Abstract
BACKGROUND
Rapid onset of epidural analgesia is an important concern for the parturient. Commonly, the local anaesthetic mixture is administered through the epidural catheter. Drugs administered through the epidural needle might decrease the onset time and enhance the spread of medication within the epidural space.
OBJECTIVES
The primary aim of this study was to compare the onset time of analgesia when a loading dose of dilute local anaesthetic opioid mixture was injected through either the Tuohy needle or a single end-hole epidural catheter.
DESIGN
A prospective, double-blinded, randomised clinical trial.
SETTING
Single university hospital, from November 2022 to August 2023.
PARTICIPANTS
A total of 200 healthy nulliparous women who requested epidural analgesia for labour were randomly allocated to the needle group (n = 100) or the catheter group (n = 100).
INTERVENTIONS
In the needle group, after identification of the epidural space, a test dose of 3 ml 0.1% ropivacaine with 0.3 μg ml−1 sufentanil was injected through the Tuohy needle followed 3 min later by a 15 ml loading dose of the same mixture over 30 s. Then the catheter was inserted into the epidural space. In the catheter group, after identification of the epidural space, a catheter was advanced into the epidural space and the ropivacaine/sufentanil mixture was injected in an identical manner though the catheter.
MAIN OUTCOME MEARSURES
The primary outcome was the onset time of labour analgesia (defined as the time from drug administration to adequate analgesia). Adequate analgesia was defined as a visual analogue score 10 mm or less during uterine contractions.
RESULTS
Median [IQR] onset time of labour analgesia did not differ significantly between the two groups (needle group: 20 [16 to 30] minutes; catheter group: 20 [15 to 25] minutes, P = 0.232).
CONCLUSION
Compared with bolus injection though a single end-hole epidural catheter, injection through the epidural needle did not shorten the analgesia onset time for adequate labour analgesia.
TRIAL REGISTRATION
ClinicalTrials.gov (NCT05594771).
KEY POINTS
What is already known on this topic: drugs administered through the epidural needle may increase the speed and degree of spread within the epidural space. We hypothesised that using a large volume of dilute local anaesthetic injected through the epidural needle over a short time interval may result in a wider spread of medication and hasten the onset of analgesia.
How this study might affect research, practice, or policy: based on the outcomes of this study, injecting a bolus loading dose of dilute local anaesthetic drug through the epidural needle did not result in a shorter analgesia onset time compared with the same injection through the catheter.
Introduction
Epidural anaesthesia is used to provide analgesia for the intense pain experienced by many women during labour. Both epidural anaesthesia and combined spinal–epidural anaesthesia are widely used for labour analgesia. Although the combined technique results in a more rapid onset of labour analgesia than the normal epidural anaesthesia technique, it is associated with a higher incidence of maternal pruritus, hypotension and foetal bradycardia.1,2 However, it can take up to 45 min for low-dose epidural anaesthesia to achieve satisfactory pain relief. Studies have shown that drugs administered through the epidural needle may increase the degree of drug spread within the epidural space.3 Compared with an epidural catheter, injecting the anaesthetic drug through the epidural needle may lead to a faster onset of analgesia. Ristev et al.4 reported that the injection of 10 ml local anaesthetic (two 5 ml injections at 2-min intervals) resulted in a similar onset time of analgesia whether injected through the epidural needle or the catheter. For safety reasons, the full dose of local anaesthetic is typically administered into the epidural space in divided doses over several minutes in most studies.5,6 Different volumes of local anaesthetics affect their spread in the epidural space, which may affect the onset of anaesthesia. The use of low-concentration local anaesthetics and opioid solutions for labour analgesia is effective with few side effects.7,8 It is possible to use a large volume of dilute local anaesthetic injected through the needle over a short time interval, which may result in a wider spread of medication and hasten the onset of analgesia.
To our knowledge, studies comparing the onset of labour analgesia with a large-volume bolus of local anaesthetic as loading dose administered through an epidural needle or an epidural catheter are lacking. We hypothesised that a high-volume loading dose of ropivacaine combined with sufentanil injected into the epidural space through the Tuohy needle would shorten the onset of analgesia, reduce anaesthetic drug consumption, and improve the quality of labour analgesia compared with catheter injection.
Methods
This prospective, double-blind, randomised controlled study (approval number IRB-20220306-R) was approved by the Research Ethics Committee of Women's Hospital, Zhejiang University School of Medicine (Chairperson Professor Wang Hui) on 26 September 2022 and registered at ClinicalTrials.gov (identifier: NCT05594771; date of registration: 26 October 2022). Patients were recruited from November 2022 until August 2023. Our reporting adhered to the CONSORT 2010 guidelines.
After written informed consent was obtained, 200 parturients who requested labour analgesia were enrolled in this study. Healthy nulliparous women who were term (37 to 42 weeks), aged 20 to 40 years, in active labour with a cervical dilation 5 cm or less at the time when the epidural analgesia was requested were included in this study. The following conditions were excluded: contraindications to neuraxial anaesthesia, body mass index greater than 50 kg m−2, visual analogue scale (VAS) 50 mm or less (0 mm = no pain, 100 mm = worst imaginable pain) during an active contraction, pregnancy-related diseases (e.g. gestational diabetes, gestational hypertension, preeclampsia, heart disease during pregnancy, hyperthyroidism during pregnancy), inadvertent dural puncture during the epidural procedure, cardiotocographic anomalies before initiation of labour analgesia and inadvertent subarachnoid catheter placement.
Patients were randomly allocated to the needle group (n = 100) or the catheter group (n = 100) according to a computer-generated randomisation sequence, which was placed in sealed opaque envelopes. The anaesthesiologist who was to perform the epidural opened the sealed opaque envelope with the group allocation information immediately before the procedure. This anaesthesiologist did not take part in subsequent patient care or data collection. After the epidural procedure, another investigator performed the observations, assessments, treatment, and data collection. The participants and the researchers who performed the observation were unaware of the group allocation.
Initiation of labour analgesia
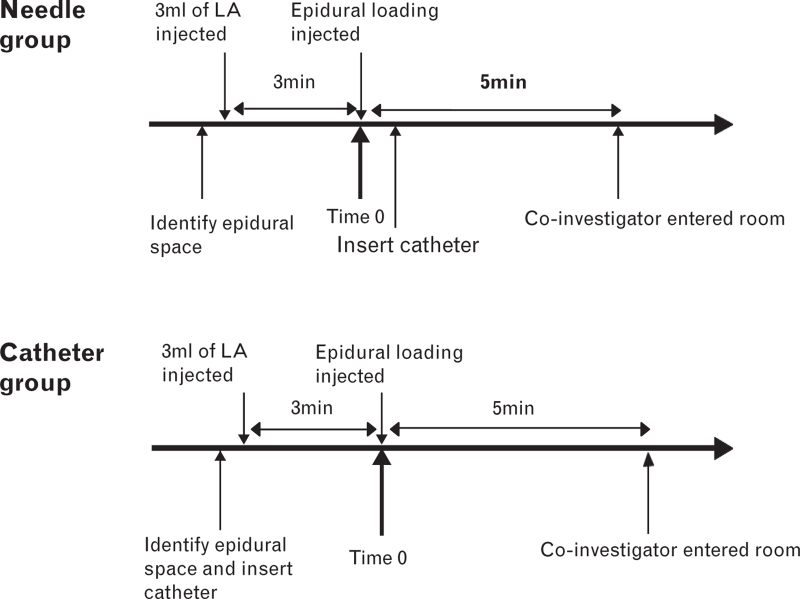
Before epidural analgesia, peripheral intravenous access with an 18-gauge cannula was established, and 500 ml of Ringer's lactate solution was connected and commenced at 10 ml kg−1 h−1: no preload was administered. The participants received maternal monitoring (noninvasive blood pressure, electrocardiography, pulse oximetry) and foetal monitoring (cardiotocography) in the predelivery room. Epidural analgesia was performed in the left lateral decubitus position at the either the L2–3 or L3–4 interspace using a 17-guage Tuohy needle with a midline approach and loss of resistance to saline (2 ml or less). If frank blood or cerebrospinal fluid was detected on aspiration of the needle or catheter, this was accepted as indicating subarachnoid placement. The same ropivacaine/sufentanil mixture was used in both groups for induction and maintenance of analgesia (0.1% ropivacaine combined with 0.3 μg ml−1 sufentanil) and the doses administered, speed of injection, and their timings were also the same. In the needle group, after identification of the epidural space by loss of resistance to saline, 3 ml of the ropivacaine/sufentanil mixture was injected through the epidural needle. If the mothers did not experience sudden numbness or warmth in their legs within 3 min, analgesia was initiated with a bolus dose of 15 ml of the solution injected through the epidural needle over 30 s. Following this, the epidural catheter (single end hole, 19-guage with a threading assist device, FlexTip Plus) was inserted 3–5 cm into the epidural space. In the catheter group, after identification of the epidural space by loss of resistance to saline the catheter was inserted into the epidural space, and then the ropivacaine/sufentanil mixture was injected via the epidural catheter. In both groups, the patients were placed in a supine position after the catheter was fixed and taped to the skin, and the epidural pump was connected to the catheters. The administration of the 15 ml loading dose marked time zero. The coinvestigator was then asked to enter the room and begin to assess and record the data 5 min after time zero (Fig. 1): by this time, all patients had their epidural catheters in place.
Fig. 1.
Timing of initial dose, catheter insertion and injection of local anaesthetic (T0). LA, local anaesthetic.
Maintenance of labour analgesia
In both groups, epidural analgesia was maintained with the solution ropivacaine/sufentanil solution. The epidural analgesia pump was set in a dual programmed intermittent epidural bolus (PIEB) + patient-controlled analgesia (PCEA) mode: background infusion 2 ml h−1; PIEB bolus 6 ml; PIEB lockout out 60 min; PCEA bolus 5 ml; PCEA lockout 15 min; 30 ml maximum volume per hour.
Breakthrough pain management
Following two PCEA boluses, if the patient still experienced breakthrough pain (VAS score > 30 mm), the anaesthesiologist was consulted, and if appropriate, the patient was provided 10 ml of 0.2% ropivacaine with 0.3 μg ml−1 sufentanil.
Patient baseline characteristics
The baseline characteristics included maternal age, height, weight, body mass index, gestational age, cervical dilation at the time of the request for epidural analgesia, induction of labour, VAS during uterine contractions, maternal blood pressure, heart rate, and pulse oximetry.
Primary outcome assessment
The primary outcome was the onset time of analgesia, defined as the duration from time zero (drug administration) to adequate analgesia (VAS score ≤10 mm during a uterine contraction).
Secondary outcomes
The following secondary outcomes were assessed: VAS score; sensory dermatome blockade level to ice (cold sensation diminished); Bromage score (0 = ability to move hips, ankles, and knees; 1 = inability to raise an extended leg; 2 = inability to flex the knee; and 3 = inability to flex the ankle, foot or knee); number of PCEA boluses requested (obtained from the pump); number of additional provider boluses; ropivacaine consumption; duration of labour; mode of delivery; Apgar scores at 1 and 5 min; and patient satisfaction (using a 100 mm VAS: 0 mm = completely unsatisfied and 100 mm = extremely satisfied) within 24 h after delivery).
VAS scores and sensory blockade levels were measured at 2 min interval from 5 min after time zero to 20 min; subsequently, measurements were collected at 25 min, 30 in and every hour until delivery.
The following additional parameters were recorded to assess safety: maternal heart rate, blood pressure, and oxygen saturation (pulse oximetry); foetal bradycardia; side effects, maternal pruritus, nausea, vomiting, back pain, headache and high sensory block (T4).
Maternal hypotension after analgesia was defined as a SBP less than 90 mmHg or a at least 20% decrease from baseline. Hypotension was treated by placing the mother in the left lateral decubitus position, rapid intravenous fluid and/or intravenous norepinephrine (4 μg). Foetal bradycardia was defined as a rate of less than 110 bpm.
Statistical analysis
The Kolmogorov–Smirnov test was used to test for the normal distribution of continuous variables. The Student's t test was used to analyse normally distributed continuous data, and the Mann–Whitney U test was used to analyse nonnormally distributed continuous data. The χ2 test or Fisher's exact test was used to analyse categorical variables. Statistical significance was assumed at a P value of 0.05 or less. Onset time of analgesia was the primary outcome. Kaplan–Meier curves and the log-rank test were used to analyse the primary outcome.
SPSS version 19.0 and GraphPad Prism were used for statistical analysis.
No previously published studies were available to perform sample size calculations. According to our institution's data, we used a median time of 20 min as the onset time of analgesia, and defined as a 1.5-fold difference in the onset time between the two groups be clinically meaningful. Using a log-rank test, 200 events in total (100 per group) were needed to detect a difference in the survival curves between the groups at 80% power (2-tailed) with α = 0.05.
Results
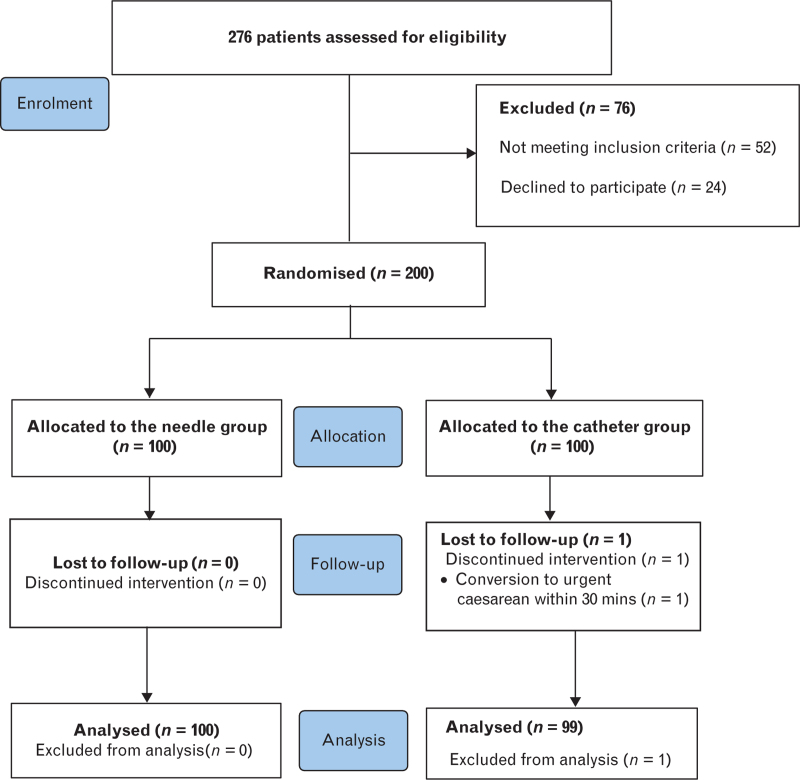
From the 276 patients who were assessed for eligibility, 200 patients were enrolled in the study (100 patients randomised to each group; Fig. 2). One patient in the catheter group was excluded because of conversion to an urgent caesarean delivery within 30 min after epidural analgesia initiation. Therefore, 100 parturients in the needle group and 99 in the catheter group were included for assessment of the primary outcome. All patients’ data were included as part of the intention-to-treat analysis. The baseline patient characteristics are shown in Table 1. Both groups were similar at baseline.
Fig. 2.
CONSORT flow diagram.
Table 1.
Baseline patient characteristics
| Needle group [n = 100] | Catheter group [n = 99] | P value | |
| Age (years) | 30.5 ± 3.8 | 30.2 ± 3.8 | 0.646 |
| Height (cm) | 161.4 ± 5.4 | 162.6 ± 4.8 | 0.114 |
| Weight (kg) | 67.5 ± 7.7 | 68.6 ± 8.5 | 0.365 |
| BMI (kg m−2) | 26.0 ± 3.1 | 25.9 ± 3.0 | 0.962 |
| Gestational age (weeks) | 39 [38 to 40] | 39 [38 to 40] | 0.329 |
| Cervical dilation (cm) | 2.5 [2.5 to 3.0] | 2.5 [2.5 to 3.0] | 0.329 |
| VAS pain scores (0–100 mm) | 90 [72.5 to 90] | 80 [70 to 90] | 0.363 |
| Induction of labour | 47 (47) | 37 (37.4) | 0.169 |
Data are mean ± SD, median [IQR], n (%). BMI, body mass index; VAS, visual analogue scale.
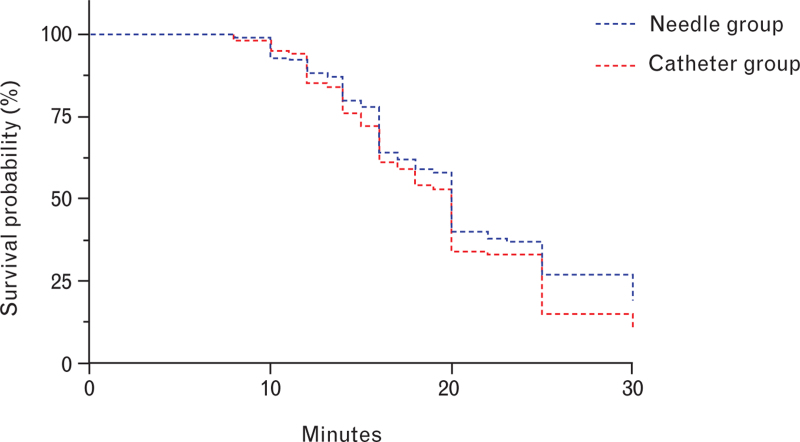
There was no significant difference in the time to VAS score 10 or less between the needle group and the catheter group. The median [IQR] time to a VAS score 10 or less was 20 [16 to 30] min for the needle group and 20 [15 to 25] min for the catheter group (P = 0.232; Fig. 3 and Table 2). The median [IQR] time to a sensory block of dermatome T10 was similar between the groups: 8 [6 to 10] min for the needle group and 8 [6 to 11] min for the catheter group (P = 0.118) (Table 2).
Fig. 3.
Kaplan–Meier curves for the time to achieve a visual analogue scale score ≤10 following an initial bolus delivered via epidural needle or catheter. The survival probability indicates the probability of a subject surviving with a VAS score >10 mm at a given time. VAS, visual analogue scale.
Table 2.
Anaesthetic outcomes, maternal and foetal outcomes
| Needle group (n = 100) | Catheter group (n = 99) | P value | |
| Onset time to VAS ≤10 (min) | 20 [16 to 30] | 20 [15 to 25] | 0.232 |
| No. of patients VAS ≤10 mm at 30 min | 81 (81) | 89 (89.9) | 0.075 |
| No. of patients VAS ≤30 mm at 30m in | 95 (95) | 95 (96.0) | 1.000 |
| Onset time to T10 (min) | 8 [6 to 10] | 8 [6 to 11] | 0.118 |
| Sensory block level at 30 min | 0.836 | ||
| T12 | 1 (1.0) | 1 (1.0) | |
| T10 | 2 (2.0) | 1 (1.0) | |
| T8 | 77 (77.0) | 79 (79.8) | |
| T6 | 18 (18.0) | 14 (14.1) | |
| T4 | 2 (2.0) | 4 (4.0) | |
| Incidence of breakthrough pain | 6 (6) | 7 (7.1) | 0.760 |
| Analgesia time of the first stage (h) | 4.87 [3.1 to 7.3] | 5.35 [3.0 to 8.0] | 0.462 |
| Total anaesthesia time (h) | 5.47 [3.9 to 8.2] | 6.07 [4.0 to 8.3] | 0.180 |
| Total ropivacaine consumption (mg) | 42.0 [27.5 to 60.0] | 46.0 [31.0 to 71.0] | 0.250 |
| PCEA boluses requested | 2 (0 to 3) | 1 (0 to 3) | 0.968 |
| PCEA boluses delivered | 1 (0 to 2) | 1 (0 to 2) | 0.927 |
| Mode of delivery | 0.136 | ||
| Vaginal delivery | 57 (57) | 69 (69.7) | |
| Instrumental delivery | 16 (16) | 15 (15.2) | |
| Caesarean delivery | 27 (27) | 15 ((15.2)) | |
| Apgar score at 1 min | 1.000 | ||
| <8 | 3 (3) | 3 (3.0) | |
| 9 to 10 | 97 (97) | 96 (97.0) | |
| Apgar score at 5 min | 0.497 | ||
| <8 | 0 (0) | 1 (1.0) | |
| 9 to 10 | 100 (100) | 98 (99.0) | |
| Patient satisfaction VAS score | 98 [95 to 100] | 98 [92 to 100] | 0.765 |
Data are median [IQR], n (%). PCEA, patient-controlled epidural analgesia; VAS, visual analogue scale.
The total ropivacaine consumption was not different between the two groups (Table 2). The sensory level and degree of motor block were not affected by the loading dose injected through the epidural needle or epidural catheter (Tables 2 and 3).
Table 3.
Adverse effects within 30 min after the epidural initiation
| Needle group (n = 100) | Catheter group (n = 99) | P value | |
| Hypotension | 0 | 0 | NA |
| Foetal bradycardia | 3 | 2 | 0.621 |
| Backpain | 0 | 0 | NA |
| Nausea | 0 | 1 | 0.497 |
| Vomiting | 1 | 0 | 1.0 |
| Pruritus | 0 | 1 | 0.497 |
| High sensory block level (T4) | 2 | 4 | 0.445 |
| Modified Bromage score 0/1/2/3 | 100/0/0/0 | 99/0/0/0 | NA |
| Intravenous placement | 0 | 0 | NA |
| Subarachnoid placement | 0 | 0 | NA |
Data are n. NA, not applicable.
The side effects, including hypotension, nausea, vomiting, pruritus, high sensory block (T4) and foetal bradycardia, did not differ between the groups (Table 3). No patients were excluded because of intrathecal or intravascular catheterisation, and no participants experienced back pain within the first 30 min after epidural insertion. The VAS scores did not differ between the groups during labour (Table 4).
Table 4.
Pain visual analogue scale score at various time points
| Hour of VAS score assessment | Needle group (n = 100) | Catheter group (n = 99) | P value |
| 1 | 1 [1 to 2]: n = 100 | 1 [1 to 2]: n = 98 | 0.342 |
| 2 | 1 [1 to 2]: n = 96 | 1 [1 to 2]: n = 97 | 0.521 |
| 3 | 1 [1 to 2]: n = 87 | 1 [1 to 2]: n = 89 | 0.748 |
| 4 | 1 [1 to 2]: n = 76 | 1 [1 to 2]: n = 76 | 0.120 |
| 5 | 1 [1 to 2]: n = 64 | 2 [1 to 2]: n = 65 | 0.625 |
| 6 | 2 [1 to 2]: n = 48 | 2 [1 to 2]: n = 53 | 0.819 |
| 7 | 2 [1 to 2]: n = 33 | 1 [1 to 2]: n = 46 | 0.818 |
| 8 | 1 [1 to 2]: n = 22 | 1 [1 to 2]: n = 36 | 0.229 |
Values are median [IQR]. VAS, visual analogue scale.
Discussion
The main finding of this study was that a loading dose administered through the epidural needle did not shorten the onset time of adequate labour analgesia compared with an identical injection through the single end-hole epidural catheter. The consumption of ropivacaine was similar, and equivalent labour analgesia was provided. The incidence of side effects did not differ between the two groups.
Epidural analgesia is one of the most effective methods for relieving pain during labour.9 One of the disadvantages of epidural analgesia is its slow onset.2 Previous researchers have conducted several studies on how to shorten the onset time of epidural anaesthesia without increasing side effects or compromising maternal and foetal safety. The pharmacodynamic and pharmacokinetic properties of local anaesthetic dose,10 concentration,11 and infusion volume12 modify the spread of anaesthetic drugs in the epidural space, and can thus affect the onset of analgesia and the intensity of the sensory blockade. Several studies have indicated that local anaesthetics injected through the needle before catheter insertion results in a faster onset compared with those injected through the epidural catheter.3,13 Husain et al.14 compared local anaesthetic injected through an epidural catheter or needle for the induction of anaesthesia for caesarean section and reported that medications administered through the epidural needle did not shorten the time to achievement of surgical anaesthesia. Another study found that 10 ml of local anaesthetic injected through the epidural needle did not shorten the onset of pain relief compared with that injected through the catheter; in addition, the sensory blockade and quality of labour analgesia were similar.4
In the aforementioned studies, local anaesthetic was injected in increments, which may limit the spread of local anaesthetic in the epidural space. Currently, low-concentration local anaesthetic and opioid solutions are commonly used for labour analgesia with few side effects.15 Therefore, we hypothesised that a large volume of dilute local anaesthetic injected over a short time interval might achieve a greater spread of medication and shorten the onset of analgesia. In our study, we used a volume of 15 ml of dilute local anaesthetic and opioid solution as a bolus injected through the needle or the catheter over 30 s for initiation of labour analgesia. Under the conditions of the current study, the primary findings were that the onset time to adequate analgesia was similar and that the sensory blockade level was comparable, indicating that the large volume loading dose administered through the Tuohy needle did not increase the spread of medication compared with that through the catheter. Secondary outcomes VAS score, sensory blockade level, Bromage score, number of PCEA boluses, ropivacaine consumption and obstetric and foetal outcomes were all comparable between the groups, indicating that the loading dose injected through the epidural needle had no significant adverse effects on overall analgesia quality nor on maternal and infant outcomes.
In previous studies comparing onset times of injection through a needle or catheter,3,4,14 multiholed catheters were usually used. Yi et al.16 found that a single-hole catheter produced earlier onset of analgesia than multiorifice catheters. Based on this, we adopted single-orifice catheters in the present study. Our findings provide information that a bolus of large-volume dilute local anaesthetic as a loading dose injected through the epidural needle did not shorten the time from drug administration to adequate labour analgesia compared with that administered through a single-orifice epidural catheter.
In normal clinical practice, the insertion and fixation of the epidural catheter requires time, especially in those cases where there are difficulties inserting the catheter such as intravenous catheters requiring withdrawal until aspiration was negative, or when the whole epidural procedure has to be undertaken again. In these situations, injecting a loading dose of local anaesthetic through the epidural needle before catheter insertion would enable the onset of analgesia while technical problems were being solved.
Although injecting a loading dose through an epidural needle did not shorten the onset time of analgesia in the present study, several studies have reported that local anaesthetics injected through the epidural needle before catheter insertion reduced catheter-related complications and enhanced the quality of anaesthesia.17 According to Cesur et al.,17 the injection of 20 ml of 2% lidocaine via the epidural needle before catheter insertion could increase the quality of anaesthesia for surgery and reduce the occurrence of paraesthesia and intravenous catheter placement. Even the injection of 0.9% saline to distend the epidural space before catheter insertion decreases the occurrence of intravenous catheter placement.18 It was found that the administration of an incompressible fluid (0.9% saline) through an epidural needle provides lubrication and distention of the epidural space, which reduces the incidence of accidental intravenous catheterisation.18
In the needle group, after negative aspiration, the loading dose was injected in the epidural space through the needle before catheter insertion. This catheter was also aspirated but no test dose was administered. When providing labour epidural analgesia, Norris Mark et al.19 assessed the ability of aspiration to identify the intravenous placement of multiholed catheters. The results suggested that traditional intravenous test doses for labour epidural analgesia are not necessary. In the present study, we used a single-orifice wire-embedded polyurethane catheter, which has been shown to reduce the incidence of intravascular catheterisation compared with a multiholed polyamide catheter.20,21 In addition, the co-investigator stood by the patients and assessed the sensory or motor blockade when the first bolus of low-dose local anaesthetic was administered by the pump. If the catheter was inserted intrathecally, the programmed PIEB of 6 ml or the PCEA bolus of 5 ml of low-concentrated local anaesthetic would result in rapid-onset analgesia and motor weakness/block without total spinal anaesthesia. No patients in either group showed any signs or symptoms of spinal anaesthesia. There were no signs of motor block during analgesia in this study.
In contrast to injecting loading doses of low-dose local anaesthetic solutions through the needle, loading doses with concentrated local anaesthetic solutions has the potential to cause harm. The safety of the loading dose administered through the epidural needle technique for induction of labour analgesia should be taken into consideration. Research investigating low-concentrated local anaesthetic solutions injected through needles are rare. A retrospective study investigated the injection of low-dose, high-volume local anaesthetic solution via an epidural needle for labour analgesia in 957 parturients. In that study, there were was no evidence of intrathecal or intravascular injection with the high-volume low-dose local anaesthetic loading technique: statistically, the calculated predicted risk of complications was less than 0.3%.8 Likewise, our study observed that 15 ml of diluted local anaesthetic solution did not cause severe side effects. The incidence of side effects was similar between the two groups in terms of maternal hypotension, heart rate, sensory block level, and foetal bradycardia. These results are consistent with the incidence of these side effects reported in previous studies of labour analgesia, in which the loading dose was administered in increments through the epidural catheter.6 Although there were no cases of intravenous or subarachnoid placement of the needle or the catheter in the present study, it was not adequately powered to address the safety of the loading dose injected through the needle compared with that through the catheter. Further studies are warranted to establish the safety of the loading dose administered via the epidural needle.
There are several limitations of this study. First, epinephrine was not used in the test dose to identify intravenous placement of the needle or catheter. Second, we studied only a low concentration of 0.1% ropivacaine with 0.3 μg ml−1 sufentanil. The concentration of local anaesthetic may affect the onset of anaesthesia. Third, for safety reasons, the loading dose used for labour analgesia was administered over 30 s. Rapid injection of local anaesthetic via the epidural needle could enhance the diffusion of medication in the epidural space. Fourth, we included only patients whose cervical dilation was 5 cm or less at the time when an epidural analgesic was requested. The onset of analgesia with medication administered via the epidural needle in more advanced labour requires further exploration.
Conclusion
There was no difference in the onset time of labour analgesia when the high-volume loading dose was administered via the epidural needle or catheter. The consumption of ropivacaine was the same while providing equivalent labour analgesia.
Acknowledgements relating to this article
Assistance with the study: the authors wish to thank the nurses and midwives at the obstetrics department, Women's Hospital, School of Medicine, Zhejiang University, for their support in this study.
Financial support and sponsorship: this work was supported by the Zhejiang Medical and Health Science and Technology Project [No. WKJ-ZJ-2319].
Conflicts of interest: none.
Presentation: the abstract of this study was presented by Dr Lihong Sun at the Research Abstract oral presentation section of the 2024 annual meeting of the Society for Obstetric Anesthesia and Perinatology (SOAP) committee, 3 May 2024, in the United States.
This manuscript was handled by Marc Van de Velde.
Footnotes
XC and YT contributed equally to this work.
References
- 1.Hattler J, Klimek M, Rossaint R, et al. The effect of combined spinal-epidural versus epidural analgesia in laboring women on nonreassuring fetal heart rate tracings: systematic review and meta-analysis. Anesth Analg 2016; 123:955–964. [DOI] [PubMed] [Google Scholar]
- 2.Wilson SH, Wolf BJ, Bingham K, et al. Labor analgesia onset with dural puncture epidural versus traditional epidural using a 26-gauge whitacre needle and 0.125% bupivacaine bolus. Anesth Analg 2018; 126:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omote K, Namiki A, Iwasaki H. Epidural administration and analgesic spread: Comparison of injection with catheters and needles. J Anesth 1992; 6:289–293. [DOI] [PubMed] [Google Scholar]
- 4.Ristev G, Sipes AC, Mahoney B, et al. Initiation of labor analgesia with injection of local anesthetic through the epidural needle compared to the catheter. J Pain Res 2017; 10:2789–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chau A, Bibbo C, Huang CC, et al. Dural puncture epidural technique improves labor analgesia quality with fewer side effects compared with epidural and combined spinal epidural techniques: a randomized clinical trial. Anesth Analg 2017; 124:560–569. [DOI] [PubMed] [Google Scholar]
- 6.Song Y, Du W, Zhou S, et al. Effect of dural puncture epidural technique combined with programmed intermittent epidural bolus on labor analgesia onset and maintenance: a randomized controlled trial. Anesth Analg 2021; 132:971–978. [DOI] [PubMed] [Google Scholar]
- 7.Acog practice bulletin no. 209: obstetric analgesia and anesthesia. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics and the Society for Maternal-Fetal Medicine. Obstet Gynecol 2019; 133:e208–e225. [DOI] [PubMed] [Google Scholar]
- 8.Paniagua O, Newman J, Orlando B, et al. Evaluation of the safety of the epidural needle loading technique for initiation of labor analgesia. Int J Obstet Anesth 2022; 50:103275. [DOI] [PubMed] [Google Scholar]
- 9.Callahan EC, Lee W, Aleshi P, et al. Modern labor epidural analgesia: Implications for labor outcomes and maternal-fetal health. Am J Obstet Gynecol 2023; 228:S1260–S1269. [DOI] [PubMed] [Google Scholar]
- 10.Danelli G, Venuti FS, Zasa M, et al. Continuous lumbar epidural infusion of levobupivacaine: effects of small-or large-volume regimen of infusion. Acta Anaesthesiol Scand 2009; 53:483–488. [DOI] [PubMed] [Google Scholar]
- 11.Duggan J, Bowler GM, McClure JH, et al. Extradural block with bupivacaine: influence of dose, volume, concentration and patient characteristics. Br J Anaesth 1988; 61:324–331. [DOI] [PubMed] [Google Scholar]
- 12.Sakura S, Sumi M, Kushizaki H, et al. Concentration of lidocaine affects intensity of sensory block during lumbar epidural anesthesia. Anesth Analg 1999; 88:123–127. [PubMed] [Google Scholar]
- 13.Lee EK, Tian H, Lee J, et al. Investigating a needle-based epidural procedure in obstetric anesthesia. AMIA Annu Symp Proc 2018; 2018:720–729. [PMC free article] [PubMed] [Google Scholar]
- 14.Husain FJ, Herman NL, Karuparthy VR, et al. A comparison of catheter vs needle injection of local anesthetic for induction of epidural anesthesia for cesarean section. Int J Obstet Anesth 1997; 6:101–106. [DOI] [PubMed] [Google Scholar]
- 15.Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology 2016; 124:270–300. [DOI] [PubMed] [Google Scholar]
- 16.Yi J, Li Y, Yuan Y, et al. Comparison of labor analgesia efficacy between single-orifice and multiorifice wire-reinforced catheters during programmed intermittent epidural boluses: a randomized controlled clinical trial. Reg Anesth Pain Med 2023; 48:61–66. [DOI] [PubMed] [Google Scholar]
- 17.Cesur M, Alici HA, Erdem AF, et al. Administration of local anesthetic through the epidural needle before catheter insertion improves the quality of anesthesia and reduces catheter-related complications. Anesth Analg 2005; 101:1501–1505. [DOI] [PubMed] [Google Scholar]
- 18.Evron S, Gladkov V, Sessler DI, et al. Predistention of the epidural space before catheter insertion reduces the incidence of intravascular epidural catheter insertion. Anesth Analg 2007; 105:460–464. [DOI] [PubMed] [Google Scholar]
- 19.Norris Mark C, Fogel Steven T, Dalman H, et al. Labor epidural analgesia without an intravascular “test dose”. Anesthesiology 1998; 88:1495–1501. [DOI] [PubMed] [Google Scholar]
- 20.Banwell BR, Morley-Forster P, Krause R. Decreased incidence of complications in parturients with the arrow (FlexTip Plus) epidural catheter. Can J Anaesth 1998; 45:370–372. [DOI] [PubMed] [Google Scholar]
- 21.Mhyre JM, Greenfield MLVH, Tsen LC, et al. A systematic review of randomized controlled trials that evaluate strategies to avoid epidural vein cannulation during obstetric epidural catheter placement. Anesth Analg 2009; 108:1232–1242. [DOI] [PubMed] [Google Scholar]