6]. However, in a study by Salama et al. [97], 21 generations were recorded per year in Egypt. It has been proposed that one pair of RPW could potentially generate over 53 million offspring across four successive generations if control factors are absent [14]. Since there are no complex behaviors required for mating of RPW and due to the similarity of variation in genitalia among the genera, interspecific mating (between Rhynchophorus spp.) is possible [36].
5. Management
5.1. Biotechnic: Semiochemicals/Trapping
The use of traps baited with aggregation pheromones represents a promising method for capturing and killing the RPW [7]. The current management approach for RPW involves aggregation pheromones used for both monitoring and mass trapping [60,98,99,100]. In general, for weevils, semiochemical-based trap systems consist of three main parts: a trap, an aggregation pheromone, and a co-attractant (kairomone) [101]. Under natural conditions, males of RPW secrete the aggregation pheromone consisting of ferrugineol (i.e., 4-methyl-5-nonanol) and ferrugineone (i.e., 4-methyl-5-nonanone) at a 9:1 ratio, with ferrugineol comprising the primary component [102]. The aggregation pheromone instigates synchronized mass attacks of RPW adults against the tree host, which frequently results in the collapse or demise of the palm [102,103]. Males and females are attracted to the aggregation pheromone, with a notable preference for females, which makes it extremely advantageous for the mass trapping method. The sex ratio of captured RPW has been reported to be 1 male: 2 females [14,76,104,105,106]. Furthermore, it has been found that most RPW individuals captured in the traps are young and fertile, which suggests a significant reduction in the local population due to this method [35,107]. Both components of the RPW aggregation pheromone have been produced in a commercial lure called Ferrolure+, while the main component ferrugineol is commercially produced with the name Ferrolure [108].
Several research efforts have shown that RPW is highly attracted to ferrugineol, especially when a food source is mixed with the pheromone [35,109]. Studies have shown that natural palm baits exhibit weak attractive properties on their own, but can greatly enhance the effectiveness of the RPW aggregation pheromone [9]. In addition, the palm esters ethyl butyrate, ethyl isobutyrate, ethyl acetate, and ethyl propionate are among the volatile compounds released from fermented tissues of various hosts to which RPW responds, as has been determined by electroantenographic (EAG) bioassays [110,111,112]. For instance, RPW captures were doubled when a 1:3 mixture of ethyl acetate and ethanol was used with the aggregation pheromone compared to the pheromone alone [111]. Abdel-Azim et al. [113] reported that the efficacy of Ferrolure+ was enhanced with the addition of ethyl acetate. In Table 2 we review in detail the attractants and food baits used to capture RPW. After RPW adults enter the pheromone trap, it is important to prevent their escape by immobilizing or killing them with an insecticide mixed into the bait [27,113,114,115].
Table 2.
Attractants and food baits used for the capture of Rhynchophorus ferrugineus.
| Substances | Conditions | Target Plantation | References |
|---|---|---|---|
| 1 Ferrolure+ + food baits (ripe date fruits or date palm stems) | Field | Date palm | [116] |
| Male aggregation pheromones + food baits | Field | Date palm | [117] |
| 2 Ferrugineol + date fruits | Field | Date palm | [76] |
| Ferrugineol + pineapple fruit | Field | Coconut | [109] |
| Ferrugineol + sago palm stem | |||
| Ferrugineol + sugarcane stem | |||
| Yellow funnel type trap + Pherodis | Field | Palm trees | [118] |
| Yellow pitfall trap + Pherodis | |||
| Ferrolure+ + ethyl acetate | Field | Canary Palm | [119] |
| Ethyl acetate | Field | Date palm | [120] |
| Ferugineol + sugar beet molasses | Field | Date Palm | [101] |
| Male aggregation pheromones + water or paraffin | Field | Date Palm | [121] |
| Ferrolure+ | Field | Date Palm | [122] |
| Male aggregation pheromone + food baits (sugarcane or pineapple or coconut fruit or oil palm petiole) + ethanol + ethyl acetate +water | Field | Coconut | [123] |
| Ferrolure+ | Field | Date Palm | [106] |
| Ferrugineol + fermenting date fruits+ ethyl acetate | Field | Date Palm | [124] |
| Ferrolure+ + ethyl acetate + different trap colors | Field | Date Palm | [125] |
| Ferrugineol + ethyl acetate + food bait (sugar cane) | Field | Coconut | [126] |
| Ferrolure+ + date fruits | Field | Date palm | [99] |
| 3 Rhylure-700 + date fruits | |||
| Ferrugineol + ethyl acetate | Field | Date Palm | [127] |
| Pineapple or sugar cane or coconut or oil palm | Field | Date Palm | [128] |
| Ethyl acetate or ethyl butyrate or ethyl propionate or ethylene glycol | |||
| Ferrugineol + aqueous solution of sugar beet + ethyl acetate + ethyl propionate | Field | Date Palm | [129] |
| Ferrolure++ sugar beet molasses + ethyl acetate | Field | Canary Island Palms | [130] |
| Ferrolure+ + ethyl acetate + sugarcane sticks | Field | Coconut | [131] |
| Ferrolure+ + ethyl acetate + date fruits | Field | Date Palm | [132] |
| Ferrugineol + date palm tissue | Field | Date Palm | [133] |
| Ferrolure+ + ethyl acetate + date fodder | Field | Date Palm | [134] |
| Ferrugineol + ethyl acetate | Field | Date palm | [135] |
| Ferrolure+ + date fodder | Field | Date Palm | [136] |
| 1 Rhyfer 700 | Field | Date Palm | [137] |
| 3 Pherocon RPW lure | |||
| 1 Ferrugitom 700 | |||
| 1 Weevil lure | |||
| Ferrolure+ | |||
| Ferrugineol + food baits (date fruits or palm fronds) | Field | Date Palm | [138] |
| Ferrugineol | Laboratory, Field | Coconut | [139] |
| Ferrugineol + fermented date fruits solution | Laboratory, Field | Date Palm | [35] |
1 4-Methyl-5-nonanol (9 parts) + 4-methyl-5-nonanol (1 part). 2 4-Methyl-5-nonanol. 3 4-Methyl-5-nonanol (31.5%) + 4-Methyl-5-nonanone (3.5%).
The location of pheromone traps is a critical factor for enhancing their efficiency [99]. For instance, Hallett et al. [140] reported that traps placed at ground level captured a significantly greater number of RPW adults than those placed at 5 m; however, the effectiveness of traps at 2 m did not differ significantly from the aforementioned heights. More recently, Al Ansi et al. [99] documented that traps positioned in shaded areas with relatively high soil moisture caught a greater number of RPW compared to traps positioned in the sun. In addition, color has been reported to affect the effectiveness of traps [119,140,141,142,143,144,145]. Hallett et al. [140] reported a higher number of RPWs were captured in black traps than in white traps, whereas Al-Saoud et al. [145] caught significantly more RPWs in red than in white or yellow traps. Additional research efforts revealed that black traps were more effective than brown, red, yellow or white traps [119,141,142].
5.2. Agronomic Methods
Different farming practices are imperative for managing the population of RPW, its subsequent population build-up and attack on new plantations [7,91]. In 2011, RPW infestation in Al-Ahsa Oasis were related to how farmers used three different watering methods: basin, flood, and drip. The results showed noticeable differences, with maximum (88%) RPW infestation occurring under flood irrigation followed by 9.6% and 2.4% with basin and drip irrigation, respectively [86,146]. Additionally, removing offshoot and fronds resulted in 79% weevil infestation because; 6- and 10-year-old palms were highly susceptible. In addition, the treatments for cryptic hidden breeding sites are also crucial for controlling this pest; particularly if it is found in closed gardens and other locations that are difficult to access [147]. These factors should be considered for future RPW management programs when implemented over a wide area since management on individual farms is less effective because weevils readily immigrate from neighboring fields.
Using a novel method for the surveillance of RPW-infested palms based on street-level imagery data is a new technique for the pest’s early management [148]. The application of historical aerial photos, remote sensing images, and field surveys, integrated in a GIS environment showed that the exponential increase of RPW populations is correlated to the spatial spread model [149]. Removal of infected damaged or fallen palm trees along with trimming of dead fronts also prevents the spread of this pest by eliminating the breeding sites [6,150].
5.3. Biological Control
A vast array of RPW natural enemies (insects, vertebrates, and mites), and microbial control agents, entomopathogenic fungi, entomopathogenic nematodes, entomopathogenic bacteria and entomopathogenic viruses have been reported from many countries of the world [151,152]. But their implementation under field conditions has not been very successful against RPW due to its cryptic behavior.
5.3.1. Natural Enemies
The predatory and parasitic potential of insects from various orders has been well-documented against a huge variety of insect pests for centuries [151]. Several species of RPW natural enemies belong to the orders Heteroptera, Hymenoptera, Dermaptera, Coleoptera, and Diptera [152]. Platymeris laevicollis Distant (Hemiptera: Reduviidae) is a predator of Oryctes rhinoceros (L.) (Coleoptera: Scarabaeidae), which was reported to attack RPW [153]. In India, a common predator of RPW is Chelisoches morio (F.) (Dermaptera: Chelisochidae), which has been recorded to attack eggs and larvae of RPW in coconut tree crowns [154]. In Sicily, Euborellia annulipes (Lucas) (Dermaptera: Anisolabididae) was found in infested palm trees with RPW, and under laboratory conditions, demonstrated that it can predate on eggs of RPW [151,155]. Additionally in Sicily, a native parasitoid Megascolia flavifrons (Fabricius) (Hymenoptera: Scoliidae) has been recorded in infested palms but more studies on its biology are still needed to assess its potential as a biocontrol agent used against the different RPW life stages [151]. A few dipteran species belonging to Tachinidae and Sarcophagidae families also prey on Rhynchophorus spp. [152]. Lastly, Billaea maritima (Schiner) (Diptera: Tachinidae), a parasitoid of cetonid beetles, has been observed parasitizing RPW pupae in Sicily [156].
There are reports of some vertebrates (mammals and birds) feeding on RPW life stages. Two palm-dwelling mammals, Apodemus sylvaticus (L.) (Rodentia: Muridae) and Rattus rattus (L.) (Rodentia: Muridae), have been reported to eat RPW larvae, pupae and adults [151]. Concerning avian predators, Dendrocitta vagabunda parvula (Whistler and Kinnear) (Passeriformes: Corvidaewas) was found to prey on adults of RPW [156]. Another avian predator, Pica pica L. (Passeriformes: Corvidae), was reported to feed on RPW in Italy [157]. There are also reports of Turdus merula L. (Passeriformes: Turdidae) and Falco tinnunculus L. (Falconiformes: Falconidae) as potential avian predators of RPW life stages [158].
Mites and RPW have phoretic associations in which the mites are actively carried on the beetle body for a short period to accomplish their dispersal strategy in favorable environments [155,159,160]. These phoretic mite species belong mainly to suborder Uropodina [161,162,163,164]. So far, 25 species of mites belonging to 21 genera in 18 families have been reported to be associated with RPW [87]. Among these mites, 21 identified species (84%) belong to the order Mesostigmata followed by orders Trombidiformes (12%) and Sarcoptiformes (4%) [87]. Regarding their parasitization habit, experiments revealed that Centrouropoda almerodai Hiramatsu & Hirschmann (Mesostigmata: Uropodidae) reduced their host’s lifespan to 1.4 times compared to the uninfested RPWs [155]. Facultative parasitic mites Aegyptus rhynchophorus (Elbishlawi and Allam) and A. alhassa (Al-Dhafar and Al-Qahtani) (Mesostigmata: Trachyuropodidae) have been suggested as potential agents to control RPW in the field since these mites were found to feed on different developmental stages of RPW [165,166,167,168]. Apart from predation, the sheer number of phoretic mites inhabiting the RPW’s body can hinder its foraging efficiency, rendering it more vulnerable to predation [87,159]. Furthermore, a significant mite infestation can impede the host’s ability to fly and move, potentially leading to exhaustion or even death [169]. An interesting observation is the presence of more phoretic mites in male RPW than the females [87]. This fact might be attributed to male-biased association [87].
5.3.2. Microbial Control Agents
The use of microorganisms to control insect pests is an alternative to the use of single-mode synthetic chemical insecticides. Naturally occurring biological control agents have a high degree of host specificity, low environmental toxicity, self-persistence, and minimal non-target effects. The control of RPW by using such environmentally friendly biocontrol agents would be welcomed in many date-producing regions.
Entomopathogenic Fungi (EPF)
Entomopathogenic fungi play a role in regulating the population of insects in natural environments and typically pose no harm to the environment, humans, and most importantly, non-target organisms [170,171]. EPF are widespread in both forest ecosystems and agricultural settings, commercially available, and used effectively in the management of several insect pests, including RPW [172,173,174]. Extensive research has been conducted in the laboratory to assess the effectiveness of EPF against RPW [175,176,177]. Various aspects of EPF have been examined for different species, i.e. doses, strains, or application methods to determine their impact on the infection rate, field efficacy, population decrease, and mortality of RPW [58,178,179,180]. Furthermore, eggs, larvae, and adults of RPW have been assessed to determine the virulence and pathogenicity of EPF [176,177,181,182,183]. Numerous EPF species have been utilized, with different strains and isolates demonstrating notable efficacy and virulence against RPW under laboratory conditions [176,179,180,181,184]. In the field, Beauveria bassiana (Balsamo Crivelli) Vuillemin (Hypocreales: Cordycipitaceae) and Metarhizium anisopliae (Metschnikoff) Sorokin (Hypocreales: Clavicipitaceae) are commonly used in IPM programs against RPW [47,151,175,185,186,187]. For instance, B. bassiana significantly reduced the RPW population under field conditions [188]. In the field, B. bassiana and M. anisopliae may become an integral part of successful IPM programs targeting larvae of RPW by injecting suspensionof both fungi into infested trees targeting larvae [47,151,175,185,186,187,189]. A comprehensive list of microbial agents used for the management of RPW is provided in Table 3.
Entomopathogenic Nematodes (EPNs)
Over the past two decades, there has been a considerable interest in using EPNs as biological control agents against harmful insect pests [190]. EPNs, such as those from the genera Steinernema and Heterorhabditis, are effective for the management of numerous agricultural insect pests [191,192]. The third juvenile stage of EPNs, known as the infective juvenile (IJ), lives freely in the soil, harboring endosymbiotic bacteria responsible for killing their hosts. After the individual penetrates the host through natural body openings, it releases symbiotic bacteria into the host’s haemocoel. Then, the bacteria rapidly multiply and produce lethal toxins, capable of killing the host within 48 hours. Afterwards, the IJs feed on the surplus of bacterial cells, develop into adults, reproduce and when the resources are depleted, their progenies evacuate the cadaver [193]. These EPNs, along with their symbiotic bacteria are target-specific, minimizing harm to non-target organisms [193,194]. Numerous studies have evaluated the effectiveness of commonly used EPNs in both laboratory and field settings, around the Mediterranean basin, the Middle East, and southern Asia against RPW [190,194,195,196,197,198,199,200] (Table 3). In laboratory experimental efforts against RPW, several EPN species have been tested against different life stages of this pest and provided high mortality rates [194,197,199]. In most laboratory cases, Steinernema carpocapsae (Weiser) (Rhabditida: Steinernematidae) and Heterorhabditis bacteriophora Poinar (Rhabditida: Heterorhabditidae) cause high mortalities depending on the life stage of the host. Within addition to causing high mortality in RPW larvae, the use of EPNs can also cause a reduction in their feeding/foraging behavior, thus hindering growth and overall development of the adult stage. Additionally, negative effects on the fecundity of RPW adult beetles have been observed and subsequently RPW population growth is negatively affected over time [201]. In field and semi-field settings, S, carpocapsae, Steinernema feltiae (Filipjev) (Rhabditida: Steinernematidae), and H. bacteriophora are usually the most studied species, in regard to controlling RPW infestations [195,196,197]. Specifically, in field research settings conducted by Abbas et al. [195], larval mortality did not exceed 66,7%, among the ten local species/strains that were used. Accordingly, most of the EPN species did not manage to suppress the life stages of the RPW pest. The decrease in efficacy of EPNs can be attributed to environmental conditions, such as temperature range, as well as RPW behavior, i.e., tunneling and excessive sap production, hindering the foraging ecology of the EPNs. However, Santhi et al [198] recorded variable mortality rates in a study simulating a natural setting where the larval stages showed reduced susceptibility as their size increased, and adults or pupae were extremely susceptible to S. carpocapsae. One other important factor that determines the efficacy of an EPN application is the foraging behavior of IJs. For example, S. carpocapsae exhibited high activity in locating and infecting tunneling larvae. In order to minimize the impact of environmental constraints on EPN activity in field conditions, a chitosan adjuvant was used to protect S. carpocapsae, providing elevated mortality (>80%) in both preventive and curative procedures [196].
Entomopathogenic Bacteria (EPB)
EPB that have primarily been used to combat RPW belong to the families Enterobacteriaceae, Streptococcaceae, Pseudomonadaceae, and Bacillaceae [202]. The insecticidal activity of EPB originates from metabolic products that procure severe symptoms in their hosts upon infection. Notably, species from the genera Photorhabdus, Xenorhabdus, Bacillus, Pseudomonas, and Serratia have extensively been studied for their insecticidal properties against important agricultural pests. They infect their target and produce secondary metabolites, like enzymes and toxins that cause a variety of negative effects, including development inhibition, antifeedant behavior, and most importantly, mortality in all developmental stages [203,204,205,206,207,208,209,210,211]. In the case of RPW, Francesca et al. [212] documented the application of a highly effective Bacillus thuringiensis Berliner (Bacillales: Bacillaceae) strain in field trials, achieving mortality rates between 70 and 85%. The authors highlighted this strain as a key and impactful component of IPM strategies against RPW. Francesca et al. [212] efficacy finding corroborates with the results of Almasoudi et al. [213] after they tested the three isolated strains of Serratia marcescens Bizio (Enterobacteriales: Yersiniaceae), Klebsiella pneumoniae (Schroeter) Trevisan (Enterobacteriales: Enterobacteriaceae) and B. thuringiensis against RPW larvae (Table 3). Interestingly, only B. thuringiensis provided 100% mortality [213]. Previously Pu et al. [214] reported an extension of egg hatching time, high mortality rates of B. thuringiensis against second and fourth instar larvae of RPW, and a reduction in the observed boring activity of treated larvae.
Entomopathogenic Viruses (EPVs)
The only EPV found in RPW is the highly potent cytoplasmic polyhedrosis virus (CPV). The CPV was first discovered in Kerala, India, where it infected all stages of the RPW [151]. Salama et al [215] suggested the use of this pathogen as part of a biological control strategy would not be efficient, mainly due to its low virulence. However, a recent study result exhibited a high efficacy of CPV against larvae of RPW. Specifically, a viral dose of 80 million PIB (Polyhedral Inclusion Body)/larva is highly potent, resulting in 80-100% mortality against tested larvae. Results also showed that during the larval stage, mortality generally decreased with the increase in larval developmental stage, whereas from infected pupae no adults emerged or malformed adults appeared [216].
Table 3.
Entomopathogens used against Rhynchophorus ferrugineus.
| Microbial Control Agent | Target Developmental Stage | Conditions | References |
|---|---|---|---|
|
Bacillus amyloliquefaciens (ex Fukomoto) Priest et al. (Bacillales: Bacillaceae), B. cereus Frankland & Frankland, B. licheniformis (Weigmann) Chester, B. megaterium (de Bary) Gupta et al., B. pumilus Meyer and Gottheil, B. subtilis (Ehrenberg) Cohn, Lysinibacillus sphaericus (Meyer & Neide) Ahmed et al. (Bacillales: Bacillaceae) |
Eggs and larvae | Laboratory | [212] |
| Beauveria bassiana (Balsamo Crivelli) Vuillemin (Hypocreales: Cordycipitaceae), Metarhizium anisopliae (Metchnikoff) Sorokin (Hypocreales: Clavicipitaceae) | Eggs and larvae | Laboratory | [177] |
| Bacillus thurigiensis Berliner (Bacillales: Bacillaceae) | Eggs and larvae | Laboratory | [217] |
| Serratia marcescens Bizio (Enterobacterales: Enterobacteriaceae), Mammaliicoccus sciuri (Kloos et al.) Madhaiyan et al. (Bacillales: Staphylococcaceae), Klebsiella pneumonia ssp. pneumonia (Schroeter) Trevisan (Enterobacterales: Enterobacteriaceae), Proteus vulgaris Hauser (Enterobacterales: Enterobacteriaceae), P. mirabilis Hauser | Larvae | Laboratory | [217] |
| Steinernema carpocapsae (Weiser) (Rhabditida: Steinernematidae) | Larvae | Laboratory | [218] |
| B. bassiana, Cordyceps fumosorosea Kepler, B. Shrestha & Spatafora (Hypocreales: Clavicipitaceae) | Larvae | Laboratory | [179] |
| B. bassiana | Larvae | Laboratory | [47] |
| B. bassiana | Larvae | Laboratory | [178] |
| M. anisopliae | Larvae | Laboratory | [219] |
| Steinernema affine (Bovien) Wouts, Mracek, Gerdin & Bedding (Rhabditida: Steinernematidae), S. carpocapsae, S. feltiae (Filipjev) (Rhabditida: Steinernematidae) Heterorhabditis bacteriophora Poinar (Rhabditida: Heterorhabditidae) | Larvae | Laboratory | [197] |
| B. bassiana | Larvae | Semi-field | [47] |
| S. carpocapsae | Larvae | Laboratory | [200] |
| B. bassiana, M. anisopliae, H. bacteriophora | Larvae | Laboratory | [174] |
| B. thuringiensis, B. cereus | Larvae and adults | Laboratory | [61] |
| B. bassiana | Larvae and adults | Laboratory | [220] |
| B. bassiana, H. bacteriophora, B. thuringiensis serovar kurstaki | Larvae and adults | Laboratory | [221] |
| S. carpocapsae, H. bacteriophora, S. feltiae | Larvae and adults | Laboratory | [190] |
| Steinernema scapterisci Nguyen & Smart (Rhabditida: Steinernematidae), S. abbasi Elawad, Ahmad & Reid, S. glaseri (Steiner) Wouts, Mracek, Gerdin & Bedding, H. bacteriophora | Larvae and adults | Laboratory | [199] |
| S. glaseri, Steinernema arenarium (Artyukhovsky) Wouts, Mracek, Gerdin & Bedding (Rhabditida: Steinernematidae), S. carpocapsae, S. feltiae, S. riobravae Cabanillas, Poinar & Raulston, S. abbasi, S. ritteri Doucet & Doucet, S. kushidai Mamiya, Heterorhabditis spp. (Rhabditida: Heterorhabditidae) | Larvae, pupae, and adults | Laboratory | [222] |
| S. carpocapsae | Larvae, pupae, and adults | Semi-field and field | [223] |
| B. bassiana | Eggs, larvae, and adults | Laboratory | [58] |
|
B. bassiana, B. brongniartii (Sacc.) Petch (Hypocreales: Cordycipitaceae), M. anisopliae, Purpureocillium lilacinum (Thom) Luangsa-ard, Houbraken, Hywel-Jones & Samson (Hypocereales: Ophiocordycipitaceae) |
Eggs, larvae, and adults | Laboratory and semi-field | [176] |
| B. bassiana | Adults | Laboratory and semi-field | [224] |
| M. anisopliae, B. bassiana, Paecilomyces sp. | Adults | Laboratory | [184] |
| B. subtilis, B. thuringiensis, M. anisopliae, B. bassiana, Akanthomyces lecanii (Zimm.) Spatafora, Kepler & B. Shrestha (Hypocreales: Cordycipitaceae) | All stages | Laboratory | [225] |
| C. fumosorosea | All stages | Laboratory and field | [226] |
| A. lecanii | All stages | Field | [227]. |
| S. carpocapsae, H. bacteriophora | All stages | Field | [198] |
| Heterorhabditis indica Poinar, Karunakar & David (Rhabditida: Steinernematidae), S. carpocapsae | All stages | Laboratory | [228] |
| H. bacteriophora, M. anisopliae, B. thuringiensis serovar kurstaki | All stages | Laboratory and field | [229] |
6. Effect of Temperature on Development
Among abiotic factors, temperature is a keyfactor that influences on all the developmental stages and population growth of poikilothermic organisms including insects such as RPW [230,231]. Oviposition rates and developmental stages are significantly affected by low temperatures [11,90]. For instance, the highest fecundity was recorded at 25 °C, while no egg production was observed at 15 °C when the effect of temperature which ranged between 10 and 25 °C was studied on the development of RPW under laboratory conditions [90]. In the same study, a lower temperature limit for oviposition was 15.45 °C, whereas for egg hatching it was 13.95 °C [90]. Over a wider temperature range (i.e., 21–36 °C), Peng et al. [29] reported the highest fecundity of RPW adults at 27 °C; however, there was no significant variation between temperatures that ranged from 24 to 33 °C. However, at 36 °C a significant reduction in female fecundity was observed. This relationship between temperature and fecundity ultimately affects population growth and the number of generations per year [11,232]. In areas where the mean annual temperature is lower than 15 °C, less than 1 generation per year is expected, and >2 generations can be expected when the mean annual temperature is higher than 19 °C [11].
7. Conclusions
Early detection of RPW infestations is critical for effective pest management, and this can be achieved through the implementation of pheromone traps. Establishing extensive monitoring systems will provide palm growers with timely warnings; thus, they will be able to take prompt actions against this noxious species. Additionally, a deep understanding of RPW biology and its interaction with host palms is essential for devising effective IPM management strategies, offering palm tree owners valuable insights into the mitigation of infestations. The implementation of plant quarantine protocols is necessary to prevent the spread of RPW populations to non-infested areas, thereby confining and mitigating the problem before it escalates. Regarding pest control, biological agents such as EPF, EPB, and EPNs have shown positive results in combating RPW populations. Continuing research into the mass production of these agents and further enhancement of their efficacy and persistence in the field could significantly improve the prospects for long-term management strategies against RPW infestations. Such strategies would prioritize environmentally friendly approaches, offering more sustainable solutions that minimize the ecological footprint of pest management methods. Overall, a multifaceted plan combining early detection, biological control, and preventive measures is imperative for the successful management of RPW infestations. The conventional management of RPW for the last twenty years has depended upon techniques like, early detection, cultural practices, biological control agents, insecticides, male-produced aggregation pheromones and mass trapping, sterile insect techniques, push–pull and attract and kill, population eradication through phytosanitation and augmentation of all management tactics [22]. However, the most recent techniques used for the detection of this pest include acoustic systems, data mining, remote sensing systems, radio telemetry, thermal and digital cameras, tree radar units (TRUs), seismic sensor-based techniques, a combination of male sterile and biocontrol agents, genomics, metabolomics, proteomics transcriptomics, volatilomics, X-ray, and microwave technology [13,22,91,233,234].
Author Contributions
Conceptualization, W.W. and N.G.K.; validation, W.W., M.C.B., N.G.K., C.S.F., N.E., M.U.G., M.Y. and M.A.Q.; investigation, W.W., M.C.B., N.G.K., C.S.F., N.E., M.U.G., M.Y. and M.A.Q.; resources, W.W.; data curation, W.W., M.C.B., N.G.K., C.S.F., N.E., M.U.G., M.Y. and M.A.Q. writing—original draft preparation, W.W., M.C.B., N.G.K., C.S.F., N.E., M.U.G., M.Y., M.A.Q. and P.B.A.; writing—review and editing, W.W., M.C.B., N.G.K., C.S.F., N.E., M.U.G., M.Y., M.A.Q. and P.B.A.; visualization, W.W., M.C.B., N.G.K., C.S.F., N.E., M.U.G., M.Y., M.A.Q. and P.B.A.; supervision, W.W. and N.G.K.; project administration, W.W.; funding acquisition, W.W. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was partly funded by Project 3244 of the Higher Education Commission, Islamabad, Pakistan.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kaiser B.A., Burnett K.M. Spatial economic analysis of early detection and rapid response strategies for an invasive species. Resour. Energy Econ. 2010;32:566–585. doi: 10.1016/j.reseneeco.2010.04.007. [DOI] [Google Scholar]
- 2.Van-Driesche R.G., Carruthers R.I., Center T., Hoddle M.S., Hough-Goldstein J., Morin L., Smith L., Wagne D.L. Classical biological control for the protection of natural ecosystems. Biol. Control. 2010;54:2–33. doi: 10.1016/j.biocontrol.2010.03.003. [DOI] [Google Scholar]
- 3.McLaughlin G.M., Dearden P.K. Invasive insects: Management methods explored. J. Insect Sci. 2019;19:17. doi: 10.1093/jisesa/iez085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wai Y.K., Bakar A.A., Azmi W.A. Fecundity, fertility and survival of red palm weevil (Rhynchophorus ferrugineus) larvae reared on sago palm. Sains Malaysiana. 2016;44:1371–1375. [Google Scholar]
- 5.Nurashikin-Khairuddin W., Abdul-Hamid S.N.A., Mansor M.S., Bharudin I., Othman Z., Jalinas J. A review of entomopathogenic nematodes as a biological control agent for red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) Insects. 2022;13:245. doi: 10.3390/insects13030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naveed H., Andoh V., Islam W., Chen L., Chen K. Sustainable pest management in date palm ecosystems: Unveiling the ecological dynamics of red palm weevil (Coleoptera: Curculionidae) infestations. Insects. 2023;14:859. doi: 10.3390/insects14110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faleiro J.R., Al-Shawaf A.M., Al-Dandan A.M., Al-Odhayb A., Al-Rudayni A., Abdallah A.B., Peixoto M.P., Vargas R., Bottom M., Chidi S., et al. Controlled release products for managing insect pests. Outlooks Pest Manag. 2016;27:175–180. doi: 10.1564/v27_aug_07. [DOI] [Google Scholar]
- 8.Buxton P.A. Insect pests of dates and the date palm in Mesopotamia and elsewhere. Bull. Entom. Res. 1920;11:287–304. doi: 10.1017/S0007485300044709. [DOI] [Google Scholar]
- 9.Giblin-Davis R.M., Oehlschlager A.C., Pérez A., Gries G., Gries R., Weissling T.J., Chinchilla C.M., Peña J.E., Hallett R.H., Pierce H.D., Jr., et al. Chemical and behavioral ecology of palm weevils (Curculionidae: Rhynchophorinae) Fla Entomol. 1996;79:153–167. doi: 10.2307/3495812. [DOI] [Google Scholar]
- 10.Yasin M., Wakil W., Kavallieratos N.G., Eleftheriadou N., Naeem A., Qayyum M.A., Muhammad A., Alhewairini S.S., Shapiro-Ilan D. Dual-strategy approach for Rhynchophorus ferrugineus control: Endophytic Beauveria bassiana and Bacillus thuringiensis topical application. Crop Prot. 2024:106954. doi: 10.1016/j.cropro.2024.106954. [DOI] [Google Scholar]
- 11.Dembilio Ó., Jacas J.A. Basic bioecological parameters of the invasive red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae), in Phoenix canariensis under Mediterranean climate. Bull. Entom. Res. 2011;101:153–163. doi: 10.1017/S0007485310000283. [DOI] [PubMed] [Google Scholar]
- 12.Sanz-Aguilar A., Cortés I., Gascón I., Martínez O., Ginard S., Tavecchia G. Modelling pest dynamics under uncertainty in pest detection: The case of the red palm weevil. Biol. Invasions. 2020;22:1635–1645. doi: 10.1007/s10530-020-02208-6. [DOI] [Google Scholar]
- 13.Mohammed M.E., El-Shafie H.A., Alhajhoj M.R. Recent trends in the early detection of the invasive red palm weevil, Rhynchophorus ferrugineus (Olivier) In: El-Shafie H.A.F., editor. Invasive Species-Introduction Pathways, Economic Impact, and Possible Management Options. IntechOpen; London, UK: 2020. pp. 1–17. [Google Scholar]
- 14.Faleiro J.R. A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Insect Sci. 2006;26:135–154. [Google Scholar]
- 15.Fiaboe K.K.M., Mankin R.W., Roda A.L., Kairo M.T.K., Johanns C. Pheromone-food-bait trap and acoustic surveys of Rhynchophorus ferrugineus (Coleoptera: Curculionidae) in Curacao. Fla Entomol. 2011;94:766–773. doi: 10.1653/024.094.0406. [DOI] [Google Scholar]
- 16.Ávalos J.A., Martí-Campoy A., Soto A. Study of the flying ability of Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) adults using a computer-monitored flight mill. Bull. Entom. Res. 2014;104:462–470. doi: 10.1017/S0007485314000121. [DOI] [PubMed] [Google Scholar]
- 17.Ávalos J.A., Balasch S., Soto A. Flight behaviour and dispersal of Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) adults using mark-release-recapture method. Bull. Entomol. Res. 2016;106:606–614. doi: 10.1017/S0007485316000341. [DOI] [PubMed] [Google Scholar]
- 18.Rochat D., Dembilio O., Jaques J.A., Suma P., Pergola A.L., Hamidi R., Kontodimas D., Soroker V. Rhynchophorus ferrugineus: Taxonomy, distribution, biology, and life cycle. In: Soroker V., Colazza S., editors. Handbook of Major Palm Pests: Biology and Management. John Wiley and Sons; Oxford, UK: 2017. pp. 69–104. [Google Scholar]
- 19.Suhriani S., Soomro F., Kanwal R., Mal B., Larik S.A., Mahar M.A., Soomro F.A., Shaikh A.M., Panhwar W.A. Sexual dimorphism and morphometric analysis of red palm weevil (Rhynchophorus ferrugineus) of Khairpur, Sindh, Pakistan. J. Wildlife Biodivers. 2024;8:313–324. [Google Scholar]
- 20.Rugman-Jones P.F., Hoddle C.D., Hoddle M.S., Stouthamer R. The lesser of two weevils: Molecular-genetics of pest palm weevil populations confirm Rhynchophorus vulneratus (Panzer 1798) as a valid species distinct from R. ferrugineus (Olivier 1790), and reveal the global extent of both. PLoS ONE. 2013;8:e78379. doi: 10.1371/journal.pone.0078379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manee M.M., Alqahtani F.H., Al-Shomrani B.M., El-Shafie H.A., Dias G.B. Omics in the red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae): A bridge to the pest. Insects. 2023;14:255. doi: 10.3390/insects14030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoddle M.S., Antony B., El-Shafie H.A., Chamorro M.L., Milosavljević I., Löhr B., Faleiro J.R. Taxonomy, biology, symbionts, omics, and management of Rhynchophorus palm weevils (Coleoptera: Curculionidae: Dryophthorinae) Annu. Rev. Entomol. 2024;69:455–479. doi: 10.1146/annurev-ento-013023-121139. [DOI] [PubMed] [Google Scholar]
- 23.Dembilio Ó., Jaques J.A. Bio-ecology and integrated management of the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae), in the region of Valencia (Spain) Hell. Plant Prot. J. 2012;5:1–12. [Google Scholar]
- 24.Niode N.J., Kepel B.J., Hessel S.S., Kairupan T.S., Tallei T.E. Rhynchophorus ferrugineus larvae: A novel source for combating broad-spectrum bacterial and fungal infections. Vet. World. 2024;17:156. doi: 10.14202/vetworld.2024.156-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Zoghby I.R.M. Rearing of the red palm weevil, Rhynchophorus ferrugineus (Olivier) on different natural diets. Ann. Agric. Sci. Moshtohor. 2018;56:509–518. doi: 10.21608/assjm.2018.214674. [DOI] [Google Scholar]
- 26.Al-Dosary N.M.N., Al-Dobai S., Faleiro J.R. Review on the management of red palm weevil Rhynchophorus ferrugineus Olivier in date palm Phoenix dactylifera L. Emir. J. Food Agric. 2016;28:34–44. doi: 10.9755/ejfa.2015-10-897. [DOI] [Google Scholar]
- 27.Giblin-Davis R.M., Faleiro J.R., Jacas J.A., Peña J.E., Vidyasagar P.S.P.V. Coleoptera: Biology and management of the red palm weevil, Rhynchophorus ferrugineus. In: Peña J., editor. Potential Invasive Pests of Agricultural Crops. CABI; Wallingford, UK: 2013. pp. 1–34. [Google Scholar]
- 28.Pu Y.C., Xiang H.J., Liang X.Y., Hou Y.M., Fu L., Wang R. External immune inhibitory efficiency of external secretions and their metabolic profiling in red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) Front. Physiol. 2020;10:476488. doi: 10.3389/fphys.2019.01624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng L., Miao Y., Hou Y. Demographic comparison and population projection of Rhynchophorus ferrugineus (Coleoptera: Curculionidae) reared on sugarcane at different temperatures. Sci. Rep. 2016;6:31659. doi: 10.1038/srep31659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dembilio Ó., Karamaouna F., Kontodimas D.C., Nomikou M., Jaques J.A. Susceptibility of Phoenix theophrasti (Palmae: Coryphoideae) to Rhynchophorus ferrugineus (Coleoptera: Curculionidae) and its control using Steinernema carpocapsae in a chitosan formulation. Span. J. Agric. Res. 2011;9:623–626. doi: 10.5424/sjar/20110902-413-10. [DOI] [Google Scholar]
- 31.Llácer E., Negre M., Jacas J.A. Evaluation of an oil dispersion formulation of imidacloprid as a drench against Rhynchophorus ferrugineus (Coleoptera: Curculionidae) in young palm trees. Pest Manag. Sci. 2012;68:878–882. doi: 10.1002/ps.3245. [DOI] [PubMed] [Google Scholar]
- 32.El-Mergawy R.A.A.M., Al-Ajlan A.M. Red palm weevil, Rhynchophorus ferrugineus (Olivier): Economic importance, biology, biogeography and integrated pest management. J. Agric. Sci. Tech. 2011;1:1–23. [Google Scholar]
- 33.Cristofaro M., Fornari C., Mariani F., Cemmi A., Guedj M., Ben Jamaa M.L., Msaad Guerfali M., Tabone E., Castellana R., Sasso R., et al. Effects of γ-irradiation on mating behavior of red palm weevil, Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera: Dryophthoridae) Insects. 2023;14:661. doi: 10.3390/insects14070661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inghilesi A.F., Mazza G., Cervo R., Cini A. A Network of sex and competition: The promiscuous mating system of an invasive weevil. Curr. Zool. 2015;61:85–97. doi: 10.1093/czoolo/61.1.85. [DOI] [Google Scholar]
- 35.El-Shafie H.A.F., Faleiro J.R. Optimizing components of pheromone-baited trap for the management of red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) in date palm agro-ecosystem. J. Plant Dis. Prot. 2017;124:279–287. doi: 10.1007/s41348-017-0097-5. [DOI] [Google Scholar]
- 36.El-Shafie H.A.F. Area-wide integrated management of red palm weevil, Rhynchophorus ferrugineus (Olivier 1790) (Coleoptera: Curculionidae) in date palm plantations: A review. Persian Gulf Crop Prot. 2014;3:92–118. [Google Scholar]
- 37.EPPO (European and Mediterranean Plant Protection Organization) Rhynchophorus ferrugineus. EPPO Datasheets on Pests Recommended for Regulation. [(accessed on 25 July 2024)]. Available online: https://gd.eppo.int.
- 38.Gailce Leo Justin C., Leelamathi M., Thangaselvabai T., Nirmal Johnson S.B. Bioecology and management of the red palm weevil, Rhynhophorus ferrugineus Oliv. (Coleoptera: Curculionidae) on coconut-A review. Agric. Rev. 2008;29:117–124. [Google Scholar]
- 39.Hetzroni A., Soroker V., Cohen V. Toward practical acoustic red palm weevil detection. Comput. Electron. Agric. 2016;124:100–116. doi: 10.1016/j.compag.2016.03.018. [DOI] [Google Scholar]
- 40.Veeraprathap V., Ramya B.K., Narendra Kumar G. Modified efficient protection of palm disaster from RPW larvae using WSNs. Int. J. Recent Technol. Eng. 2020;8:245–251. [Google Scholar]
- 41.Dembilio Ó., Jaques J.A. Biology and management of red palm weevil. In: Wakil W., Faleiro J.R., Miller T.A., editors. Sustainability in Plant and Crop Protection. Sustainable Pest Management in Date: Current Status and Emerging Challenges. Springer; Cham, Switzerland: 2015. pp. 13–36. [Google Scholar]
- 42.Suma P., La Pergola A., Longo S., Soroker V. The use of sniffing dogs for the detection of Rhynchophorus ferrugineus. Phytoparasitica. 2014;42:269–274. doi: 10.1007/s12600-013-0330-0. [DOI] [Google Scholar]
- 43.Soroker V., Suma P., La Pergola A., Cohen Y., Cohen Y., Alchanatis V., Golomb O., Goldshtein E., Hetzroni A., Galazan L., et al. Early detection and monitoring of red palm weevil: Approaches and challenges. AFPP-Palm Pest Mediterr. Conf. 2013 [Google Scholar]
- 44.Nakash J., Osem Y., Kehat M. A suggestion to use doges for detecting red palm weevil (Rhynchophorus ferrugineus) infestation in date palms in Israel. Phytoparasitica. 2000;28:153–155. doi: 10.1007/BF02981745. [DOI] [Google Scholar]
- 45.Mankin R.W. Recent development in the use of acoustic sensors and signal processing tools to target early infestations of red palm weevils in agricultural environments. Fla Entomol. 2011;94:761–765. doi: 10.1653/024.094.0405. [DOI] [Google Scholar]
- 46.Mankin R.W., Al-Ayedh H.Y., Aldryhim Y., Rohde B. Acoustic detection of Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) and Oryctes elegans (Coleoptera: Scarabaeidae) in Phoenix dactylifera (Arecales: Arecacae) trees and offshoots in Saudi Arabian orchards. J. Econ. Entomol. 2016;109:622–628. doi: 10.1093/jee/tov398. [DOI] [PubMed] [Google Scholar]
- 47.Jalinas J., Güerri-Agullo B., Mankin R.W., Lopez-Follana R., Lopez-Llorca L.V. Acoustic assessment of Beauveria bassiana (Hypocreales: Clavicipitaceae) effects on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) larval activity and mortality. J. Econ. Entomol. 2015;108:444–453. doi: 10.1093/jee/tov023. [DOI] [PubMed] [Google Scholar]
- 48.Potamitis I., Rigakis I., Vidakis N., Petousis M., Weber M. Affordable bimodal optical sensors to spread the use of automated insect monitoring. J. Sens. 2018:949415. doi: 10.1155/2018/3949415. [DOI] [Google Scholar]
- 49.Martin B., Shaby S.M., Premi M.G. Studies on acoustic activity of red palm weevil the deadly pest on coconut crops. Procedia Mater. Sci. 2015;10:455–466. doi: 10.1016/j.mspro.2015.06.081. [DOI] [Google Scholar]
- 50.Nangai V.L. Interpreting the acoustic characteristics of RPW towards its detection-A Review. IOP Conf. Ser. Mater. Sci. Eng. 2017;225:1–9. [Google Scholar]
- 51.Martin B., Juliet V. A novel approach to identify red palm weevil on palms. Adv. Mater. Res. 2013;634:3853–3857. doi: 10.4028/www.scientific.net/AMR.634-638.3853. [DOI] [Google Scholar]
- 52.Pinhas J., Soroker V., Hetzroni A., Mizrach A., Teicher M., Goldberger J. Automatic acoustic detection of the red palm weevil. Comput. Electron. Agric. 2008;63:131–139. doi: 10.1016/j.compag.2008.02.004. [DOI] [Google Scholar]
- 53.Hussein W., Hussein M., Becker T. Detection of the red palm weevil Rhynchophorus ferrugineus using its bioacoustics features. Bioacoustics. 2010;19:177–194. doi: 10.1080/09524622.2010.9753623. [DOI] [Google Scholar]
- 54.Rach M.M., Gomis H.M., Granado O.L., Malumbres M.P., Campoy A.M., Martín J.J.S. On the design of a bioacoustic sensor for the early detection of the red palm weevil. Sensors. 2013;13:1706–1729. doi: 10.3390/s130201706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khudri N.A.F.R.S., Mohd Masri M.M., Maidin M.S.T., Kamarudin N., Hussain M.H., Abd Ghani I., Jalinas J. Preliminary evaluation of acoustic sensors for early detection of red palm weevil, Rhynchophorus ferrugineus incidence on oil palm and coconut in Malaysia. Int. J. Trop. Insect Sci. 2021;41:3287–3292. doi: 10.1007/s42690-021-00499-3. [DOI] [Google Scholar]
- 56.Kurdi H., Al-Aldawsari A., Al-Turaiki I., Aldawood A.S. Early detection of red palm weevil, Rhynchophorus ferrugineus (Olivier), infestation using data mining. Plants. 2021;10:95. doi: 10.3390/plants10010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dembilio Ó., Jacas J.A., Llacer E. Are the new palms Washintonia filifera and Chamaerops humilis suitable hosts for the red palm weevil, Rhynchophorus ferrugineus (Col. Curculionidae) J. Appl. Entomol. 2009;133:565–567. doi: 10.1111/j.1439-0418.2009.01385.x. [DOI] [Google Scholar]
- 58.Dembilio Ó., Quesada-Moraga E., Santiago-Álvarez C., Jacas J.A. Potential of an indigenous strain of the entomopathogenic fungus Beauveria bassiana as a biological control agent against the red palm weevil, Rhynchophorus ferrugineus. J. Invertebr. Pathol. 2010;104:214–221. doi: 10.1016/j.jip.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 59.Malumphy C., Moran H. Red Palm Weevil, Rhynchophorus ferrugineus. Plant Pest Factsheet. [(accessed on 25 July 2024)];2009 Available online: www.fera.defra.gov.uk/plants/publications/documents/factsheets/redPalmWeevil.pdf.
- 60.Jaques J.A., Riolo P., Audsley N., Barroso J.M., Dembilio O., Isidoro N., Minuz R.L., Nardi S., Llopis V.N., Beaudoin-Ollivier L., et al. Control measures against Rhynchophorus ferrugineus and Paysandisia archon. In: Soroker V., Colazza S., editors. Handbook of Major Palm Pests: Biology and Management. John Wiley and Sons; Oxford, UK: 2017. pp. 255–279. [Google Scholar]
- 61.Elsharkawy M.M., Almasoud M., Alsulaiman Y.M., Baeshen R.S., Elshazly H., Kadi R.H., Shawer R. Efficiency of Bacillus thuringiensis and Bacillus cereus against Rhynchophorus ferrugineus. Insects. 2022;13:905. doi: 10.3390/insects13100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dutta R., Thakur N.S.A., Bag T.K., Anita N., Chandra S., Ngachan S.V. New record of red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) on Arecanut (Areca catechu) from Meghalaya, India. Fla Entomol. 2010;93:446–448. doi: 10.1653/024.093.0320. [DOI] [Google Scholar]
- 63.Kumara A.D.N.T., Chandrashekharaiah M., Kandakoor S.B., Chakravarthy A.K. Status and management of three major insect pests of coconut in the tropics and subtropics. In: Chakravarthy A.K., editor. New Horizons in Insect Science: Towards Sustainable Pest Management. Springer; Berlin, Germany: 2015. pp. 359–381. [Google Scholar]
- 64.Saneera E.K., Chandrika M., Thube S.H., Jose C.T. Beware of red palm weevil, a destructive pest on Arecanut. Indian J. Arecanut Spices Med. Plants. 2019;21:27–30. [Google Scholar]
- 65.Manjunatha H., Niranjana K.S., Ravikumar M. Incidence of red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) on arecanut from Karnataka, India. Curr. Biotica. 2013;7:92–95. [Google Scholar]
- 66.European Commission . EU Efforts to Stop the Red Palm Weevil. Office for Official Publications of the European Communities; Luxembourg: 2011. The insect killing our palm trees; pp. 1–32. [DOI] [Google Scholar]
- 67.Cocco A., Pusceddu M., Lentini A., Floris I. Can increasing infestations by Rhynchophorus ferrugineus threaten endemic Chamaerops humilis in Sardinia (Italy)? EPPO Bull. 2019;49:405–413. doi: 10.1111/epp.12583. [DOI] [Google Scholar]
- 68.Bombi P. Potential conflict extent between two invasive alien pests, Rhynchophorus ferrugineus and Paysandisia archon, and the native populations of the Mediterranean fan palm. J. Nat. Conserv. 2020;58:125927. doi: 10.1016/j.jnc.2020.125927. [DOI] [Google Scholar]
- 69.El-Shafie H.A.F., Faleiro J.R. Red palm weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae): Global invasion, current management options, challenges and future prospects. In: El-Shafie H.A.F., editor. Invasive Species-Introduction Pathways, Economic Impact, and Possible Management Options. IntechOpen; London, UK: 2020. pp. 1–30. [Google Scholar]
- 70.Ferry M., Gomez S. The red palm weevil in the Mediterranean area. Palms. 2002;46:172–178. [Google Scholar]
- 71.Roda A., Kairo M., Damian T., Franken F., Heidweiller K., Johanns C., Mankin R. Red palm weevil (Rhynchophorus ferrugineus), an invasive pest recently found in the Caribbean that threatens the region. EPPO Bull. 2011;41:116–121. doi: 10.1111/j.1365-2338.2011.02446.x. [DOI] [Google Scholar]
- 72.Hallett R.H., Crespi B.J., Borden J. Synonymy of Rhynchophorus ferrugineus (Olivier), 1790 and R. vulneratus (Panzer), 1798 (Coleoptera: Curculionidae: Rhynchophorinae) J. Nat. Hist. 2004;38:2863–2882. doi: 10.1080/00222930310001657874. [DOI] [Google Scholar]
- 73.Azmi W.A., Lian C.J., Zakeri H.A., Yusuf N., Omar W.B.W., Wai Y.K., Husasin M. The red palm weevil, Rhynchophorus ferrugineus: Current issues and challenges in Malaysia. Oil Palm Bull. 2017;74:17–24. [Google Scholar]
- 74.Kontodimas D.C., Milonas P.G., Vassiliou V., Thymakis N., Economou D. The occurrence of Rhynchophorus ferrugineus in Greece and Cyprus and the risk against the native Greek palm tree Phoenix theophrasti. Entomol. Hell. 2006;16:11–15. doi: 10.12681/eh.11621. [DOI] [Google Scholar]
- 75.Ju R.T., Wang F., Wan F.H., Li B. Effect of host plants on development and reproduction of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) J. Pest Sci. 2010;84:33–39. doi: 10.1007/s10340-010-0323-4. [DOI] [Google Scholar]
- 76.Aldryhim Y.N., Al Ayedh H.Y. Diel flight activity patterns of the red palm weevil (Coleoptera: Curculionidae) as monitored by smart traps. Fla Entomol. 2015;98:1019–1024. doi: 10.1653/024.098.0402. [DOI] [Google Scholar]
- 77.Karut K., Kazak C. A new pest of date palm trees (Phoenyx dactylifera L.): Rynchophorus ferrugineus (Olivier, 1790) (Coleoptera: Curculionidae) in Mediterranean region of Turkey. Türk. Entomol. Derg. 2005;29:295–300. [Google Scholar]
- 78.Liao Q., Guo Y., Zhou J., Wan Y., Carballar-Lejarazú R., Sheng L., Wu S., Zou S. Characterization of bacterial communities associated with Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) and its host Phoenix sylvestris. Curr. Microbiol. 2020;77:3321–3329. doi: 10.1007/s00284-020-02196-9. [DOI] [PubMed] [Google Scholar]
- 79.Fiorello A., Speciale M., Lo Verde G., Massa B. Strelitzia nicolai (Strelitziaceae), new host plant for Rhynchophorus ferrugineus (Coleoptera: Curculionidae) in Sicily. Naturalista Sicil. 2015;39:435–438. [Google Scholar]
- 80.Giovino A., Scibetta S., Gugliuzza G., Longo S., Suma P., La Mantia T. Attacks of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) on natural specimens of dwarf fan palm Chamaerops humilis L. (Arecaceae) in Sicily. Naturalista Sicil. 2012;36:427–433. [Google Scholar]
- 81.Abe F., Hata K., Sone K. Life history of the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Dryophtoridae), in Southern Japan. Fla Entomol. 2009;92:421–425. [Google Scholar]
- 82.Faleiro J.R., Ben Abdullah A., El-Bellaj M., Al Ajlan A.M., Oihabi A. Threat of red palm weevil, Rhynchophorus ferrugineus (Olivier) to date palm plantations in North Africa. Arab J. Plant Prot. 2012;30:274–280. [Google Scholar]
- 83.Abraham V.A., Faleiro J.R., Nair C.P.R., Nair S.S. Present management technologies for red palm weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) in palms and future thrusts. Pest Manag. Hortic. Ecosyst. 2002;8:69–82. [Google Scholar]
- 84.Ferry M. The world situation and the main lessons of 30 years of fight against the red palm weevil. Arab J. Plant Prot. 2019;37:109–118. doi: 10.22268/AJPP-037.2.109118. [DOI] [Google Scholar]
- 85.Metwally H.A.A., Basheer A.M. The behavior and activity of the red palm weevil Rhynchophorus ferrugineus throughout the year under Baharia Oasis conditions. Egypt. Middle East J. 2019;8:797–807. [Google Scholar]
- 86.Abdel-Banat B.M.A., El-Shafie H.A.F. Management of the red palm weevil in date palm plantations in Al-Ahsa oasis of Saudi Arabia. Plant Health Cases. 2023;14:phcs20230001. doi: 10.1079/planthealthcases.2023.0001. [DOI] [Google Scholar]
- 87.Dilipkumar M., Ahadiyat A., Mašán P., Chuah T.S. Mites (Acari) associated with Rhynchophorus ferrugineus (Coleoptera: Curculionidae) in Malaysia, with a revised list of the mites found on this weevil. J. Asia Pac. Entomol. 2015;18:169–174. doi: 10.1016/j.aspen.2014.12.010. [DOI] [Google Scholar]
- 88.Salama H.S., Zaki F.N., Abdel-Razek A.S. Ecological and biological studies on the red palm weevil Rhynchophorus ferrugineus (Olivier) Arch. Phytopathol. Plant Prot. Pflanzenschutz. 2009;42:392–399. doi: 10.1080/03235400601121521. [DOI] [Google Scholar]
- 89.Ince S., Porcelli F., Al-Jboory I. Egg laying and egg laying behavior of red palm weevil, Rhynchophorus ferrugineus (Olivier) 1790 (Coleoptera: Curculionidae) Agric. Biol. J. N. Am. 2011;2:1368–1374. doi: 10.5251/abjna.2011.2.11.1368.1374. [DOI] [Google Scholar]
- 90.Dembilio Ó., Tapia G.V., Téllez M.M., Jacas J.A. Lower temperature thresholds for oviposition and egg hatching of the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae), in a Mediterranean climate. Bull. Entomol. Res. 2012;102:97–102. doi: 10.1017/S0007485311000411. [DOI] [PubMed] [Google Scholar]
- 91.Aziz A.T. Red palm weevil, Rhynchophorus ferrugineus, a significant threat to date palm tree, global invasions, consequences, and management techniques. J. Plant Dis. Protection. 2024;131:9–26. doi: 10.1007/s41348-023-00805-w. [DOI] [Google Scholar]
- 92.Witt A., Hula V., Suleiman A.S., Van Damme K. First record of the red palm weevil Rhynchophorus ferrugineus (Olivier) on Socotra Island (Yemen), an exotic pest with high potential for adverse economic impacts. Rend. Lincei Sci. Fis. Nat. 2020;31:645–654. doi: 10.1007/s12210-020-00918-6. [DOI] [Google Scholar]
- 93.Esteban-Durán J., Yela J.L., Crespo F.B., Alvarez A.J. Biology of red palm weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae: Rhynchophorinae), in the laboratory and field life cycle, biological characteristics in its zone of introduction in Spain, biological method of detection and possible control. Bol. San. Veg. Plagas. 1998;24:737–748. [Google Scholar]
- 94.Martín M.M., Cabello T. Manejo de la cría del picudo rojo de la palmera, Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera: Dryophthoridae), en dieta artificial y efectos en su biometría y biología. Bol. San. Veg. Plagas. 2006;32:631–641. [Google Scholar]
- 95.Dalbon V.A., Acevedo J.P.M., Ribeiro Junior K.A.L., Ribeiro T.F.L., Silva J.M.D., Fonseca H.G., Santana A.E.G., Porcelli F. Perspectives for synergic blends of attractive sources in South American palm weevil mass trapping: Waiting for the red palm weevil Brazil invasion. Insects. 2021;12:828. doi: 10.3390/insects12090828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Al-Nujiban A.A., Aldosari S.A., Al-Suhaibani A.M., Abdel-Azim M.M., Ibrahim S.M.M., Paraj Shukla P.S. Effect of date palm cultivar on fecundity and development of Rhynchophorus ferrugineus. Bull. Insectol. 2015;68:199–206. [Google Scholar]
- 97.Salama H., Hamdy H.M., Magd El-Din M. The thermal constant for timing the emergence of the red palm weevil, Rhynchophorus ferrugineus (Oliv.) (Coleoptera: Curculionidae) J. Pest Sci. 2002;75:26–29. doi: 10.1046/j.1439-0280.2002.02004.x. [DOI] [Google Scholar]
- 98.Faleiro J.R., El-Saad M.A., Al-Abbad H. Pheromone trap density to mass trap Rhynchophorus ferrugineus (Coleoptera: Curculionidae/Rhynchophoridae/Dryophthoridae) in date plantations of Saudi Arabia. Int. J. Trop. Insect Sci. 2012;31:75–77. doi: 10.1017/S1742758411000099. [DOI] [Google Scholar]
- 99.Al Ansi A.N., Aldryhim Y.N., Al Janobi A.A., Aldawood A.S. Effects of trap locations, pheromone source, and temperature on red palm weevil surveillance (Coleoptera: Dryophthoridae) Fla Entomol. 2022;105:58–64. doi: 10.1653/024.105.0109. [DOI] [Google Scholar]
- 100.Suma P., Peri E., La Pergola A., Soroker V., Dembilio O., Riolo P., Nardi S. Action programs for Rhynchophorus ferrugineus and Paysandisia archon. In: Soroker V., Colazza S., editors. Handbook of Major Palm Pests: Biology and Management. John Wiley and Sons; Oxford, UK: 2017. pp. 280–299. [Google Scholar]
- 101.Navarro-Llopis V., Primo J., Vacas S. Improvements in Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) trapping systems. J. Econ. Entomol. 2018;111:1298–1305. doi: 10.1093/jee/toy065. [DOI] [PubMed] [Google Scholar]
- 102.Oehlschlager A.C. Palm weevil pheromones–discovery and use. J. Chem. Ecol. 2016;42:617–630. doi: 10.1007/s10886-016-0720-0. [DOI] [PubMed] [Google Scholar]
- 103.Antony B., Johny J., Montagné N., Jacquin-Joly E., Capoduro R., Cali K., Persaud K., Al-Saleh M.A., Pain A. Pheromone receptor of the globally invasive quarantine pest of the palm tree, the red palm weevil (Rhynchophorus ferrugineus) Mol. Ecol. 2021;30:2025–2039. doi: 10.1111/mec.15874. [DOI] [PubMed] [Google Scholar]
- 104.Vidyasagar P.S.P.V., Hagi M., Abozuhairah R.A., Al-Mohanna O.E., Al-Saihati A.A. Impact of mass pheromone trapping on red palm weevil adult population and infestation level in date palm gardens of Saudi Arabia. Planter. 2000;76:347–355. [Google Scholar]
- 105.Vacas S., Primo J., Navarro-Llopis V. Advances in the use of trapping systems for Rhynchophorus ferrugineus (Coleoptera: Curculionidae): Traps and attractants. J. Econ. Entomol. 2013;106:1739–1746. doi: 10.1603/EC13105. [DOI] [PubMed] [Google Scholar]
- 106.Soomro M.H., Mari J.M., Nizamani I.A., Gilal A.A. Performance of Ferrolure+ pheromone in the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) management in date palm growing areas of Sindh, Pakistan. J. Saudi Soc. Agric. Sci. 2022;21:114–124. doi: 10.1016/j.jssas.2021.07.004. [DOI] [Google Scholar]
- 107.Faleiro J.R., Abraham V.A., Al-Shuaibi M.A., Kumar T.P. Field evaluation of red palm weevil, Rhynchophorus ferrugineus Oliv. pheromone (ferrugineol) lures. Indian J. Entomol. 2000;62:427–433. [Google Scholar]
- 108.Mahmud A.I., Farminhao J., Viez E.R. Red palm weevil (Rhynchophorus ferrugineus Olivier, 1790): Threat of palms. J. Biol. Sci. 2015;15:56–67. doi: 10.3923/jbs.2015.56.67. [DOI] [Google Scholar]
- 109.Azmi W., Daud S., Hussain M., Wai Y., Zazali C., Sajap A. Field trapping of adult red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) with kairomone-releasing food baits and synthetic pheromone lure in a coconut. Philipp. Agric. Sci. 2014;97:409–415. [Google Scholar]
- 110.Guarino S., Lo Bue P., Peri E., Colazza S. Responses of Rhynchophorus ferrugineus adults to selected synthetic palm esters: Electroantennographic studies and trap catches in an urban environment. Pest Manag. Sci. 2011;67:77–81. doi: 10.1002/ps.2035. [DOI] [PubMed] [Google Scholar]
- 111.Vacas S., Abad-Payá M., Primo J., Navarro-Llopis V. Identification of pheromone synergists for Rhynchophorus ferrugineus trapping systems from Phoenix canariensis palm volatiles. J. Agric. Food Chem. 2014;62:6053–6064. doi: 10.1021/jf502663y. [DOI] [PubMed] [Google Scholar]
- 112.Gries G., Gries R., Perez A.L., Gonzalez L.M., Pierce H.D.R., Oehlschlager A.C., Rhainds M., Zebeyou M., Kouame B. Ethyl propionate: Synergistic kairomone for African palm weevil, Rhynchophorus phoenicis L., (Coleoptera: Curculionidae) J. Chem. Ecol. 1994;20:889–897. doi: 10.1007/BF02059585. [DOI] [PubMed] [Google Scholar]
- 113.Abdel-Azim M.M., Aldosari S.A., Mumtaz R., Vidyasagar P.S., Shukla P. Pheromone trapping system for Rhynchophorus ferrugineus in Saudi Arabia: Optimization of trap contents and placement. Emir. J. Food Agric. 2017;29:936–948. [Google Scholar]
- 114.Abraham V.A., Nair S.S. Evaluation of five insecticides for use in the red palm weevil pheromone traps. Pestology. 2001;25:31–33. [Google Scholar]
- 115.Faleiro J.R. Pheromone technology for the Management of Red Palm Weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Rhynchophoridae) – A Key Pest of Coconut. Technical Bulletin. ICAR Research Complex for Goa; Ela, Old Goa, India: 2005. [Google Scholar]
- 116.Hallett R.H., Oehlschlager A.C., Borden J.H. Pheromone trapping protocols for the Asian palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) Int. J. Pest Manag. 1999;45:231–237. doi: 10.1080/096708799227842. [DOI] [Google Scholar]
- 117.Al-Saoud A.H. Effect of ethyl acetate and trap colour on weevil captures in red palm weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae) pheromone traps. Int. J. Trop. Insect Sci. 2013;33:202–206. doi: 10.1017/S1742758413000167. [DOI] [Google Scholar]
- 118.Abuagla A.M., Al-Deeb M.A. Effect of bait quantity and trap color on the trapping efficacy of the pheromone trap for the red palm weevil, Rhynchophorus ferrugineus. J. Insect Sci. 2012;12:120. doi: 10.1673/031.012.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ajlan A.M., Abdulsalam K.S. Efficiency of pheromone traps for controlling the red palm weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae), under Saudi Arabia conditions. Bull. Entomol. Soc. Egypt (Econ. Ser.) 2000;27:109–120. [Google Scholar]
- 120.Al-Saoud A.H. Effect of red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) aggregation pheromone, trap height and colors on the number of weevils captured. Acta Hortic. 2010;882:419–429. doi: 10.17660/ActaHortic.2010.882.47. [DOI] [Google Scholar]
- 121.Al-Saoud A.H., Al-Deeb M.A., Murchie A.K. Effect of color on the trapping effectiveness of red palm weevil pheromone traps. J. Entomol. 2010;7:54–59. doi: 10.3923/je.2010.54.59. [DOI] [Google Scholar]
- 122.Ávalos J.A., Soto A. Study of chromatic attraction of the red palm weevil, Rhynchophorus ferrugineus using bucket traps. Bull. Insectol. 2015;68:83–90. [Google Scholar]
- 123.Sallam A.A., El-Shafie H.A.F., Al-Abdan S. Influence of farming practices on infestation by red palm weevil Rhynchophorus ferrugineus (Olivier) in date palm: A case study. Int. Res. J. Agric. Sci. Soil Sci. 2012;2:370–376. [Google Scholar]
- 124.Abraham V.A., Al-Shuaibi M.A., Faleiro J.R., Abozuhairah R.A., Vidyasagar P.S.P.V. An integrated management approach for red palm weevil Rhynchophorus ferrugineus Oliv: A key pest of date palm in the Middle East. Agric. Sci. 1998;3:77–83. doi: 10.24200/jams.vol3iss1pp77-83. [DOI] [Google Scholar]
- 125.Kagan D., Alpert G.F., Fire M. Automatic large scale detection of red palm weevil infestation using street view images. J. Photogramm. Remote Sens. 2021;182:122–133. doi: 10.1016/j.isprsjprs.2021.10.004. [DOI] [Google Scholar]
- 126.Cinnirella A., Bisci C., Nardi S., Ricci E., Palermo F.A., Bracchetti L. Analysis of the spread of Rhynchophorus ferrugineus in an urban area, using GIS techniques: A study case in Central Italy. Urban Ecosyst. 2020;23:255–269. doi: 10.1007/s11252-019-00920-3. [DOI] [Google Scholar]
- 127.Kassem H.S., Alotaibi B.A., Ahmed A., Aldosri F.O. Sustainable management of the red palm weevil: The nexus between farmers’ adoption of integrated pest management and their knowledge of symptoms. Sustainability. 2020;12:9647. doi: 10.3390/su12229647. [DOI] [Google Scholar]
- 128.Vidyasagar P.S.P.V., Aldosari S.A., Sultan E.M., Al Saihati A., Khan R.M. Efficiency of optimal pheromone trap density in management of red palm weevil, Rhynchophorus ferrugineus Olivier. Afr. J. Agric. Res. 2016;11:1071–1078. [Google Scholar]
- 129.Sewify G.H., Belal M.H., Qaed M.S. Food-baited aggregation pheromone traps for management of the red palm weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) Egypt. J. Biol. Pest Control. 2014;24:431 [Google Scholar]
- 130.Aggelakopoulos K., Alissandrakis E., Kollaros D., Liantraki Z. Comparison of two types of pheromone traps for the capture of the red palm weevil (Rhynchophorus ferrugineus) Entomol. Hell. 2014;23:45–51. doi: 10.12681/eh.11535. [DOI] [Google Scholar]
- 131.Al-Saoud A.H. Factors affecting the efficacy of ethyl acetate in the red palm weevil aggregation pheromone traps; Proceedings of the 5th International Date Palm Conference; Emirates Palace-Abu Dhabi, UAE. 16–18 March 2014. [Google Scholar]
- 132.Dhouibi M.H., Haouari W., Khrissi I., Guerret O., Chaar H., de Cozar K. Effect of different concentrations of M2ITM pheromone dispensers and the impact of water and paraffin in pheromone traps for Rhynchophorus ferrugineus (Coleoptera: Curculionidae) management in Tunisia. Int. J. Agric. Innov. Res. 2018;6:152–157. [Google Scholar]
- 133.El-Wahab A.A., El-Fattah A.A., El-Shafei W.K.M., Helaly A.E. Efficacy of aggregation nano gel pheromone traps on the catchability of Rhynchophorus ferrugineus (Olivier) in Egypt. Brazilian J. Biol. 2020;81:452–460. doi: 10.1590/1519-6984.231808. [DOI] [PubMed] [Google Scholar]
- 134.Firdaus M.M., Chuah T.S., Wahizatul A.A. Synergistic effect of synthetic pheromone and kairomone-releasing food baits in mass trapping system of red palm weevil, Rhynchophorus ferrugineus. IOP Conf. Ser. Earth Environ. Sci. 2020;494:012015. doi: 10.1088/1755-1315/494/1/012015. [DOI] [Google Scholar]
- 135.Sabbahi R., Azzaoui K., Hammouti B. An assessment of the efficacy of pheromone traps in managing the red palm weevil. Indones. J. Sci. Technol. 2021;6:371–384. doi: 10.17509/ijost.v6i2.34556. [DOI] [Google Scholar]
- 136.Arafa O.E. Evaluation of the effectiveness of attractant trap colors on attractive red palm weevil, Rhynchophorus ferrugineus (Olivier) in Egypt. Egyptian Int. J. Palms. 2021;1:1–9. doi: 10.21608/esjp.2021.247969. [DOI] [Google Scholar]
- 137.Shelke R. Field evaluation of red palm weevil Rhynchophorus ferrugineus Olivier pheromone lures. Indian J. Entomol. 2023;85:776–779. doi: 10.55446/IJE.2021.363. [DOI] [Google Scholar]
- 138.Salman T.A.A., Abbas M.K., Mandour N.S.A., Osman M.A.M., El-Kady G.A. Fluctuations in the population density of the red palm weevil Rhynchophorus ferrugineus (Curculionidae: Coleoptera) in Ismailia Governorate, Egypt. Egypt. J. Plant Prot. Res. 2020;3:977–985. [Google Scholar]
- 139.Haris-Hussain M., Kamarudin N.W.A. Efficacy of baits for red palm weevil (RPW), Rhynchophorus ferrugineus Olivier under constant laboratory condition. J. Oil Palm Res. 2020;32:355–364. [Google Scholar]
- 140.Arafa O.E. Field evaluation of synthetic pheromone, allomone, palm kairomone and ester in capturing adult red palm weevils, Rhynchophorus ferrugineus (Olivier) by aggregation pheromone traps in date palm plantations. Plant Arch. 2020;20:1857–1862. [Google Scholar]
- 141.Guarino S., Colazza S., Peri E., Bue P.L., Germanà M.P., Kuznetsova T., Gindin G., Soroker V. Behaviour-modifying compounds for management of the red palm weevil (Rhynchophorus ferrugineus Oliver) Pest Manag. Sci. 2015;71:1605–1610. doi: 10.1002/ps.3966. [DOI] [PubMed] [Google Scholar]
- 142.Gonzalez F., Kharrat S., Rodríguez C., Calvo C., Oehlschlager A. Red palm weevil (Rhynchophorus ferrugineus Olivier): Recent advances. Arab. J. Plant Prot. 2019;37:178–187. doi: 10.22268/AJPP-037.2.178187. [DOI] [Google Scholar]
- 143.Al-Saoud A.H. Effect of trap colour and stirring of contents of pheromone-baited traps on the capture of the adult red palm weevil in the United Arab Emirates. Int. J. Trop. Insect Sci. 2018;38:224–231. doi: 10.1017/S1742758418000048. [DOI] [Google Scholar]
- 144.Mohammadpour K., Avand-Faghih A., Hosseini-Gharalari A. The effect of date palm tissue and aggregation pheromone on attraction and trapping of red palm weevil, Rhynchophorus ferrugineus Oliv. (Coleoptera: Dryophthoridae) Acta Phytopathol. Entomol. Hung. 2018;53:233–239. doi: 10.1556/038.53.2018.013. [DOI] [Google Scholar]
- 145.Junejo R., Memon S., Shar M.U., Memon A.A. Effect of various parameters for trapping red palm weevil (Rhynchophorus ferrugineus L.) in date palm orchards at Khairpur Mir’s Sindh, Pakistan. Pak. J. Zool. 2022;54:1475. doi: 10.17582/journal.pjz/20200201180253. [DOI] [Google Scholar]
- 146.Abdel Kareim A.I., Rashed A.A., Mohamed A.M., Ahmed F.M.S. Seasonal activity of red palm weevil (RPW), Rhynchophorus ferrugineus (Olivier) in response to some olfactory stimulants. J. Plant Prot. Pathol. 2018;9:387–392. [Google Scholar]
- 147.Al-Saoud A.H., Yusta R., Sarto i Monteys V. Effect of trap colour and trap height above the ground on pheromone mass-trapping of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Dryophoridae) in date palm groves in Abu Dhabi, UAE. Bol. Soc. Entomol. Aragonesa. 2016;59:247–253. [Google Scholar]
- 148.Jaddou M.I., Al Kaabi A.J., Agla A.M.A., Al Kaabi A.S., Al Kayoumi K.N. Field Evaluation to the attraction efficiency for the different sources of the red palm weevil aggregation pheromone. Open Sci. J. 2017;2 doi: 10.23954/osj.v2i4.1019. [DOI] [Google Scholar]
- 149.Hajjar M.J., Ajlan A.M., Al-Ahmad M.H. Integration of repellency effect of neem-based insecticide and pheromone Bio-Trap® with Beauveria bassiana (Hypocreales: Cordycipitaceae) to control the red palm weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) Afr. Entomol. 2021;29:611–619. doi: 10.4001/003.029.0611. [DOI] [Google Scholar]
- 150.Chakravarthy A.K., Chandrashekharaiah M., Kandakoor S.B., Nagaraj D.N. Efficacy of aggregation pheromone in trapping red palm weevil (Rhynchophorus ferrugineus Olivier) and rhinoceros beetle (Oryctes rhinoceros Linn.) from infested coconut palms. J. Environ. Biol. 2014;35:479. [PubMed] [Google Scholar]
- 151.Mazza G., Francardi V., Simoni S., Benvenuti C., Cervo R., Faleiro J.R., Llácer E., Longo S., Nannelli R., Tarasco E., et al. An overview on the natural enemies of Rhynchophorus palm weevils, with focus on R. ferrugineus. Biol. Control. 2014;77:83–92. doi: 10.1016/j.biocontrol.2014.06.010. [DOI] [Google Scholar]
- 152.Murphy S.T., Briscoe B.R. The red palm weevil as an alien invasive: Biology and the prospects for biological control as a component of IPM. Biocontrol. 1999;20:35–46. [Google Scholar]
- 153.El-Shafie H.A.F., Abdel-Banat B.M.A., Al-Hajhoj M.R. Arthropod pests of date palm and their management. CABI Reviews. 2018;12:1–18. doi: 10.1079/PAVSNNR201712049. [DOI] [Google Scholar]
- 154.Abraham V.A., Kurian C., Nayer N.M. Chelisoches morio F. (Forficulidae: Dermaptera), a predator on eggs and early instar grubs of the red palm weevil Rhynchophorus ferrugineus F. (Curculionidae: Coleoptera) J. Plant. Crops. 1973;1:147–152. [Google Scholar]
- 155.Mazza G., Cini A., Cervo R., Longo S. Just phoresy? Reduced lifespan in red palm weevils Rhynchophorus ferrugineus (Coleoptera: Curculionidae) infested by the mite Centrouropoda almerodai (Uroactiniinae: Uropodina) Ital. J. Zool. 2011;78:101–105. doi: 10.1080/11250003.2010.509135. [DOI] [Google Scholar]
- 156.Lo Verde G., Massa B. Note sul Punteruolo della palma Rhynchophorus ferrugineus (Olivier, 1790) in Sicilia (Coleoptera Curculionidae) Boll. Zool. Agraria Bach. 2007;39:131–149. [Google Scholar]
- 157.Lo Verde G., Caldarella C.G., La Mantia G., Sauro G. Punteruolo rosso delle palme, l’emergenza continua. Inf. Agr. 2008;64:74–77. [Google Scholar]
- 158.Sarto i Monteys V., Aguilar L. The castniid palm borer, Paysandisia archon (Burmeister, 1880), in Europe: Comparative biology, pest status and possible control methods (Lepidoptera: Castniidae) Nachr. Entomol. Ver. Apollo. 2005;26:61–94. [Google Scholar]
- 159.Al-Deeb M.A., Muzaffar S., Abuagla A.M., Sharif E.M. Distribution and abundance of phoretic mites (Astigmata, Mesostigmata) on Rhynchophorus ferrugineus (Coleoptera: Curculionidae) Fla Entomol. 2011;94:748–755. doi: 10.1653/024.094.0403. [DOI] [Google Scholar]
- 160.Hassan M.F., Nasr A.K., Allam S.F., Taha H.A., Mahmoud R.A. Biodiversity and seasonal fluctuation of mite families associated with the red palm weevil, Rhynchophorus ferrugineus Oliver (Coleoptera: Curculionidae) in Egypt. Egypt. J. Biol. Pest Control. 2011;21:317–323. [Google Scholar]
- 161.Longo S., Ragusa S. Presenza e diffusione in Italia dell’acaro Centrouropoda almerodai (Uroactiniinae: Uropodina) Boll. Zool. Agr. Bachic. 2006;38:265–269. [Google Scholar]
- 162.Atakan E., Çobanoğlu S., Yüsel O., Ali Bal D. Phoretic uropodid mites (Acarina: Uropodidae) on the red palm weevil [Rhynchophorus ferrugineus (Oliver, 1790) (Coleoptera: Curculionidae)] Türk. Entomol. Derg. 2009;33:93–105. [Google Scholar]
- 163.Porcelli F., Ragusa E., D’Onghia A.M., Mizzi S., Mifsud D. Occurrence of Centrouropoda almerodai and Uroobovella marginata (Acari: Uropodina) phoretic on the red palm weevil in Malta. Bull. Entomol. Soc. Malta. 2009;2:61–66. [Google Scholar]
- 164.El-Sharabasy H.M. A survey of mite species associated with the red palm weevil, Rhynchophorus ferrugineus Oliver in Egypt. Egypt. J. Biol. Pest Control. 2010;20:67–70. [Google Scholar]
- 165.Allam S.F., Elbadawy A.R. Mass production of the facultative parasitic mite, Aegyptus rhynchophorus, as a natural enemy against the red palm weevil in Egypt; Proceedings of the 8th International Agriculture Symposium; Jahorina, Bosnia and Herțegovina. 2017; pp. 1172–1177. [Google Scholar]
- 166.Allam S.F., Hassan M.F., Taha H.A., Mahmoud R.A. Hyperphoresy of phoretic deutonymph of Aegyptus rhynchophorus (El-Bishlawi & Allam) (Acari: Uropodidae: Trachyuropodidae) with the red palm weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) in Egypt. Acarines. 2013;7:3–6. [Google Scholar]
- 167.Allam S.F., El-Bishlawi S.M.O. Description of immature stages of Aegyptus rhynchophorus (Elbishlawy & Allam), (Uropodina: Trachyuropodidae) Acarines. 2010;4:3–5. [Google Scholar]
- 168.Al-Dhafar Z.M., Al-Qahtani A.M. Mites associated with the red palm weevil, Rhynchophorus ferrugineus Oliver in Saudi Arabia with a description of a new species. Acarines. 2012;6:3–6. doi: 10.21608/ajesa.2012.163616. [DOI] [Google Scholar]
- 169.Bajerlein D., Bloszyk J. Phoresy of Uropoda orbicularis (Acari: Mesostigmata) by beetles (Coleoptera) associated with cattle dung in Poland. Eur. J. Entomol. 2004;101:185–188. doi: 10.14411/eje.2004.022. [DOI] [Google Scholar]
- 170.Zimmermann G. Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci. Technol. 2007;17:879–920. doi: 10.1080/09583150701593963. [DOI] [Google Scholar]
- 171.Roy H.E., Brodie E.L., Chandler D., Goettel M.S., Pell J.K., Wajnberg E., Vega F.E. Deep space and hidden depths: Understanding the evolution and ecology of fungal entomopathogens. In: Roy H.E., Vega F.E., Chandler D., Goettel M.S., Pell J.K., Wajnberg E., editors. The Ecology of Fungal Entomopathogens. Springer; Dordrecht, The Netherlands: 2010. pp. 1–6. [Google Scholar]
- 172.Tahir M., Wakil W., Ali A., Sahi S.T. Pathogenicity of Beauveria bassiana and Metarhizium anisopliae isolates against larvae of the polyphagous pest Helicoverpa armigera. Entomol. Gen. 2019;38:225–242. doi: 10.1127/0171-8177/2019/0460. [DOI] [Google Scholar]
- 173.Yasin M., Wakil W., Ghazanfar M.U., Qayyum M.A., Tahir M., Bedford G.O. Virulence of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae against red palm weevil, Rhynchophorus ferrugineus (Olivier) Entomol. Res. 2019;49:3–12. doi: 10.1111/1748-5967.12260. [DOI] [Google Scholar]
- 174.Wakil W., Kavallieratos N.G., Ghazanfar M.U., Usman M., Habib A., El-Shafie H.A.F. Efficacy of different entomopathogenic fungal isolates against four key stored-grain beetle species. J. Stored Prod. Res. 2021;93:101845. doi: 10.1016/j.jspr.2021.101845. [DOI] [Google Scholar]
- 175.Sabbahi R., Hock V. Entomopathogenic fungi against the red palm weevil: Lab and field evidence. Crop Prot. 2024;177:106566. doi: 10.1016/j.cropro.2023.106566. [DOI] [Google Scholar]
- 176.Alwaneen W.S., Wakil W., Kavallieratos N.G., Qayyum M.A., Tahir M., Rasool K.G., Shapiro-Ilan D. Efficacy and persistence of entomopathogenic fungi against Rhynchophorus ferrugineus on date palm: Host-to-host transmission. Agronomy. 2024;14:642. doi: 10.3390/agronomy14040642. [DOI] [Google Scholar]
- 177.Al-Keridis L.A., Gaber N.M., Aldawood A.S. Pathogenicity of Saudi Arabian fungal isolates against egg and larval stages of Rhynchophorus ferrugineus under laboratory conditions. Int. J. Trop. Insect Sci. 2020;40:845–853. doi: 10.1007/s42690-020-00141-8. [DOI] [Google Scholar]
- 178.Ahmed R., Freed S. Virulence of Beauveria bassiana Balsamo to red palm weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) Egypt. J. Biol. Pest Control. 2021;31:77. doi: 10.1186/s41938-021-00422-5. [DOI] [Google Scholar]
- 179.Yang T.H., Wu L.H., Liao C.T., Li D., Shin T.Y., Kim J.S., Nai Y.S. Entomopathogenic fungi-mediated biological control of the red palm weevil Rhynchophorus ferrugineus. J. Asia-Pac. Entomol. 2023;26:102037. doi: 10.1016/j.aspen.2023.102037. [DOI] [Google Scholar]
- 180.Hussain A., Rizwan-ul-Haq M., Al-Ayedh H., AlJabr A.M. Susceptibility and immune defense mechanisms of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) against entomopathogenic fungal infections. Int. J. Mol. Sci. 2016;17:1518. doi: 10.3390/ijms17091518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Abdel-Raheem M.A., Alghamdi H.A., Reyad N.F. Virulence of fungal spores and silver nanoparticles from entomopathogenic fungi on the red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) Egypt. J. Biol. Pest Control. 2019;29:97. doi: 10.1186/s41938-019-0200-2. [DOI] [Google Scholar]
- 182.El Husseini M.M. Efficacy of the fungus Beauveria bassiana (Balsamo) Vuillemin on the red palm weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) larvae and adults under laboratory conditions. Egypt. J. Biol. Pest Control. 2019;29:58. doi: 10.1186/s41938-019-0155-3. [DOI] [Google Scholar]
- 183.Gindin G., Levski S., Glazer I., Soroker V. Evaluation of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana against the red palm weevil Rhynchophorus ferrugineus. Phytoparasitica. 2006;34:370–379. doi: 10.1007/BF02981024. [DOI] [Google Scholar]
- 184.Hou F.J., Addis S.N.K., Azmi W.A. Virulence evaluation of entomopathogenic fungi against the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Dryopthoridae) Malays. Appl. Biol. 2018;47:25–30. [Google Scholar]
- 185.Al-Zyoud F., Shibli R., Ghabeish I. Current status, challenges, management and future perspectives of the red palm weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) eradication—A review. J. Exp. Biol. Agric. Sci. 2021;9:697–714. doi: 10.18006/2021.9(6).697.714. [DOI] [Google Scholar]
- 186.Cito A., Mazza G., Strangi A., Benvenuti C., Barzanti G.P., Dreassi E., Turchetti T., Francardi V., Roversi P.F. Characterization and comparison of Metarhizium strains isolated from Rhynchophorus ferrugineus. FEMS Microbiol. Lett. 2014;355:108–115. doi: 10.1111/1574-6968.12470. [DOI] [PubMed] [Google Scholar]
- 187.Francardi V., Benvenuti C., Barzanti G.P., Roversi P.F. Autocontamination trap with entomopathogenic fungi: A possible strategy in the control of Rhynchophorus ferrugineus (Olivier) (Coleoptera Curculionidae) Redia. 2013;96:57–67. [Google Scholar]
- 188.Sewify G.H., Belal M.H., Al-Awash S.A. Use of the entomopathogenic fungus Beauveria bassiana for the biological control of the red palm weevil Rhynchophorus ferrugineus Olivier. Egypt. J. Biol. Pest Control. 2009;19:157–163. [Google Scholar]
- 189.Sutanto K.D., Al-Shahwan I.M., Husain M., Rasool K.G., Mankin R.W., Aldawood A.S. Field evaluation of promising indigenous entomopathogenic fungal isolates against red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) J. Fungi. 2023;9:68. doi: 10.3390/jof9010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Manzoor M., Ahmad J.N., Sharif M.Z., Majeed D., Kiran H., Jafir M., Ali H. Comparative effectiveness of entomopathogenic nematodes against red palm weevil (Rhynchophorus ferrugineus) in Pakistan. J. Entomol. Zool. Stud. 2017;5:756–760. [Google Scholar]
- 191.Bhat A.H., Chaubey A.K., Askary T.H. Global distribution of entomopathogenic nematodes, Steinernema and Heterorhabditis. Egypt. J. Biol. Pest Control. 2020;30:31. doi: 10.1186/s41938-020-0212-y. [DOI] [Google Scholar]
- 192.Liu W.T., Chen T.L., Hou R.F., Chen C.C., Tu W.C. The invasion and encapsulation of the entomopathogenic nematode, Steinernema abbasi, in Aedes albopictus (Diptera: Culicidae) larvae. Insects. 2020;11:832. doi: 10.3390/insects11120832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Stock S.P., Hunt D.J. Morphology and systematics of nematodes used in biocontrol. In: Grewal P.S., Ehlers R.-U., Shapiro-Ilan. D.I., editors. Nematodes as Biocontrol Agents. CABI; Wallingford, UK: 2005. pp. 3–43. [Google Scholar]
- 194.Lacey L.A., Georgis R. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J. Nematol. 2012;44:218. [PMC free article] [PubMed] [Google Scholar]
- 195.Abbas M.S.T., Saleh M.M.E., Akil A.M. Laboratory and field evaluation of the pathogenicity of entomopathogenic nematodes to the red palm weevil Rhynchophorus ferrugineus (Oliv.) (Col.: Curculionidae) Anz. Schädlingskd. 2001;74:167–168. doi: 10.1046/j.1439-0280.2001.d01-1.x. [DOI] [Google Scholar]
- 196.Llácer E., Martínez de Altube M.M., Jacas J.A. Evaluation of the efficacy of Steinernema carpocapsae in a chitosan formulation against the red palm weevil, Rhynchophorus ferrugineus, in Phoenix canariensis. BioControl. 2009;54:559–565. doi: 10.1007/s10526-008-9208-3. [DOI] [PubMed] [Google Scholar]
- 197.Gözel U., Gözel Ç., Yurt Ç., İnci D. Efficacy of entomopathogenic nematodes on the red palm weevil Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera: Curculionidae) larvae. Int. J. Bioassays. 2015;4:4436–4439. [Google Scholar]
- 198.Santhi V.S., Salame L., Nakache Y., Koltai H., Soroker V., Glazer I. Attraction of entomopathogenic nematodes Steinernema carpocapsae and Heterorhabditis bacteriophora to the red palm weevil (Rhynchophorus ferrugineus) Biol. Control. 2015;83:75–81. doi: 10.1016/j.biocontrol.2015.01.003. [DOI] [Google Scholar]
- 199.El Sadawy H.A., Namaky A.H.E., Al Omari F., Bahareth O.M. Susceptibility of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) to entomopathogenic nematodes with regard to its immune response. Biol. Control. 2020;148:104308. doi: 10.1016/j.biocontrol.2020.104308. [DOI] [Google Scholar]
- 200.Binda-Rossetti S., Mastore M., Protasoni M., Brivio M.F. Effects of an entomopathogen nematode on the immune response of the insect pest red palm weevil: Focus on the host antimicrobial response. J. Invertebr. Pathol. 2016;133:110–119. doi: 10.1016/j.jip.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 201.Wakil W., Yasin M., Shapiro-Ilan D. Effects of single and combined applications of entomopathogenic fungi and nematodes against Rhynchophorus ferrugineus (Olivier) Sci. Rep. 2017;7:5971. doi: 10.1038/s41598-017-05615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mazza G., Arizza V., Baracchi D., Barzanti G.P., Benvenuti C., Francardi V., Frandi A., Gherardi F., Longo S., Manachini B., et al. Antimicrobial activity of the red palm weevil Rhynchophorus ferrugineus. Bull. Insectol. 2011;64:33–41. [Google Scholar]
- 203.Zeng T., Bai X., Liu Y.L., Li J.F., Lu Y.Y., Qi Y.X. Intestinal responses of the oriental fruit fly Bactrocera dorsalis (Hendel) after ingestion of an entomopathogenic bacterium strain. Pest Manag. Sci. 2020;76:653–664. doi: 10.1002/ps.5563. [DOI] [PubMed] [Google Scholar]
- 204.Thanwisai A., Tandhavanant S., Saiprom N., Waterfield N.R., Ke Long P., Bode H.B., Peacock S.J., Chantratita N. Diversity of Xenorhabdus and Photorhabdus spp. and their symbiotic entomopathogenic nematodes from Thailand. PloS ONE. 2012;7:e43835. doi: 10.1371/journal.pone.0043835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Machado R.A., Wüthrich D., Kuhnert P., Arce C.C., Thönen L., Ruiz C., Zhang X., Robert C.A.M., Karimi J., Kamali S., et al. Whole-genome-based revisit of Photorhabdus phylogeny: Proposal for the elevation of most photorhabdus subspecies to the species level and description of one novel species Photorhabdus bodei sp. nov., and one novel subspecies Photorhabdus laumondii subsp. clarkei subsp. nov. Int. J. Syst. Evol. Microbiol. 2018;68:2664–2681. doi: 10.1099/ijsem.0.002820. [DOI] [PubMed] [Google Scholar]
- 206.Sajnaga E., Kazimierczak W. Evolution and taxonomy of nematode-associated entomopathogenic bacteria of the genera Xenorhabdus and Photorhabdus: An οverview. Symbiosis. 2020;80:1–13. doi: 10.1007/s13199-019-00660-0. [DOI] [Google Scholar]
- 207.Heckel D.G. How do toxins from Bacillus thuringiensis kill insects? An evolutionary perspective. Arch. Insect Biochem. Physiol. 2020;104:e21673. doi: 10.1002/arch.21673. [DOI] [PubMed] [Google Scholar]
- 208.Paliwal D., Hamilton A.J., Barrett G.A., Alberti F., Van Emden H., Monteil C.L., Mauchline T.H., Nauen R., Wagstaff C., Bass C., et al. Identification of novel aphid-killing bacteria to protect plants. Microb. Biotechnol. 2022;15:1203–1220. doi: 10.1111/1751-7915.13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Paliwal D., Rabiey M., Mauchline T.H., Hassani-Pak K., Nauen R., Wagstaff C., Jackson R.W. Multiple toxins and a protease contribute to the aphid-killing ability of Pseudomonas fluorescens PpR24. Environ. Microbiol. 2024;26:e16604. doi: 10.1111/1462-2920.16604. [DOI] [PubMed] [Google Scholar]
- 210.Pineda-Castellanos M.L., Rodríguez-Segura Z., Villalobos F.J., Hernández L., Lina L., Nuñez-Valdez M.E. Pathogenicity of isolates of Serratia marcescens towards larvae of the scarab Phyllophaga blanchardi (Coleoptera) Pathogens. 2015;4:210–228. doi: 10.3390/pathogens4020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Aggarwal C., Paul S., Tripathi V., Paul B., Khan M.A. Characterization of putative virulence factors of Serratia marcescens Strain SEN for pathogenesis in Spodoptera litura. J. Invertebr. Pathol. 2017;143:115–123. doi: 10.1016/j.jip.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 212.Francesca N., Alfonzo A., Lo Verde G., Settanni L., Sinacori M., Lucido P., Moschetti G. Biological activity of Bacillus spp. evaluated on eggs and larvae of red palm weevil Rhynchophorus ferrugineus. Ann. Microbiol. 2015;65:477–485. doi: 10.1007/s13213-014-0881-4. [DOI] [Google Scholar]
- 213.Almasoudi N.M., Asiry K.A., Abo-Elyousr K.A. Isolation, Identification and efficacy of three bacterial isolates against the red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) Egypt. J. Biol. Pest Control. 2022;32:52. doi: 10.1186/s41938-022-00551-5. [DOI] [Google Scholar]
- 214.Pu Y.C., Hou Y.M. Isolation and identification of bacterial strains with insecticidal activities from Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) J. Appl. Entomol. 2016;140:617–626. doi: 10.1111/jen.12293. [DOI] [Google Scholar]
- 215.Salama H.S., Foda M.S., El-Bendary M.A., Abdel-Razek A. Infection of red palm weevil, Rhynchophorus ferrugineus, by spore-forming bacilli indigenous to its natural habitat in Egypt. J. Pest Sci. 2004;77:27–31. doi: 10.1007/s10340-003-0023-4. [DOI] [Google Scholar]
- 216.Mahmoud Y.A., Salama H.S., Moawed S.M., Ebadah I.M.A., Sadek H.E., Khalifa I.A. Virulence of a new isolate of Cytoplasmic Polyhedrosis Virus against the red palm weevil, Rhynchophorus ferrugineus (Oliv.) (Order: Coleoptera, Family: Curculionidae) Asian J. Agric. Hortic. Res. 2018;2:1–10. doi: 10.9734/AJAHR/2018/44385. [DOI] [Google Scholar]
- 217.Pu Y.C., Ma T.L., Hou Y.M., Sun M. An entomopathogenic bacterium strain, Bacillus thuringiensis, as a biological control agent against the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) Pest Manag. Sci. 2017;3:1494–1502. doi: 10.1002/ps.4485. [DOI] [PubMed] [Google Scholar]
- 218.Mastore M., Arizza V., Manachini B., Brivio M.F. Modulation of immune responses of Rhynchophorus ferrugineus (Insecta: Coleoptera) induced by the entomopathogenic nematode Steinernema carpocapsae (Nematoda: Rhabditida) Insect Sci. 2015;22:748–760. doi: 10.1111/1744-7917.12141. [DOI] [PubMed] [Google Scholar]
- 219.Sun X., Yan W., Zhang J., Niu X., Li F., Qin W., Ma G. Frozen section and electron microscopy studies of the infection of the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) by the entomopathogenic fungus Metarhizium anisopliae. Springerplus. 2016;5:1748. doi: 10.1186/s40064-016-3416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lo Verde G., Torta L., Mondello V., Caldarella C.G., Burruano S., Caleca V. Pathogenicity bioassays of isolates of Beauveria bassiana on Rhynchophorus ferrugineus. Pest Manag. Sci. 2014;71:323–328. doi: 10.1002/ps.3852. [DOI] [PubMed] [Google Scholar]
- 221.Yasin M., Wakil W., Qayyum M.A., Ali S., Sajjad A., Aqueel M.A., Shakeel M. Biocontrol potential of entomopathogenic fungi, nematodes, and bacteria against Rhynchophorus ferrugineus (Olivier) Egypt. J. Biol. Pest Control. 2021;31:138. doi: 10.1186/s41938-021-00484-5. [DOI] [Google Scholar]
- 222.Atwa A.A., Hegazi E.M. Comparative susceptibilities of different life stages of the red palm weevil (Coleoptera: Curculionidae) treated by entomopathogenic nematodes. J. Econ. Entomol. 2014;107:1339–1347. doi: 10.1603/EC13438. [DOI] [PubMed] [Google Scholar]
- 223.Tapia G., Ruiz M.A., Téllez M.M. Recommendations for a preventive strategy to control red palm weevil (Rhynchophorus ferrugineus, Olivier) based on the use of insecticides and entomopathogenic nematodes. EPPO Bull. 2011;41:136–141. doi: 10.1111/j.1365-2338.2011.02448.x. [DOI] [Google Scholar]
- 224.Hajjar M.J., Ajlan A.M., Al-Ahmad M.H. New approach of Beauveria bassiana to control the red palm weevil (Coleoptera: Curculionidae) by trapping technique. J. Econ. Entomol. 2015;108:425–432. doi: 10.1093/jee/tou055. [DOI] [PubMed] [Google Scholar]
- 225.Abdel-Raheem M.A., Reyad N.F., Alghamdi H.A. Virulence of nano–particle preparation of entomopathogenic fungi and entomopathogenic bacteria against red palm weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) Biotechnol. Lett. 2020;25:1151–1159. doi: 10.25083/rbl/25.1/1151.1159. [DOI] [Google Scholar]
- 226.Sabbour M.M., Abdel-Raheem M.A. Evaluations of Isaria fumosorosea isolates against the red palm weevil Rhynchophorus ferrugineus under laboratory and field conditions. Curr. Sci. Int. 2014;3:179–185. [Google Scholar]
- 227.Sabbour M.M., Solieman N.Y. Preliminary investigations into the biological control of red palm weevil Rhynchophorus ferrugineus by using three isolates of the fungus Lecanicillium (Verticillium) lecanii in Egypt. Int. J. Sci. Res. 2014;3:2060–2066. [Google Scholar]
- 228.Husain M., Rasool K.G., Sutanto K.D., Omer A.O., Tufail M., Aldawood A.S. Laboratory evaluation of indigenous and commercial entomopathogenic nematodes against red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) Insects. 2024;15:290. doi: 10.3390/insects15040290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ali M.A., Bekhiet H.K., Ragheb D.A., El-Feshaway A.A. Evaluation of some entomopathogens on the red palm weevil, Rhynchophorus ferrugineus under laboratory and field conditions. Egypt. J. Agric. Res. 2018;96:415–430. doi: 10.21608/ejar.2018.133799. [DOI] [Google Scholar]
- 230.Mirhosseini M.A., Fathipour Y., Reddy G.V. Arthropod development’s response to temperature: A review and new software for modeling. Ann. Entomol. Am. 2017;110:507–520. doi: 10.1093/aesa/sax071. [DOI] [Google Scholar]
- 231.Bale J.S., Masters G.J., Hodkinson I.D., Awmack C., Bezemer T.M., Brown V.K., Butterfield J., Buse A., Coulson J.C., Farrar J., et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Change Biol. 2002;8:1–16. doi: 10.1046/j.1365-2486.2002.00451.x. [DOI] [Google Scholar]
- 232.Li L., Qin W.Q., Ma Z.L., Yan W., Huang S.C., Peng Z.Q. Effect of temperature on the population growth of Rhynchophorus ferrugineus (Coleoptera: Curculionidae) on sugarcane. Environ. Entomol. 2010;39:999–1003. doi: 10.1603/EN09316. [DOI] [PubMed] [Google Scholar]
- 233.Mendel Z., Voet H., Modan N., Naor R., Ment D. Seismic sensor-based management of the red palm weevil Rhynchophorus ferrugineus in date palm plantations. Pest Manag. Sci. 2024;80:1053–1064. doi: 10.1002/ps.7836. [DOI] [PubMed] [Google Scholar]
- 234.Massa R., Panariello G., Pinchera D., Schettino F., Caprio E., Griffo R., Migliore M.D. Experimental and numerical evaluations on palm microwave hearting for red palm weevil pest control. Sci. Rep. 2016;7:45299. doi: 10.1038/srep45299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available within the article.