Supplemental Digital Content is Available in the Text.
Key Words: Sjögren's syndrome, dry eye, RCI001, inflammation, oxidative stress
Abstract
Purpose:
The aim of this study was to investigate the efficacy of RCI001 (RCI) in a mouse model of primary Sjögren syndrome.
Methods:
Eight 12-week-old NOD.B10-H2b mice were used in this study. All experimental animals were randomly divided into phosphate-buffered saline (PBS) and RCI groups in NOD.B10-H2b mice. The eyes of mice were topically treated with PBS or RCI twice a day for a week. Ocular surface staining (OSS) and tear secretion were compared between before and after treatment. The transcript levels of inflammatory cytokines and nicotinamide adenine dinucleotide phosphate oxidase (NOX) in the conjunctiva and cornea (CC) and lacrimal gland were assayed. In addition, immunofluorescence staining of the conjunctiva was assessed.
Results:
The RCI group showed significant clinical improvement in OSS and tear secretion after 1 week of treatment compared with the baseline (both P < 0.001) and showed better improvement in OSS and tear secretion than the PBS group after 1 week of treatment (both P < 0.05). The levels of IL-1β and IL-17 in CC and IL-6 in the lacrimal gland were also significantly reduced in the RCI group compared with the PBS group (each P < 0.05). Transcript levels of NOX2 and NOX4 were also significantly reduced in CC of the RCI group compared with those of the PBS group (P < 0.05). The RCI group also resulted in lower conjunctival expression of oxidative stress markers (4-hydroxy-2-nonenal, hexanoyl-lysine, and NOX4) than the PBS group.
Conclusions:
Topical RCI001 demonstrated excellent therapeutic efficacy in a mouse model of primary Sjögren syndrome by inhibiting inflammation and oxidative stress.
Sjögren syndrome (SS) is a chronic inflammatory disorder that affects over 60 per 100,000 people globally.1 The syndrome typically manifests in middle-aged women and progresses over months to years. Dry eye (keratoconjunctivitis sicca), dry mouth, and chronic arthritis are the 3 major symptoms/signs of SS.2 Like most autoimmune diseases, SS has a female predominance with a high female-to-male ratio (9:1) and a peak incidence in the 40 to 55-year age group.1,2 Although the pathogenesis of SS is not fully understood, symptoms are known to be caused by exocrine gland inflammation and dysfunction.2 Lymphocytic infiltration of the lacrimal and salivary glands results in the classic sicca complex characterized by dry eyes (keratitis sicca or keratoconjunctivitis sicca) and dry mouth (xerostomia). Moreover, SS is divided into primary and secondary SS. Primary SS is not associated with other autoimmune diseases; meanwhile, secondary SS is associated with other autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, autoimmune cirrhosis of the liver, and mixed connective tissue disease.2 Dry eye disease (DED) in SS is associated with decreased tear secretion and severe ocular surface damage.3 The most common presentation of patients with SS is dry eyes followed by dry mouth. Therefore, ophthalmologists are often the first to diagnose patients with SS in the early clinical stage. DED in SS was initially classified as an aqueous-deficient type in the 2007 Tear Film & Ocular Surface Dry Eye WorkShop report; however, it has also demonstrated the characteristics of an evaporative component associated with meibomian gland dysfunction.3
SS is an autoimmune disorder characterized by inflammation secondary to activation of T and B lymphocytes that affect exocrine glands and produce autoantibodies. Various inflammatory cytokines produced by immunocompetent cells, including interferon (IFN)-γ and interleukin (IL)-17, are increased in SS. Autoreactive T and B cells are activated by IFN in the immune pathogenesis of SS.4 Particularly, IFN, which is involved in the early phase of the innate immunity in SS and is secreted by activated dendritic cells (DCs) and monocytes. B-cell–activating factor from DCs and monocytes is also an essential cytokine for the proliferation and differentiation of B cells in SS.4,5 In patients with SS and experimental models, B-cell hyperactivity and increased B-cell–activating factor expression were observed.5 This complex inflammatory process is also related to oxidative stress. Oxidative stress generally plays a role in the aging process, hypoxic damage, cancer, cardiovascular diseases, and neurodegenerative diseases.6 In the eye, oxidative stress is involved in DED, cataract formation, age-related macular degeneration, uveitis, premature retinopathy, keratitis, and ocular inflammation.6 Several studies have discovered that SS increases oxidative stress markers such as 4-hydroxy-2-nonenal (4-HNE), hexanoyl-lysine (HEL), and malondialdehyde.2 Furthermore, SS elevates nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), nitric oxide synthase, and xanthine oxidase, which are enzymes that produce reactive oxygen species (ROS) and reactive nitrogen species.2 Normally, they are produced to support the adaptive immune system in healing damaged cells; however, inadequate production of ROS and reactive nitrogen species in autoimmune diseases causes tissue damage by its excessive reactivity.4
Innate and adaptive immune responses and oxidative stress are involved in these complex inflammatory cascades of DED, including SS. Therefore, antiinflammatory drugs such as corticosteroids, cyclosporin A, and lifitegrast; mucin secretagogues such as diquafosol and rebamipide; artificial tears; and autoserum have been used to treat the inflammatory DED in patients with SS. However, the limited efficacy of these agents and associated side effects have led to unmet medical needs in the treatment of DED. Therefore, discovering a safe drug with potent antiinflammatory effects and multiple mechanisms of action on DED as an alternative to current therapies is essential.
RCI001 (RCI) is a novel therapeutic candidate for treating ocular surface diseases, including DED. We have demonstrated that topical RCI effectively managed ocular surface inflammation in several ocular surface inflammatory disease models.7–9 The main component in RCI is 8-oxo-2ʹ-deoxyguanosine (8-oxo-dG), and 8-oxo-dG is an oxidized derivative of deoxyguanosine, which is endogenously released when DNA guanosine is damaged. In addition, 8-oxo-dG is considered an oxidative stress biomarker.10 Previous studies also demonstrated potent antiinflammatory and antioxidative effects of exogenous applications of 8-oxo-dG in several systemic inflammatory disease models through Rac1 inhibition.10–13 To date, no studies have evaluated the effects of RCI on the inflammatory DED in SS. NOD.B10 mice were generated by replacing the NOD/ShiLtJ major histocompatibility locus with that of healthy C57BL/10 strain. Similarly to SS, the exocrine tissue of them shows histopathologic features such as exocrine gland lymphocytic infiltration composed of Thy 1.2+ T cells and B220+ B cells.14 Also, the model shows salivary flow loss with disease progression. Therefore, this study is designed to investigate the therapeutic effects of RCI on inflammation-related DED by using the NOD.B10-H2b mouse model, which mimics SS.14
MATERIALS AND METHODS
Ethics Declarations
The protocol was approved by the Institutional Animal Care and Use Committee of the Seoul National University Biomedical Research Institute (IACUC no. 20-0178-S1A0). Animal experiments were performed in accordance with the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic vision and research and Animal Research: Reporting of In Vivo Experiments guidelines.
Animals
The eight 12-week-old nonobese mice with diabetes from the B10-H2b strain (NOD.B10-H2b, male) used in this study were purchased from the Jackson Laboratory (Bar Harbor, ME). The mice were bred in a specific pathogen-free facility at the Biomedical Research Institute of Seoul National University Hospital (Seoul, Korea), maintained at 22 to 24°C with 55 ± 5% relative humidity, and provided free access to food and water. These mice were randomly divided into 2 groups of 4: the phosphate-buffered saline (PBS) group and RCI (10 mg/mL RCI001, Rudacure Co, Ltd, Seoul, Korea) group. RCI was evenly dissolved in PBS, and 5 μL of RCI or PBS was instilled into the right eye twice daily for 7 days in each group with intervals of more than 6 hours. Two mice were in the negative control group and received no treatment. The protocol was approved by the Institutional Animal Care and Use Committee of the Seoul National University Biomedical Research Institute (IACUC no. 20-0178-S1A0). Concentrations of RCI001 above 10 mg/mL were found to precipitate in PBS solution. Therefore, the concentration of 10 mg/mL was selected based on the solubility of RCI001 in PBS. In our previous studies, 10 mg/mL of RCI001 was effective in ethanol-induced corneal injury and ocular alkali-burn model.8,9
Clinical Evaluation of the Dry Eye
Ocular surface examination and the tear secretion test were performed under anesthesia (using a mixture of zoletil and xylazine in a ratio of 1:3). In addition, ocular surface staining (OSS) was blindly assessed by 2 experienced ophthalmologists (Y.J. and J.M.) using the National Eye Institute scoring scheme. Lissamine Green B (3%) (Sigma-Aldrich) was used for visualizing the OSS in the NOD.B10-H2b mice.12,13 After placing 1 drop of dye on the conjunctival sac for 30 seconds, the ocular surface was gently washed with 1 mL of normal saline. The OSS of the mice was observed under white light [LED (light emitting diode)] illumination using a microscope (Olympus SZ61; Olympus Corporation, Tokyo, Japan).12,13 The OSS score was divided into 5 zones centered on the center of the cornea (center, top, bottom, left, and right). The degree of staining in each zone was allocated a score of 0 to 3 points, which were then added for a total of 0 to 15 points. Phenol red–impregnated cotton threads (FCI Ophthalmics, Pembroke, MA) were used for the tear secretion test. Threads were placed into the lateral canthus of the mouse eye and assessed after 60 seconds.
Periodic Acid–Schiff Staining and Conjunctival Goblet Cell Evaluation
After the mice were euthanized, a whole eyeball was excised and fixed in 10% neutral buffered formalin overnight at 4°C. The eyeball tissue was embedded in paraffin, cut into 4-µm sections through the superior and inferior conjunctival fornixes, and stained with periodic acid–Schiff (PAS). Goblet cells in the conjunctiva were evaluated by 2 independent, blinded examiners (Y.J. and J.M.). Two different sections of central superior conjunctiva were randomly selected, and the average density of PAS-stained goblet cells was calculated under the light microscope.
Quantitative Real-Time Polymerase Chain Reaction
The conjunctiva and cornea (CC) and extraorbital lacrimal gland (LG) were cut into small pieces and lysed in a RNA isolation reagent. After sonication with a probe sonicator (Ultrasonic Processor, Cole Parmer Instruments, Vernon Hills, IL), total RNA was extracted using the RNeasy Mini Kit (Qiagen, Venlo, The Netherlands), and first-strand complementary DNA was synthesized by reverse transcription (High-Capacity RNA-to-cDNA Kit, Applied Biosystems, Foster City, CA). Real-time amplification was performed by TaqMan Universal PCR Master Mix (Applied Biosystems) in an automated instrument (ABI 7500 Real-Time Polymerase Chain Reaction System, Applied Biosystems) targeting TNF-α (TaqMan Gene Expression Assays ID, Mm00443260_g1), IFN-γ, IL-1β (Mm00434228_m1), IL-6 (Mm00446190_m1), IL-17a (Mm00439618_m1), IL-18 (Mm00434226_m1), and CXCL1 (Mm04207460_m1) in the CC to assess the antiinflammatory effect. Transforming growth factor-β (Mm01178820_m1), IL-10 (Mm01288386_m1), NOX2 (Mm01287743_m1), and NOX4 (Mm00627696_m1) from the CC and extraorbital LG were analyzed by RT-PCR to assess for ROS production.
Immunofluorescence Staining
LG from killed recipients was subjected to immunofluorescent staining. In the immunofluorescence staining experiment focusing on LG, a total of 8 individuals were used. Oxidative stress induced by desiccation was assessed by immunohistochemical detection of HEL (early-phase oxidative stress marker), 4-HNE (late-phase oxidative stress marker), NOX2, and NOX4. The avidin–biotin–peroxidase complex method was used in immunostaining. Tissues were fixed overnight in a 4% buffered paraformaldehyde solution and processed for paraffin embedding. Sections 4 μm thick were cut from paraffin wax blocks, mounted on precoated glass slides, deparaffinized, and rehydrated. The mean fluorescence intensity was measured in 3 regions of interest of the conjunctival fornix and cornea using ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical Analyses
The Mann–Whitney U test was used to compare the 2 groups. Statistical analyses were performed using GraphPad Prism software (version 9.0; GraphPad Software, La Jolla, CA). All statistical tests were performed using 2-tailed tests, and P-values <0.05 were considered statistically significant. The bars on the graph represent the SD.
RESULTS
RCI001 Improved Keratoepitheliopathy and Tear Secretion in a Primary Sjögren Model
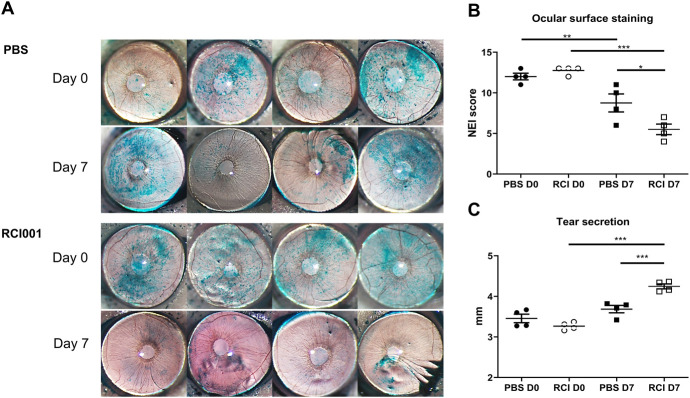
On day 7, the average OSS of the RCI group was significantly lower (5.9 ± 1.8) than that of the PBS group (8.4 ± 2.3) (P < 0.05; Figs. 1A, B). After RCI treatment, a notable improvement in OSS was observed compared with both pre-RCI instillation and post-RCI instillation (12.6 ± 0.9 vs. 5.9 ± 1.8, P < 0.001; Figs. 1A, B). In the PBS group, OSS was also significantly improved after PBS instillation (11.9 ± 1.2 vs. 8.4 ± 2.3, P < 0.05; Figs. 1A, B). Tear secretion was significantly higher in the RCI group (4.2 ± 0.2 mm) than in the PBS group (3.7 ± 0.3) after 1 week of treatment (P < 0.001; Fig. 1C). In the RCI group, tear secretion was also significantly improved when compared with the baseline (Day 0 vs. Day 7, tear secretion: 3.3 ± 0.2 mm vs. 4.2 ± 0.1 mm, P < 0.001; Fig. 1C). The PBS group exhibited no significant difference in tear secretion compared with the baseline (Day 0 vs. Day 7, tear secretion: 3.5 ± 0.3 mm vs. 3.7 ± 0.3 mm, P > 0.05; Fig. 1C). There were no notable adverse events in any of the mice during the experiments.
FIGURE 1.
Ocular surface and tear secretion are altered after RCI001 treatment in a primary Sjögren syndrome model. A, Representative images of corneal staining of BALB/C mice (6 weeks old). B, The National Eye Institute corneal staining score was significantly lower in the RCI001 group than in the PBS group (P < 0.01). The National Eye Institute score improved significantly in the RCI001 group on day 7 (P < 0.01). C, Tear secretion is significantly increased in the RCI001 group on day 7 (P < 0.001), and more tears are secreted in the RCI001 group than in the PBS group on day 7 (P < 0.001). BALB/c, an albino, laboratory-bred strain of the house mouse.
RCI001 Modulated Inflammatory Cytokines and Oxidative Stress–Related Molecules Compared With PBS
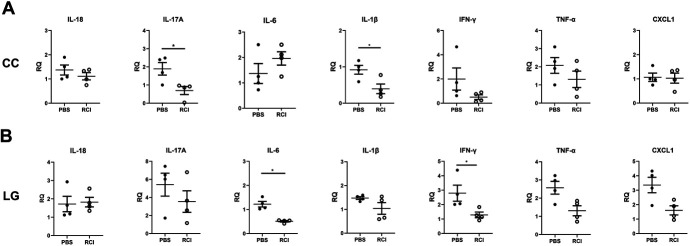
Transcript levels of IL-17A and IL-1β in CC were significantly reduced in the RCI group (each P < 0.05, Fig. 2A). Transcript levels of IL-6 and IFN-γ in LG were also markedly reduced in the RCI group (P < 0.05, Fig. 2B). Those of IL-18, tumor necrosis factor (TNF)-α, and chemokine (C-X-C motif) ligand 1 (CXCL1) in CC and IL-17A, IL-1β, IFN-γ, TNF-α, and CXCL1 in LG were not significantly different between the RCI and PBS groups; however, the RCI group displayed lower mean expression levels compared with those in the PBS group (Fig. 2). In transcript levels of oxidative stress–related molecules (NOX2, NOX4), a considerable reduction was observed in the CC of the RCI group (P < 0.05, Fig. 3A). In LG, no significant change was identified between the PBS and RCI groups (P > 0.05, Fig. 3B).
FIGURE 2.
Comparison of cytokines in the primary Sjögren syndrome model. A, Levels of cytokines on the ocular surface (corneoconjunctiva) of the PBS and RCI001 groups in an experimental dry eye model. B, Cytokine levels in the lacrimal glands of the PBS and RCI001 groups. No significant difference was observed between the 2 groups in the experimental dry eye model. Data are expressed as mean ± standard error of the mean. *P < 0.05, **P < 0.01, and ***P < 0.001.
FIGURE 3.
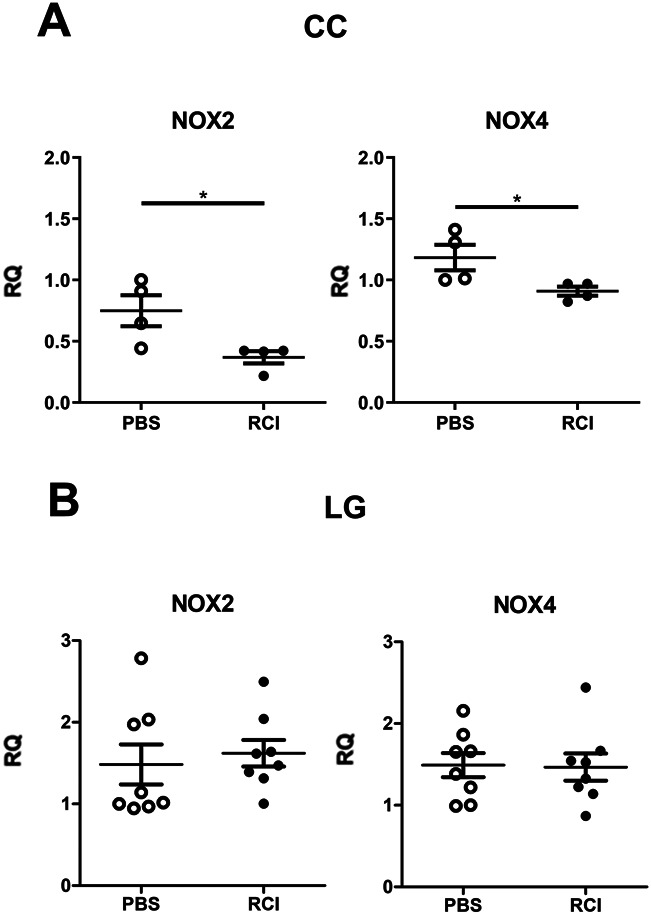
Effect of short-term RCI001 on the oxidative stress markers on the ocular surface and LG. Comparison of NOX levels in CC (A) and LG. (B) Levels of NOX2 and NOX4 in CC of the primary Sjögren syndrome model were decreased in the RCI group (P < 0.05).
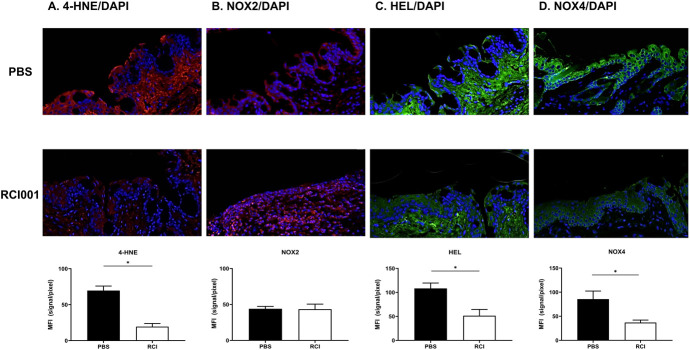
In the immunohistochemistry study, the RCI group exhibited significantly decreased expressions of 4-HNE, HEL, and NOX4 in the conjunctiva compared with those in the PBS group (A: red, C and D: green [P < 0.05, Fig. 4]). However, no significant change was observed in NOX2 between the RCI and PBS groups (B: green [P > 0.05, Fig. 4]). The mean fluorescence intensity of 4-HNE, HEL, and NOX4 was 108.4 ± 5.6, 69.6 ± 3.2, and 85.6 ± 8.4 in the PBS group and 51.4 ± 6.6, 19.4 ± 2.2, and 36.9 ± 2.6 in the RCI group, respectively.
FIGURE 4.
Effect of RCI001 on oxidative stress–related molecules. A, Representative images of immunofluorescence staining (×200) of corneoconjunctival sections in the primary Sjögren syndrome model. [4-HNE (A), NOX2 (B), HEL (C), NOX4 (D)]. Data are expressed as mean ± standard error of the mean. *P < 0.05.
Short-Term RCI Eye Drops Have Little Effect on Conjunctival Goblet Cells
Conjunctival goblet cell loss is common in DED, including SS. Conjunctival goblet cell counts did not significantly differ between the PBS and RCI groups (Fig. 5). The mean number of goblet cells in the conjunctiva was 126.5 ± 55.8 and 129.3 ± 46.1 in the PBS and RCI groups, respectively.
FIGURE 5.
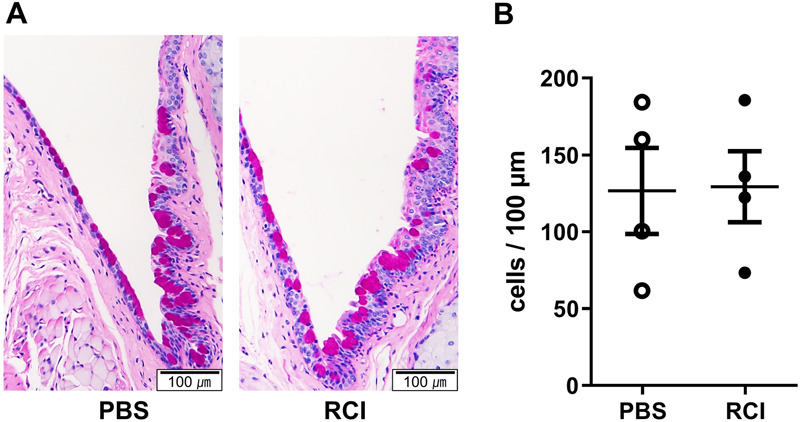
PAS histology and conjunctival goblet cell density analysis between RCI001 and PBS in the primary Sjögren syndrome model. A, Representative images of PAS staining (×100). B, Goblet cell counts revealed no significant change in the primary Sjögren syndrome model. Data are expressed as mean ± standard error of the mean. *P < 0.05, **P < 0.01, and ***P < 0.001.
DISCUSSION
In this study, administration of RCI for 7 days demonstrated excellent therapeutic effects on inflammatory dry eye in the SS mouse model. Topical RCI improved OSS and tear production by significantly reducing some inflammatory cytokines (IL-17 and IL-1β in CC and IL-6 in LG) and oxidative stress markers (4-HNE, HEL, NOX2, and NOX4) in CC.
The mechanism of action of exogenous RCI is the inhibition of Rac1, a small G protein that regulates oxidative stress and inflammatory cytokine release through NOX activation.8,9,15 NADPH oxidases are major enzymes that generate ROS, a collection of oxygen-derived short-lived messenger molecules, which can cause DNA damage and increase tyrosine kinase activity by inactivating enzymes.16,17 Rac1 protein has been demonstrated to be involved in activating NOX and producing ROS.16,17 In addition, 4-HNE is produced by activated neutrophils as part of an inflammatory response.18 4-HNE is a main product of lipid peroxidation in ocular tissues related to NOX and Rac1.19 As inflammation due to SS generates oxidative stress, reduction of those oxidative stress markers may explain the therapeutic effects of topical RCI.
The patient's immune system response is exaggerated by SS, which also causes an abnormal increase in inflammatory cytokines and autoantibodies.20,21 IL-17 is an overproduced proinflammatory cytokine involved in multiple autoimmune diseases, including SS.20,21 Overexpression of IL-17 in the tears, LG, and CC of patients with SS correlates with the severity of eye inflammation and dryness.20,21 IL-6 is another major inflammatory cytokine related to SS.22 The expression of IL-6 is elevated in the saliva and tears of patients with SS and is considered a marker of local inflammation.22 Furthermore, LG-infiltrating lymphocytes are considered a source of IL-6 in the eyes.22 This particular cytokine seems to regulate BCR-mediated Rag regulation in B cells.23
Ocular surface inflammation due to SS is generally caused by an aqueous tear deficiency. Some studies report that inflammation is a result of global tear dysfunction, including changes in meibomian gland function.24 Treatment of ocular surface inflammation in SS is aimed at maintaining the integrity of the tear film by preserving and increasing insufficient tear secretion. In this study, topical RCI displayed a remarkable therapeutic effect by increasing tear volume and improving ocular surface damage in a Sjögren mouse model. RCI did not demonstrate superior effects on the conjunctival goblet cell in the primary SS model. However, we observed a significant increase in tear volume and conjunctival goblet cell density after 2 weeks of ocular administration of topical RCI in the environmental DED mouse model (see Figure, Supplemental Digital Content 1, http://links.lww.com/ICO/B724).
DED is mainly caused by tear hyperosmolarity and an unstable tear film due to desiccating stress.3,6 As a result, DED causes stress and damage to the ocular surface epithelium, which ultimately induces a complicated, inflammatory cascade of the innate (corneal epithelial cells, DCs, neutrophils, and macrophages; acute response) and adaptive (T cells, B cells; chronic response) immune responses.6 The vicious cycle of inflammation and oxidative stress is regarded as a core pathophysiologic mechanism in DED, with the crosstalk between the neuronal system of the ocular surface and the local immune system disrupting ocular surface homeostasis.6,9 Numerous cytokines and chemokines related to this vicious cycle in DED can trigger stimulation of corneal sensory nerves that cause or aggravate ocular discomfort. This vicious cycle can be perpetuated if proper tear film stabilizing or antiinflammatory treatment is not applied, especially in inflammatory DED in SS.
Various commercial topical drugs available for Sjögren-induced inflammatory DED have certain limitations. The first-line therapies, artificial tears and ocular gels/ointment, can reduce dry eye symptoms, but they are temporary and require multiple periodic applications. Antiinflammatory agents such as topical corticosteroids, cyclosporin A, and lifitegrast can be considered, but each has its clinical limitations. Although topical corticosteroids are known as commercially available potent immunosuppressive agents, they can also cause secondary glaucoma, increased risks of infection, and cataract formation in long-term use. Cyclosporin A and lifitegrast are less potent than corticosteroids and cannot modulate innate immunity, which is one of the key regulators in ocular surface inflammation.9 In addition, cyclosporin A requires months to exert clinical effect and can cause temporary ocular pain and conjunctival congestion.9,25,26 Lifitegrast can demonstrate clinical effects faster than cyclosporin A, typically within 2 to 4 weeks. However, lifitegrast causes frequent pain, irritation, and dysgeusia lasting up to 4 hours.25 Diquafosol and rebamipide, known to help stabilize the tear film, have clinical limitations in modulating ocular surface inflammation in SS.
8-oxo-dG is a natural substance released when the guanine base of cellular DNA is damaged.8,9 An interesting clinical point is that the exogenous application of 8-oxo-dG demonstrated potent antiinflammatory and antioxidant effects in several inflammatory and chronic disease models through Rac1 inhibition.11–13 As a result, 8-oxo-dG has displayed significant clinical and histologic improvement in several preclinical disease models (lipopolysaccharide-induced sepsis model, stress-induced gastritis model, atherosclerosis model, and diabetes mellitus model). Rac1-associated functions include phagocytosis, chemotaxis, inflammatory cytokine release, and ROS production through NOX activation.9 In addition, Rac1 is known to be implicated in the regulation of mitogen-activated protein kinase, extracellular signal-regulated kinase, Janus kinase/signal transducer, activator of transcription, and nuclear factor kappa light chain enhancer of activated B cells.8,9 Therefore, as ophthalmic researchers, we aimed to investigate the potential antiinflammatory and antioxidative properties of 8-oxo-dG by applying it to an ocular surface inflammatory model. First, 8-oxo-dG inhibited the infiltration of neutrophils and macrophages into the corneal tissue and reduced the expression of key inflammatory cytokines such as IL-1β and TNF-α in an ethanol-induced corneal injury model.15 In an alkali-induced ocular chemical burn model, these antiinflammatory/antioxidative effects were superior to 1% prednisolone acetate, the most potent commercially available ophthalmic topical corticosteroid.8 These results led us to develop RCI, which may be a novel therapeutic candidate for ocular surface inflammatory diseases. We also discovered that RCI exhibited significant antiinflammatory and antioxidant effects by inhibiting the Rac1/NLRP3 inflammasome/IL-1β axis.9 In addition, TNF-α, NOX2, and NOX4 expression were significantly lower after RCI treatment compared with that after PDE treatment in an alkali-burned mouse cornea.8 Unlike topical corticosteroids, cyclosporin A, lifitegrast, rebamipide, and diquafosol, topical RCI can effectively and extensively suppress the activation of neutrophils, macrophages, Rac1, NLRP3 inflammasomes, and inflammatory cytokines in DED; thus, it can act broadly on various immune cells.8,9,15 Moreover, RCI can also inhibit the expression of NOX2 and NOX4 better than corticosteroids in ocular chemical burn and environmental dry eye models.9 Our preclinical in vivo study recently demonstrated that long-term topical application of RCI for more than 5 weeks did not induce an elevation in intraocular pressure.27 Furthermore, RCI also displayed excellent corneal epithelial healing effects compared with solcoseryl and polydeoxyribonucleotide, used as adjunctive therapy in corneal abrasions.28 Our other study also demonstrated that the effects of RCI were superior to those of 1% prednisolone acetate and 5% lifitegrast in the environmental DED model .29 Similarly, with this study, the RCI group demonstrated more reduced OSS and increased tear production compared to the PBS group. Suppression of oxidative stress was also the most potent in the RCI group in the environmental DED model. Many patients with chronic DED frequently have acute flares, which can be induced by various lifestyle and environmental factors. These inflammatory flares can exacerbate ocular discomfort and activate nonspecific innate and specific adaptive immune responses.30 Topical corticosteroids with a wide spectrum of antiinflammatory effects can be selected to rapidly cool down acute DED flares. If RCI is successfully developed, we believe that RCI might be an effective and safe therapy for ocular surface inflammatory diseases with broad-spectrum antiinflammatory/antioxidative effects.
This study had several limitations. First, the sample size was small. Second, the long-term changes were not assessed in the experimental models. Third, meibomian gland dysfunction, which is a major cause of DED, was not evaluated. Fourth, conjunctival goblet cell densities were not significantly different between the RCI and PBS groups. Fifth, oxidative stress markers were not evaluated at the protein level. Sixth, the molecular factors of the CC were not analyzed separately. Nevertheless, this study established that RCI has excellent antiinflammatory and antioxidative effects on the inflammatory DED in an experimental SS model. The number of experimental samples was limited due to the use of rare animal models (NOD.B10-H2b mice). Since this study was conducted for only 1 week, it can be considered that there was not enough time to increase goblet cell density. Topical cyclosporin also increased goblet cell density but, most studies were conducted more than a month,31,32 and 1 of the studies shows that conventional cyclosporin does not have significant difference in goblet cell density after 2 weeks.33
In conclusion, topical RCI demonstrated a remarkable therapeutic effect on the inflammatory dry eye in SS by improving clinical signs, modulating the inflammation in CC and LG, and reducing the oxidative stress of the ocular surface. Therefore, RCI may be considered a novel therapeutic candidate for ocular surface inflammatory diseases, including dry eye in SS.
Supplementary Material
Footnotes
Supported by the National Research Foundation of Korea grant funded by the Korean government (MSIT) (No. RS-2023-00243720) and a grant of Korea University Anam Hospital, Seoul, Republic of Korea (K2319651 and K2225511).
D. H. Kim invented a patent for the topical use of RCI001 as treatment of various ocular diseases (Republic of Korea: 10-1816277/United States: 10/675294). Y. H. Kim is the Chief Executive Officer of RudaCure, the company developing RCI001. The remaining authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.corneajrnl.com).
All data generated or analyzed during this study are included in this published article.
D. H. Kim and M. K. Kim contributed equally to this work.
Writing—original draft: H. Kang, Y. Jung, and D. H. Kim. Writing-review and editing: M. K. Kim and D. H. Kim. Data acquisition: Y. Jung, J. Moon, and C. H. Yoon. Formal analysis: M. K. Kim and D. H. Kim. Funding acquisition: Y. H. Kim and D. H. Kim. Supervision: M. K. Kim and D. H. Kim. All authors have contributed to the manuscript and approved the submitted version.
Contributor Information
Hyereen Kang, Email: hyereen.kang@rudacure.com.
Young-ho Jung, Email: dudghmed86@naver.com.
Jayoon Moon, Email: ja-yoon88@hanmail.net.
Jin Suk Ryu, Email: enter2357@naver.com.
Chang Ho Yoon, Email: ifree7@gmail.com.
Yong Ho Kim, Email: euro16@gachon.ac.kr.
Mee Kum Kim, Email: kmk9@snu.ac.kr.
REFERENCES
- 1.Qin B, Wang J, Yang Z, et al. Epidemiology of primary Sjögren's syndrome: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:1983–1989. [DOI] [PubMed] [Google Scholar]
- 2.Vivino FB. Sjogren's syndrome: clinical aspects. Clin Immunol. 2017;182:48–54. [DOI] [PubMed] [Google Scholar]
- 3.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–283. [DOI] [PubMed] [Google Scholar]
- 4.Youinou P, Pers JO. Disturbance of cytokine networks in Sjögren’s syndrome. Arthritis Res Ther. 2011;13:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogawa Y, Shimizu E, Tsubota K. Interferons and dry eye in Sjögren's syndrome. Int J Mol Sci. 2018;19:3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dogru M, Kojima T, Simsek C, et al. Potential role of oxidative stress in ocular surface inflammation and dry eye disease. Invest Ophthalmol Vis Sci. 2018;59:163–168. [DOI] [PubMed] [Google Scholar]
- 7.Ibáñez-Cabellos JS, Pallardó FV, García-Giménez JL, et al. Oxidative stress and epigenetics: mirna involvement in rare autoimmune diseases. Antioxidants. 2023;12:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DH, Im ST, Yoon JY, et al. Comparison of therapeutic effects between topical 8-oxo-2′-deoxyguanosine and corticosteroid in ocular alkali burn model. Sci Rep. 2021;11:6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Jang YW, Ku YA, et al. Investigating the anti-inflammatory effects of RCI001 for treating ocular surface diseases: insight into the mechanism of action. Front Immunol. 2022;13:850287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung H, Ha Y, Kim YH, et al. Ocular distribution and pharmacokinetics of 8-oxo-2′-deoxyguanosine: a novel therapeutic candidate of ocular surface diseases. J Ocul Pharmacol Ther. 2022;38:561–566. [DOI] [PubMed] [Google Scholar]
- 11.Choi S, Choi HH, Lee SH, et al. Anti-inflammatory effects of 8-hydroxy-2′-deoxyguanosine on lipopolysaccharide-induced inflammation via Rac suppression in Balb/c mice. Free Radic Biol Med. 2007;43:1594–1603. [DOI] [PubMed] [Google Scholar]
- 12.Kim DH, Cho IH, Kim HS, et al. Anti-inflammatory effects of 8-hydroxydeoxyguanosine in LPS-induced microglia activation: suppression of STAT3-mediated intercellular adhesion molecule-1 expression. Exp Mol Med. 2006;38:417–427. [DOI] [PubMed] [Google Scholar]
- 13.Ock CY, Hong KS, Choi KS, et al. A novel approach for stress-induced gastritis based on paradoxical anti-oxidative and anti-inflammatory action of exogenous 8-hydroxydeoxyguanosine. Biochem Pharmacol. 2011;81:111–122. [DOI] [PubMed] [Google Scholar]
- 14.Kiripolsky J, Shen L, Liang Y, et al. Systemic manifestations of primary Sjögren's syndrome in the NOD. B10Sn-H2b/J mouse model. Clin Immunol. 2017;183:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Im S-T, Kim HY, Yoon JY, et al. Therapeutic effects of topical 8-oxo-2ʹ-deoxyguanosine on ethanol-induced ocular chemical injury models. Cornea. 2018;37:1311–1317. [DOI] [PubMed] [Google Scholar]
- 16.Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res. 2006;98:453–462. [DOI] [PubMed] [Google Scholar]
- 17.Ago T, Kuroda J, Kamouchi M, et al. Pathophysiological roles of NADPH oxidase/nox family proteins in the vascular system–review and perspective. Circ J. 2011;75:1791–1800. [DOI] [PubMed] [Google Scholar]
- 18.Chacko BK, Wall SB, Kramer PA, et al. Pleiotropic effects of 4-hydroxynonenal on oxidative burst and phagocytosis in neutrophils. Redox Biol. 2016;9:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Zong R, Zhou J, et al. The oxidant role of 4-hydroxynonenal in corneal epithelium. Sci Rep. 2015;5:10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang LW, Zhou PR, Wei P, et al. Expression of interleukin‐17 in primary Sjögren's syndrome and the correlation with disease severity: a systematic review and meta‐analysis. Scand J Immunol. 2018;87:e12649. [DOI] [PubMed] [Google Scholar]
- 21.De Paiva CS, Hwang CS, Pitcher JD, III, et al. Age-related T-cell cytokine profile parallels corneal disease severity in Sjögren’s syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology (Oxford). 2010;49:246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tishler M, Yaron I, Geyer O, et al. Elevated tear interleukin-6 levels in patients with Sjögren syndrome. Ophthalmology. 1998;105:2327–2329. [DOI] [PubMed] [Google Scholar]
- 23.Hillion S, Garaud S, Devauchelle V, et al. Interleukin‐6 is responsible for aberrant B‐cell receptor‐mediated regulation of RAG expression in systemic lupus erythematosus. Immunology. 2007;122:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheppard JD, Nichols KK. Dry eye disease associated with meibomian gland dysfunction: focus on tear film characteristics and the therapeutic landscape. Ophthalmol Ther. 2023;12:1397–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu KY, Chen WT, Chu-Bédard YK, et al. Management of Sjogren's dry eye disease—advances in ocular drug delivery offering a new hope. Pharmaceutics. 2022;15:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos-Casals M, Brito-Zerón P, Bombardieri S, et al. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann Rheum Dis. 2020;79:3–18. [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Ku YA, Yoo C, et al. Comparison of RCI001 and corticosteroid on the effects on intraocular pressure in mice. Front Med (Lausanne). 2023;10:1256569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song MS, Ku YA, Kim S, et al. Comparison of corneal epithelial wound healing between topical RCI001, solcoseryl, and polydeoxyribonucleotide in the murine ocular alkali burn model. Korean J Ophthalmol. 2023;37:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung Y Ku Y Moon J, et al. Efficacy of RCI001 as a therapeutic candidate of dry eye disease in a modified mixed dry eye model. Eye Vis (Lond). 2024;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez V, Stern M, Pflugfelder S. Inflammatory basis for dry eye disease flares. Exp Eye Res. 2020;201:108294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pflugfelder SC, De Paiva CS, Villarreal AL, et al. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008;27:64–69. [DOI] [PubMed] [Google Scholar]
- 32.Moore CP, McHugh JB, Thorne JG, et al. Effect of cyclosporine on conjunctival mucin in a canine keratoconjunctivitis sicca model. Invest Ophthalmol Vis Sci. 2001;42:653–659. [PubMed] [Google Scholar]
- 33.Bang SP, Yeon CY, Adhikari N, et al. Cyclosporine A eyedrops with self-nanoemulsifying drug delivery systems have improved physicochemical properties and efficacy against dry eye disease in a murine dry eye model. PLoS One. 2019;14:e0224805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.