Abstract
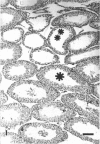
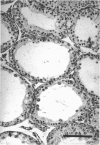
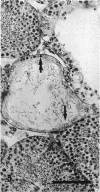
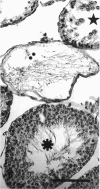
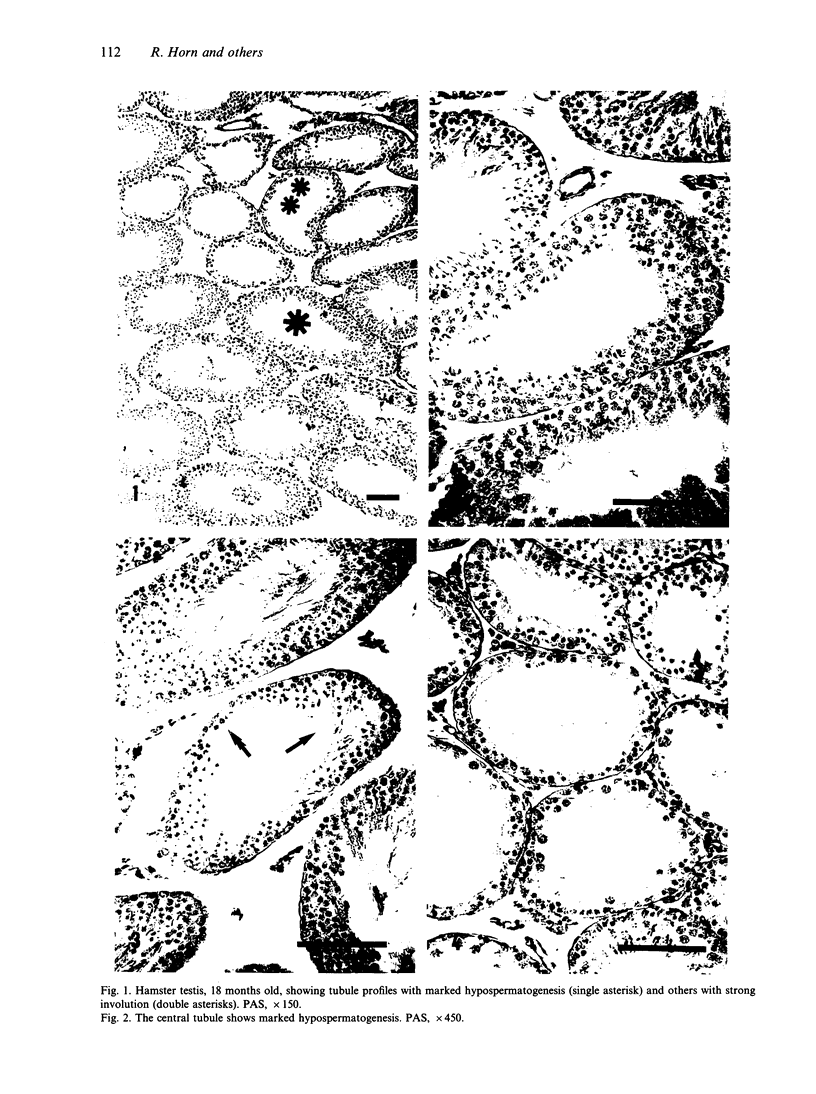
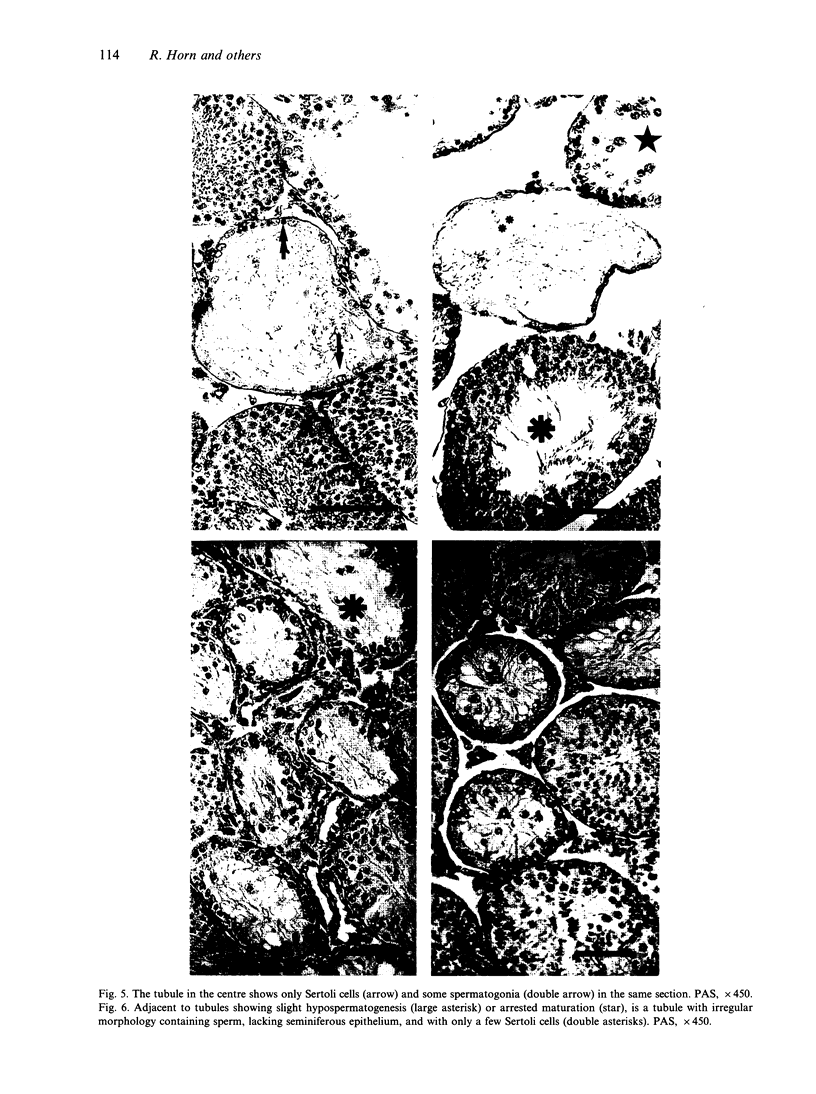
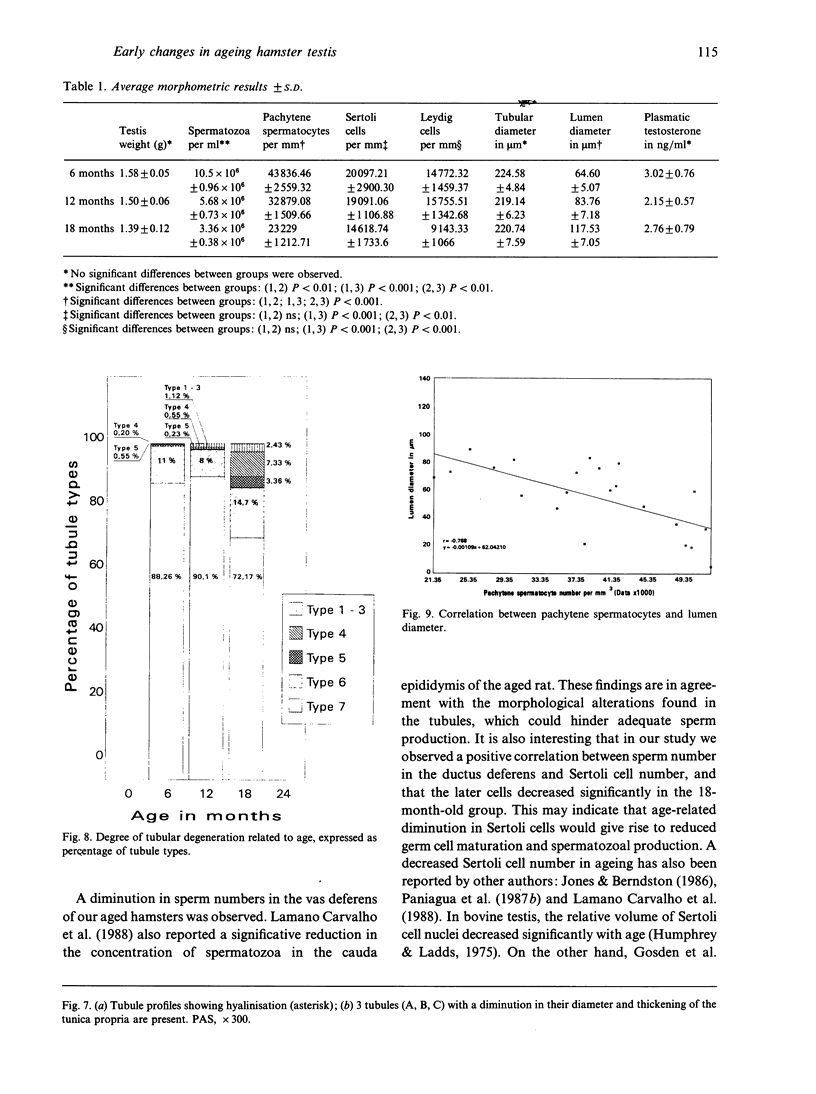
The histological and morphometric features of the aged golden hamster testis were examined and compared with those of adult animals. Three age groups (6, 12 and 18 months) were studied by light microscopy, and testosterone levels were determined. The observations showed a progressive involution of the seminiferous tubules, beginning to be perceptible at 12 months with slight hypospermatogenesis and desquamation. In 18-month-old specimens degeneration was more significant and histopathological lesions could be classified on a 6-point scale, ranging from slight hypospermatogenesis to absence of germ cells. These involutive changes were not homogeneously distributed in the testis; affected tubules close to seeming normal ones were present. The morphometric results point to a progressive diminution, in the 3 age groups, in vas deferens spermatozoa, pachytene spermatocytes, and Sertoli and Leydig cells (the latter significantly diminished only in the 18-month-old group). For morphometric purposes a 7-point scale of tubule degeneration was used, showing a significant increase, with age, in the presence of more degenerated tubule stages. Several correlations were found between the morphometric variables, outlining existing relations between age and the associated diminution of several testis cell types, and lumen diameter. No significant differences were found between groups in serum testosterone levels. In conclusion, histological changes related to age are evident in 18-month-old animals, while at 12 months a diminution in germ cell numbers and sperm production is detectable.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almahbobi G., Papadopoulos V., Carreau S., Silberzahn P. Age-related morphological and functional changes in the Leydig cells of the horse. Biol Reprod. 1988 Apr;38(3):653–665. doi: 10.1095/biolreprod38.3.653. [DOI] [PubMed] [Google Scholar]
- Baker H. W., Burger H. G., de Kretser D. M., Hudson B., O'Connor S., Wang C., Mirovics A., Court J., Dunlop M., Rennie G. C. Changes in the pituitary-testicular system with age. Clin Endocrinol (Oxf) 1976 Jul;5(4):349–372. doi: 10.1111/j.1365-2265.1976.tb01964.x. [DOI] [PubMed] [Google Scholar]
- Carvalho T. L., Favaretto A. L., Komesu M. C., Lopes R. A., Petenusci S. O., Silva-Netto C. R. Influence of age on the production of rat spermatozoa, on their concentration in the cauda epididymidis, and on FSH, LH and testosterone plasma levels. Histol Histopathol. 1988 Oct;3(4):413–417. [PubMed] [Google Scholar]
- Cutler R. G. Human longevity and aging: possible role of reactive oxygen species. Ann N Y Acad Sci. 1991;621:1–28. doi: 10.1111/j.1749-6632.1991.tb16965.x. [DOI] [PubMed] [Google Scholar]
- Flickinger C. J., Herr J. C., Howards S. S., Sisak J. R., Gleavy J. M., Fusia T. J., Vailes L. D., Handley H. H. Early testicular changes after vasectomy and vasovasostomy in Lewis rats. Anat Rec. 1990 May;227(1):37–46. doi: 10.1002/ar.1092270106. [DOI] [PubMed] [Google Scholar]
- Gosden R. G., Richardson D. W., Brown N., Davidson D. W. Structure and gametogenic potential of seminiferous tubules in ageing mice. J Reprod Fertil. 1982 Jan;64(1):127–133. doi: 10.1530/jrf.0.0640127. [DOI] [PubMed] [Google Scholar]
- Harbitz T. B. Morphometric studies of the Leydig cells in elderly men with special reference to the histology of the prostate. An analysis in an autopsy series. Acta Pathol Microbiol Scand A. 1973 May;81(3):301–314. doi: 10.1111/j.1699-0463.1973.tb03539.x. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Antecedents of cell aging research. Exp Gerontol. 1989;24(5-6):355–365. doi: 10.1016/0531-5565(89)90043-0. [DOI] [PubMed] [Google Scholar]
- Humphrey J. D., Ladds P. W. A quantitative histological study of changes in the bovine testis and epididymis associated with age. Res Vet Sci. 1975 Sep;19(2):135–141. [PubMed] [Google Scholar]
- Johnson L., Abdo J. G., Petty C. S., Neaves W. B. Effect of age on the composition of seminiferous tubular boundary tissue and on the volume of each component in humans. Fertil Steril. 1988 Jun;49(6):1045–1051. doi: 10.1016/s0015-0282(16)59959-2. [DOI] [PubMed] [Google Scholar]
- Johnson L., Neaves W. B. Age-related changes in the Leydig cell population, seminiferous tubules, and sperm production in stallions. Biol Reprod. 1981 Apr;24(3):703–712. doi: 10.1095/biolreprod24.3.703. [DOI] [PubMed] [Google Scholar]
- Johnson L. Spermatogenesis and aging in the human. J Androl. 1986 Nov-Dec;7(6):331–354. doi: 10.1002/j.1939-4640.1986.tb00943.x. [DOI] [PubMed] [Google Scholar]
- Johnson L., Zane R. S., Petty C. S., Neaves W. B. Quantification of the human Sertoli cell population: its distribution, relation to germ cell numbers, and age-related decline. Biol Reprod. 1984 Nov;31(4):785–795. doi: 10.1095/biolreprod31.4.785. [DOI] [PubMed] [Google Scholar]
- Jones L. S., Berndtson W. E. A quantitative study of Sertoli cell and germ cell populations as related to sexual development and aging in the stallion. Biol Reprod. 1986 Aug;35(1):138–148. doi: 10.1095/biolreprod35.1.138. [DOI] [PubMed] [Google Scholar]
- Kaler L. W., Neaves W. B. Attrition of the human Leydig cell population with advancing age. Anat Rec. 1978 Dec;192(4):513–518. doi: 10.1002/ar.1091920405. [DOI] [PubMed] [Google Scholar]
- Lowseth L. A., Gerlach R. F., Gillett N. A., Muggenburg B. A. Age-related changes in the prostate and testes of the beagle dog. Vet Pathol. 1990 Sep;27(5):347–353. doi: 10.1177/030098589002700507. [DOI] [PubMed] [Google Scholar]
- Nistal M., Codesal J., Paniagua R., Santamaria L. Decrease in the number of human Ap and Ad spermatogonia and in the Ap/ Ad ratio with advancing age. New data on the spermatogonial stem cell. J Androl. 1987 Mar-Apr;8(2):64–68. doi: 10.1002/j.1939-4640.1987.tb00950.x. [DOI] [PubMed] [Google Scholar]
- Paniagua R., Martín A., Nistal M., Amat P. Testicular involution in elderly men: comparison of histologic quantitative studies with hormone patterns. Fertil Steril. 1987 Apr;47(4):671–679. doi: 10.1016/s0015-0282(16)59120-1. [DOI] [PubMed] [Google Scholar]
- Paniagua R., Nistal M., Amat P., Rodriguez M. C., Martin A. Seminiferous tubule involution in elderly men. Biol Reprod. 1987 May;36(4):939–947. doi: 10.1095/biolreprod36.4.939. [DOI] [PubMed] [Google Scholar]
- Paniagua R., Nistal M., Sáez F. J., Fraile B. Ultrastructure of the aging human testis. J Electron Microsc Tech. 1991 Oct;19(2):241–260. doi: 10.1002/jemt.1060190209. [DOI] [PubMed] [Google Scholar]
- Pirke K. M., Doerr P. Age related changes and interrelationships between plasma testosterone, oestradiol and testosterone-binding globulin in normal adult males. Acta Endocrinol (Copenh) 1973 Dec;74(4):792–800. doi: 10.1530/acta.0.0740792. [DOI] [PubMed] [Google Scholar]
- Plymate S. R., Tenover J. S., Bremner W. J. Circadian variation in testosterone, sex hormone-binding globulin, and calculated non-sex hormone-binding globulin bound testosterone in healthy young and elderly men. J Androl. 1989 Sep-Oct;10(5):366–371. doi: 10.1002/j.1939-4640.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Swanson L. J., Desjardins C., Turek F. W. Aging of the reproductive system in the male hamster: behavioral and endocrine patterns. Biol Reprod. 1982 Jun;26(5):791–799. doi: 10.1095/biolreprod26.5.791. [DOI] [PubMed] [Google Scholar]
- Takano H., Abe K. Age-related histologic changes in the adult mouse testis. Arch Histol Jpn. 1987 Dec;50(5):533–544. doi: 10.1679/aohc.50.533. [DOI] [PubMed] [Google Scholar]
- Vijg J., Papaconstantinou J. Aging and longevity genes: strategies for identifying DNA sequences controlling life span. J Gerontol. 1990 Sep;45(5):B179–B182. doi: 10.1093/geronj/45.5.b179. [DOI] [PubMed] [Google Scholar]
- Wright W. W., Fiore C., Zirkin B. R. The effect of aging on the seminiferous epithelium of the brown Norway rat. J Androl. 1993 Mar-Apr;14(2):110–117. [PubMed] [Google Scholar]