Abstract
We describe two locally acquired cases of Mycobacterium ulcerans infection (Buruli ulcer) in the town of Batemans Bay on the east coast of New South Wales (NSW), Australia, 150 km north of Eden, the only other place in NSW where Buruli ulcer has likely been locally acquired. Genomic analysis showed that the bacterial isolates from the cases were identical but belonged to a phylogenetically distinct M. ulcerans clade that was most closely related to the isolate from the earlier case in Eden to the south. It is proposed that Batemans Bay is a new endemic focus of human Buruli ulcer transmission.
Author summary
Mycobacterium ulcerans causes Buruli ulcer, a progressive infection affecting skin and subcutaneous soft tissues. Although predominantly reported in central and west Africa, Australia, and Japan, new endemic regions for Buruli ulcer continue to be discovered. In Australia, the state of Victoria has seen a significant public health challenge with Buruli ulcer, where evidence points to zoonotic transmission from native possums to humans via mosquitoes. Recently, a new location of locally acquired Buruli ulcer has been identified in Batemans Bay, New South Wales (NSW), Australia. Genome sequencing of cases in this area has revealed a distinct M. ulcerans genotype, suggesting the pathogen is extant in NSW similar to the situation in Victoria. Surveys of possum excreta in Batemans Bay have confirmed the presence of M. ulcerans, indicating its establishment in a local wildlife reservoir in a new endemic region.
Introduction
Mycobacterium ulcerans is responsible for causing a toxin-mediated, progressive, destructive infection of the skin and soft tissue known as Buruli ulcer [1,2]. This ulcer was likely first described in Uganda in 1897 by the missionary physician Sir Albert Cook, but the causative organism, M. ulcerans, was not formally identified as a new mycobacterial pathogen of humans until the 1940s in Victoria, Australia [3]. While predominantly reported in central and west Africa, Australia, and Japan, new Buruli ulcer endemic locations are still being encountered [4].
In Australia, Buruli ulcer has been increasingly reported in urban areas of Victoria, where the infection has become a significant public health issue following the initial appearance of a small number of localized coastal outbreaks [5]. The reservoir and mode of transmission of M. ulcerans have remained mysterious, but in Victoria, there is now evidence that Buruli ulcer is a zoonosis, with native possums as the key reservoir and mosquitoes as an important vector for transmission to humans [6]. Whether the zoonosis/insect transmission paradigm applies in other endemic regions, particularly Africa, has yet to be determined. Confirmation of high bacterial pathogen load with Buruli ulcer in possums in Victoria (as well as their excreta) has implications for further dissemination of this zoonosis in Australia [7].
Herein, we report a new site of locally acquired Buruli ulcer, in Batemans Bay, New South Wales (NSW), Australia. Genome sequencing and phylogeographic analysis revealed a distinct M. ulcerans genotype, that is consistent with the population structure of the pathogen in the region. The new cases we report here in Batemans Bay could be a harbinger of a disease expansion in NSW similar to Victoria. Additionally, we conducted a survey of possum excreta in a small area near the reported cases to determine the presence of M. ulcerans in the local possum population.
Case 1
A 94-year-old Caucasian male reported that his left fourth finger became entrapped between the folding legs of an outdoor table at his home in Batemans Bay in November 2020. There was no history of travel outside his local area for at least four years. This trauma resulted in pain and swelling to the fourth proximal phalanx over four weeks. He did not recall a previous skin lesion or insect bite at the site. By mid-to-late December 2020, the wound progressively opened on the dorsal aspect of the fourth digit. Seeking treatment at the local hospital, intravenous cefazolin was administered for five days. Although he remained systemically well, due to ongoing progression and expansion of the skin lesion, he was transferred to Canberra Health Services in the Australian Capital Territory (ACT), Australia, for assessment.
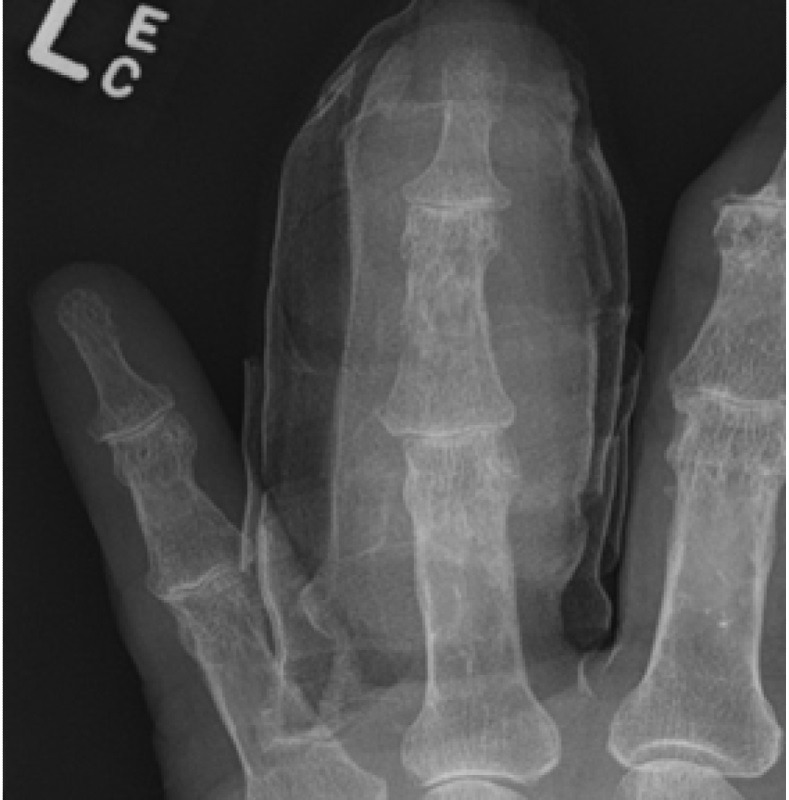
Microscopic examination and bacterial culture of the initial superficial wound swab identified a mixed growth, including multiple Pseudomonas species and coliforms. A subsequent surgical debridement was performed (Fig 1) during which sampled tissue was subjected to further microbiological testing, including being sent for acid-fast bacilli (AFB) stain and mycobacterial culture. An X-ray of the affected finger confirmed changes consistent with osteomyelitis of the middle and proximal phalanges (Fig 2).
Fig 1. Dorsal necrotic ulcer to fourth digit.
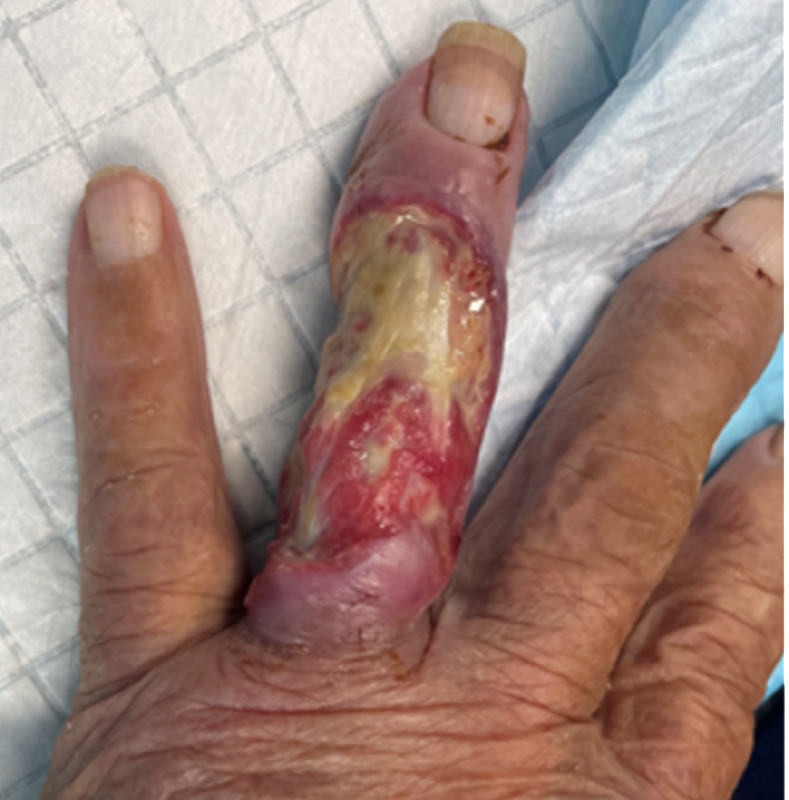
Fig 2. X-ray left fourth digit proximal and middle phalanx, showing focal bony lysis and cortical erosion.
Before results could be confirmed, the patient underwent terminalization of the left fourth finger. Histopathology of the original debrided surgical specimen revealed the presence of necrotic tissue with Ziehl-Neelsen (ZN) stain-positive bacilli. Mycobacterium ulcerans was identified by polymerase chain reaction (PCR) performed on soft tissue from the finger [8]. Following successful source control and the absence of clinical evidence of residual M. ulcerans infection, antibiotic therapy was deemed unnecessary. The patient subsequently experienced a favorable recovery at the site of the proximal wound after the amputation.
Case 2
A 71-year-old previously well Caucasian male noted a mosquito bite on the inner aspect of his right upper arm in mid-May 2023. This occurred while sitting in an armchair in his living room at home in Batemans Bay. He did not recall any interstate or international travel for at least three years. A red mark on the inner side of his right upper arm evolved into a small ulcer by late June, suggesting an incubation period of four to five weeks (Fig 3). Despite being prescribed courses of oral cephalexin and dicloxacillin, aimed at treating methicillin-susceptible Staphylococcus aureus identified from a superficial swab, there was no improvement.
Fig 3. Dry, small ulcer on the inner aspect of the right upper arm.
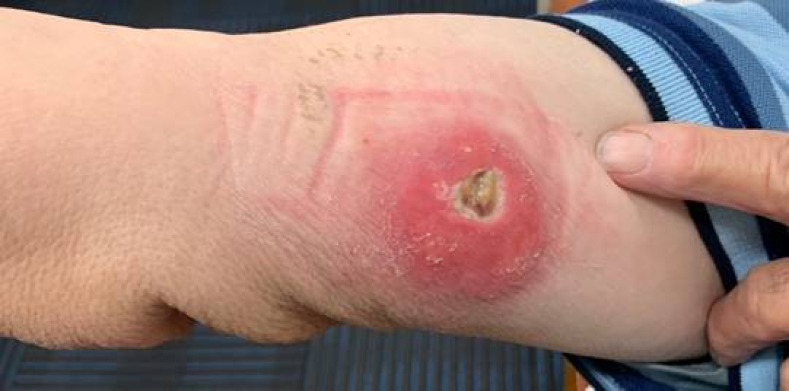
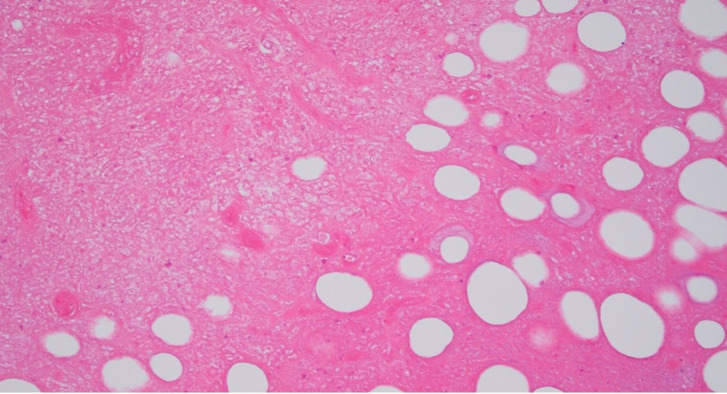
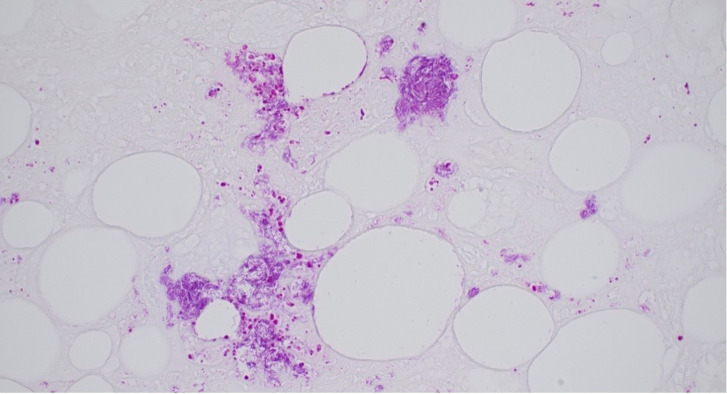
By early July, the ulcer had expanded to four by four centimeters. While it was tender to touch, it was otherwise not painful, and he did not require analgesia. There was a rubbery texture underlying the surface. The patient remained systemically well. He was referred to a general surgeon, and a biopsy in mid-July 2023 demonstrated extensive fat necrosis with numerous acid-fast bacilli (Figs 4 and 5). The 16S and internal transcribed spacer (ITS) region amplification revealed mycobacterial DNA but was unable to distinguish between M. marinum and M. ulcerans. This was subsequently confirmed to be M. ulcerans via a targeted IS2404 PCR assay performed on a swab taken from the ulcer [8].
Fig 4. Hematoxylin and eosin stains (original magnification ×200) from tissue biopsy of lesion, showing fat necrosis.
Fig 5. Ziehl-Neelsen stain (original magnification ×400) demonstrating acid-fast bacilli in the wound biopsy.
He was commenced on rifampicin 600 mg daily and clarithromycin 500 mg twice daily and experienced improvement of the ulcer over the subsequent two months. By the three-month mark, the lesion had largely resolved.
Methods
Ethics statement
Clinical samples from Buruli ulcer patient lesions were tested under Institutional Review Board (IRB) exemption for the use of de-identified pathology specimens, as per section 3.2.6 of the Australian National Health and Medical Research Council, National Statement on Ethical Conduct in Human Research (2023): “Where human biospecimens were obtained for clinical purposes and have been retained by an accredited clinical pathology service, the biospecimens may be used for research purposes if the identity of the donor is not necessary for the activity”.
Mycobacterial detection
Tissue sample from case 1 and a swab from case 2 were collected for M. ulcerans PCR (DNA extraction followed by PCR detecting the IS2404 amplicon, specific for M. ulcerans).
Tissue sample from case 1 was incubated in Mycobacteria Growth Indicator Tubes (MGIT) containing modified Middlebrook 7H9 Broth base in the liquid media culture, BD BACTEC MGIT 960 automated system (Becton, Dickinson, Sparks, MD, USA). Growth was observed at approximately six weeks. This was then referred to the Victorian Infectious Diseases Reference Laboratory (VIDRL) for identification and DNA extraction, and whole genome sequencing (WGS) was performed at the Microbiological Diagnostic Unit (MDU) Public Health Laboratory, Victoria.
For case 2, the swab was cultured on Brown and Buckle culture media and incubated at 31°C. On detection of growth, the isolate was confirmed as M. ulcerans by PCR and DNA was referred for WGS as for case 1.
Genomic sequencing
Genomic DNA was prepared from M. ulcerans cultures and sequenced using Illumina paired end whole genome sequencing technology. The resulting reads were combined with a set of 44 publicly available clinical isolates that were chosen to depict the main components in the previously defined population structure of M. ulcerans in south-eastern Australia (S1 Table) [9]. Snippy (v4.4.5) was used to map sequence read data against a finished M. ulcerans reference chromosome, isolated from a south-eastern Australian Buruli ulcer case (JKD8049; GenBank accession NZ_CP085200.1; https://github.com/tseemann/snippy). An alignment of core genome single nucleotide polymorphisms (SNP) was derived from mapped reads and used to estimate a maximum likelihood phylogeny using the GTR model of nucleotide substitution with FastTree (v.2.1.10) [10]. The R packages phytools (v.1.0–1) [11] and mapdata (v.2.3.1) [12] were used to align tree tips against a base map to visualize the geographical origins of the clinical isolates.
Possum excreta survey
Australian native possums are a well-established wildlife reservoirs of M. ulcerans. Possums develop Buruli ulcer lesions similar to human lesions, but unlike humans, they also shed the bacteria in their excreta [7]. Field surveys of possum excreta for the presence of M. ulcerans are a convenient means to assess the risk of Buruli ulcer transmission to humans [7]. We conducted a possum excreta survey over a two-day period in December 2023. The survey covered approximately 1.5 km by 1.5 km near the location of the reported cases. The survey involved roadside collection of possum excreta, following a pre-determined 200-meter grid pattern along residential streets. Excreta was not found at all pre-determined sampling locations; however, it was collected from 27 locations, providing a good representation of the survey area.
Results
Confirmation of M. ulcerans
The specimens from cases 1 and 2, collected for M. ulcerans PCR, returned with cycle threshold values of 28 and 18, respectively. Positive cultures on solid culture medium and in broth (MGIT tubes) were referred to VIDRL, and to MDU Public Health Laboratory for WGS.
Comparative genomics
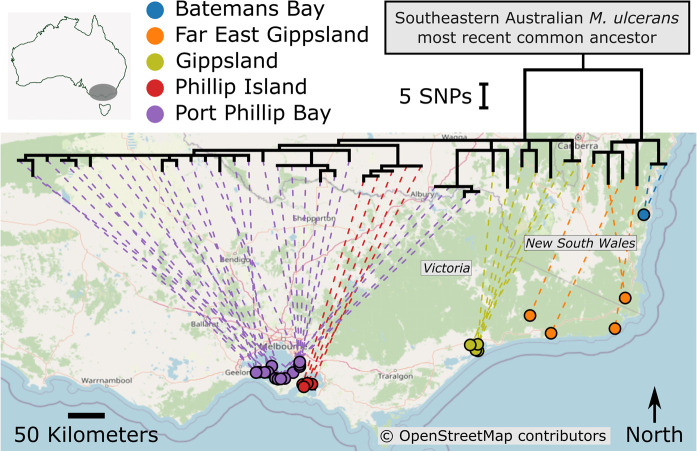
The alignment of DNA sequence reads obtained from each of the patient isolates to the JKD8049 reference chromosome facilitated the identification of 130 core genome single nucleotide polymorphisms (SNP). These SNPs were then used to infer a maximum likelihood phylogenomic tree. The core genome tree distinctly delineated the Batemans Bay isolates, setting them apart from the clinical isolates in Victoria in their own clade (Fig 6). Nevertheless, the Batemans Bay isolates exhibited a closer phylogenomic relationship with those from Far East Gippsland, the region geographically nearest to Batemans Bay (around 150 km away). Notably, the isolates from Batemans Bay and Far East Gippsland are situated on a distinct branch of the phylogenetic tree, which merges at the most recent common ancestor of M. ulcerans in south-eastern Australia (Fig 6). From this root point, the lineage harboring the Batemans Bay and Far East Gippsland isolates splits, leading away from isolates from the western endemic areas such as Gippsland, Phillip Island, and the Port Phillip Bay region. In previous research we used phylodynamic modelling and established a molecular clock of 0.422 SNPs/genome/year for M. ulcerans from southeastern Australia [9]. Applying this clock to the two Batemans Bay M. ulcerans genomes and the observation that these genomes are separated by one SNP from the east Gippsland most recent common ancestor (MRCA) that emerged in approximately 1980, we inferred that the Batemans Bay ancestor likely first emerged in the late 1970s (S1 Fig).
Fig 6. Phylogeographic analysis of Batemans Bay isolates in the context of the population structure of M. ulcerans in south-eastern Australia.
A maximum likelihood phylogenomic tree of M. ulcerans isolates was estimated using core genome SNPs, with branch lengths corresponding to SNP distances. The tree illustrates the clustering of isolates, with isolates from Batemans Bay and Far East Gippsland forming a distinct cluster. The lineage harboring the Batemans Bay and Far East Gippsland isolates traces back to the south-eastern Australian M. ulcerans most recent common ancestor, before branching out towards isolates from the more western localities of Gippsland, Phillip Island, and the Port Phillip Bay area. Link to the Open Street Map basemap: https://www.openstreetmap.org/#map=7/-36.752/144.278; link to the Open Street Map copyright and license information: https://www.openstreetmap.org/copyright.
Possum excreta survey
Out of the 27 samples collected for a possum excreta survey within a small area near the location of the reported cases, two samples tested positive for IS2404 by qPCR.
Discussion
Since the initial discovery of Mycobacterium ulcerans in the 1940s, new regions with local transmission of Buruli ulcer have continued to emerge [13]. We describe the first locally acquired cases of Buruli ulcer in Batemans Bay, NSW, Australia. The appearance of this phylogenomically divergent lineage, causing locally acquired infections, underlines the propensity for M. ulcerans to become established in new geographically separate possum populations as has been well documented in Victoria, Australia. The risk for further spread along coastal NSW is significant.
Both cases described above reside in distinct pockets of bushland within Batemans Bay, which is on the southeastern coast of Australia, approximately 520 km northeast of Melbourne and 110 km southeast of Canberra. The local permanent population at the latest census was 8,581 [14], but the region attracts many more national and international tourists annually. As a coastal town that sits at the mouth of the Clyde River, it is surrounded by densely forested native vegetation. With the convergence of native forest, saltwater, and freshwater, the area supports a broad range of aquatic and land-based wildlife, including possums. Studies have explored the possibility of mammals serving as a reservoir for Buruli ulcer [15,16]. M. ulcerans has been detected in the scat of possums at locations where human cases of the disease have been reported previously [16]. Both ringtail and brushtail possums are susceptible to natural infections, which result in skin ulcerations, and both may excrete M. ulcerans in high concentration in their feces [17]. The detection of positive possum excreta samples from Batemans Bay establishes beyond doubt that M. ulcerans is present in local possums. Larger, more systematic surveys will be required to examine the extent of possum involvement and to monitor change over time.
The phylogenomic separation of isolates from Port Phillip Bay, Phillip Island, and Gippsland from those of Far East Gippsland and Batemans Bay is traced back to the south-eastern Australian M. ulcerans most recent common ancestor. This deep divergence indicates a significant historical split and diversification in the pathogen population in the eastern part of south-eastern Australia [9]. This split has subsequently led to the emergence of the mycobacterial population in Batemans Bay which is distinct from the lineage prevalent in the most endemic areas of Victoria. The close genomic relatedness of the Batemans Bay isolates to those from Far East Gippsland suggests a pattern of spatial correlation, wherein isolates from geographically proximate regions are more closely related at the genomic level. This observed ’distance decay’ effect supports the hypothesis that the M. ulcerans in Batemans Bay has arisen from a local bacterial population, making it likely that M. ulcerans has been extant in the region for some time. Why Buruli ulcer cases have appeared in the last three years is a question that remains to be answered, but by extrapolation from observations in Victoria, expansion of the bacterial burden in a local possum population is a predictor of increased Buruli ulcer risk in humans [18].
Various transmission routes for Mycobacterium ulcerans have been proposed, with direct contact with contaminated environments suggested as one likely mode of infection in regions such as Africa and Australia [16,19,20]. In Case 1, the patient sustained blunt trauma prior to the development of Buruli ulcer and associated osteomyelitis. Given the detection of M. ulcerans in possum excreta in Batemans Bay and the potential for environmental contamination, it is conceivable that microabrasions from contact with the folding legs of an outdoor table facilitated pathogen entry. While the patient did not observe possum feces, the possibility of M. ulcerans contamination of the outdoor table remains. However, this explanation does not fit well with experimental evidence in hairless guinea pigs, where the application of cultured M. ulcerans cells, in high concentrations to experimentally created abrasions consistently failed to establish an infection [21]. Similar experiments performed in a mouse tail model of M. ulcerans infection showed the same failure to infect via direct contact [22]. Alternatively, there are reports that blunt trauma can trigger the sudden clinical manifestation of an infection that may have already been present [23].
In case 2, a mosquito bite was thought to precede the development of Buruli ulcer. In Victoria, mosquitoes are now thought to be the predominant mode of transmission to humans [18,24]. Also in Victoria, a mean incubation period for Mycobacterium ulcerans infection of 4.5–5 months has been established, with a range of 32 to 277 days [25,26]. Both of our patients experienced a relatively short incubation period of only a few weeks. The reasons for the wide variation in the incubation period remain unclear. In individual cases, there is the possibility of mistaken recall given the known long incubation period and the high frequency of more recent bites and minor trauma.
The possum excreta survey had limitations, including the small number of samples collected and the restricted geographic scope, which was confined to a single time point. While the detection of positive samples confirms the presence of Mycobacterium ulcerans in the local possum population, the limited size and scope of the survey constrain the ability to draw broader conclusions about the distribution and prevalence of the organism. Future studies incorporating larger survey areas and multiple time points would be valuable for gaining a more comprehensive understanding of the extent and distribution of affected possum populations in the region.
Given the many similarities in wildlife composition and insect presence between coastal Victoria and Eden and Batemans Bay in NSW, it is likely that NSW Public Health authorities are now facing progressive expansion of Buruli ulcer endemic areas and an increase in human infections, just as has been in Victoria. Among many unanswered questions in both Victoria and NSW is why new endemic foci of Buruli ulcer transmission appear discontinuously, sometimes hundreds of kilometers away from existing foci. The relatively long branch length of the Batemans Bay M. ulcerans genomes, revealed by the phylogenomic analysis, is consistent with—as yet—unsampled genetically diverse M. ulcerans present on other areas of Australia (Fig 6). That is, while Buruli ulcer occurrence in humans is discontinuous in its distribution, it is possible that M. ulcerans is more widely spread, harbored by wildlife reservoirs across Australia. Further research is needed to uncover the pathways of transmission that contribute to these sporadic outbreaks, which will be essential for controlling the disease’s spread and protecting communities.
Supporting information
(DOCX)
Shown is the relevant region of the M. ulcerans core-genome phylogeny and the internal tree nodes with divergence time estimations.
(DOCX)
Acknowledgments
We would like to express our sincere gratitude to our two patients and their families for their willingness to share the patients’ medical information for this journal article. We extend our appreciation to the plastic surgeons at Canberra Health Services, ACT, and Dr. Sanjay Singh (General Surgeon) at Batemans Bay, NSW. We acknowledge the invaluable support of the laboratory scientists and microbiologists at the Microbiology Department of Canberra Health Services, ACT, as well as Dr. Taryn Crighton and Professor Vitali Sintchenko at The Institute for Clinical Pathology and Medical Research (ICPMR), Westmead Hospital, NSW, for their assistance with sample processing.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Hong H, Coutanceau E, Leclerc M, Caleechurn L, Leadlay PF, Demangel C. Mycolactone Diffuses from Mycobacterium ulcerans–Infected Tissues and Targets Mononuclear Cells in Peripheral Blood and Lymphoid Organs. PLoS Negl Trop Dis. 2008. Oct 22;2(10):e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarner J. Buruli Ulcer: Review of a Neglected Skin Mycobacterial Disease. J Clin Microbiol. 2018. Apr;56(4). doi: 10.1128/JCM.01507-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacCULLUM P. A new mycobacterial infection in man; clinical aspects. J Pathol Bacteriol. 1948. Jan;60(1):93–102. [PubMed] [Google Scholar]
- 4.Pluschke G, Röltgen K. Buruli Ulcer. Pluschke G, Röltgen K, editors. Cham: Springer International Publishing; 2019. [Google Scholar]
- 5.Johnson PDR. Buruli Ulcer in Australia. In: Buruli Ulcer. Cham: Springer International Publishing; 2019. p. 61–76. [PubMed] [Google Scholar]
- 6.Röltgen K, Pluschke G, Johnson PDR, Fyfe J. Mycobacterium ulcerans DNA in Bandicoot Excreta in Buruli Ulcer–Endemic Area, Northern Queensland, Australia. Emerg Infect Dis. 2017. Dec;23(12):2042–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandelannoote K, Buultjens AH, Porter JL, Velink A, Wallace JR, Blasdell KR, et al. Statistical modeling based on structured surveys of Australian native possum excreta harboring Mycobacterium ulcerans predicts Buruli ulcer occurrence in humans. Elife. 2023. Apr 14;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fyfe JAM, Lavender CJ, Johnson PDR, Globan M, Sievers A, Azuolas J, et al. Development and Application of Two Multiplex Real-Time PCR Assays for the Detection of Mycobacterium ulcerans in Clinical and Environmental Samples. Appl Environ Microbiol. 2007. Aug;73(15):4733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buultjens AH, Vandelannoote K, Meehan CJ, Eddyani M, de Jong BC, Fyfe JAM, et al. Comparative Genomics Shows That Mycobacterium ulcerans Migration and Expansion Preceded the Rise of Buruli Ulcer in Southeastern Australia. Appl Environ Microbiol. 2018. Apr 15;84(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009. Jul;26(7):1641–50. doi: 10.1093/molbev/msp077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Revell LJ. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol. 2012. Apr 15;3(2):217–23. [Google Scholar]
- 12.Becker RA, Wilks AR. Constructing a Geographical Database. Computer Science, Geography. 1997. Available from: https://www.researchgate.net/publication/2444672_Constructing_a_Geographical_Database [Google Scholar]
- 13.Simpson H, Deribe K, Tabah EN, Peters A, Maman I, Frimpong M, et al. Mapping the global distribution of Buruli ulcer: a systematic review with evidence consensus. Lancet Glob Health. 2019. Jul;7(7):e912–22. doi: 10.1016/S2214-109X(19)30171-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batemans Bay, 2021. Census All persons QuickStats: Australian Bureau of Statistics [cited 2024 May 15]. Available from: https://www.abs.gov.au/census/find-census-data/quickstats/2021/101041017 [Google Scholar]
- 15.Singh A, McBride WJH, Govan B, Pearson M. Potential Animal Reservoir of Mycobacterium ulcerans: A Systematic Review. Trop Med Infect Dis. 2018. May 30;3(2):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fyfe JAM, Lavender CJ, Handasyde KA, Legione AR, O’Brien CR, Stinear TP, et al. A Major Role for Mammals in the Ecology of Mycobacterium ulcerans. PLoS Negl Trop Dis. 2010. Aug 10;4(8):e791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien CR, Handasyde KA, Hibble J, Lavender CJ, Legione AR, McCowan C, et al. Clinical, Microbiological and Pathological Findings of Mycobacterium ulcerans Infection in Three Australian Possum Species. PLoS Negl Trop Dis. 2014. Jan 30;8(1):e2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mee PT, Buultjens AH, Oliver J, Brown K, Crowder JC, Porter JL, et al. Mosquitoes provide a transmission route between possums and humans for Buruli ulcer in southeastern Australia. Nat Microbiol. 2024. Jan 23;9(2):377–89. doi: 10.1038/s41564-023-01553-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muleta AJ, Lappan R, Stinear TP, Greening C. Understanding the transmission of Mycobacterium ulcerans: A step towards controlling Buruli ulcer. PLoS Negl Trop Dis. 2021. Aug 26;15(8):e0009678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osei-Owusu J, Aidoo OF, Eshun F, Gaikpa DS, Dofuor AK, Vigbedor BY, et al. Buruli ulcer in Africa: Geographical distribution, ecology, risk factors, diagnosis, and indigenous plant treatment options–A comprehensive review. Heliyon. 2023. Nov;9(11):e22018. doi: 10.1016/j.heliyon.2023.e22018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williamson HR, Mosi L, Donnell R, Aqqad M, Merritt RW, Small PLC. Mycobacterium ulcerans Fails to Infect through Skin Abrasions in a Guinea Pig Infection Model: Implications for Transmission. PLoS Negl Trop Dis. 2014. Apr 10;8(4):e2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace JR, Mangas KM, Porter JL, Marcsisin R, Pidot SJ, Howden B, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017. Apr 14;11(4):e0005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkin GA, Smith M, Fairley M, Johnson PDR. Acute, oedematous Mycobacterium ulcerans infection in a farmer from far north Queensland. Medical Journal of Australia. 2002. Feb 18;176(4):181–2. [DOI] [PubMed] [Google Scholar]
- 24.Buultjens AH, Tay EL, Yuen A, Friedman ND, Stinear TP, Johnson PDR. Mosquitoes as Vectors of Mycobacterium ulcerans Based on Analysis of Notifications of Alphavirus Infection and Buruli Ulcer, Victoria, Australia. Emerg Infect Dis. 2024. Sep;30(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trubiano JA, Lavender CJ, Fyfe JAM, Bittmann S, Johnson PDR. The Incubation Period of Buruli Ulcer (Mycobacterium ulcerans Infection). PLoS Negl Trop Dis. 2013. Oct 3;7(10):e2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loftus MJ, Trubiano JA, Tay EL, Lavender CJ, Globan M, Fyfe JAM, et al. The incubation period of Buruli ulcer (Mycobacterium ulcerans infection) in Victoria, Australia–Remains similar despite changing geographic distribution of disease. PLoS Negl Trop Dis. 2018. Mar 19;12(3):e0006323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Shown is the relevant region of the M. ulcerans core-genome phylogeny and the internal tree nodes with divergence time estimations.
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.