Abstract
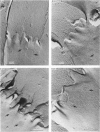
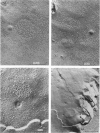
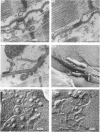
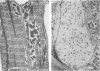
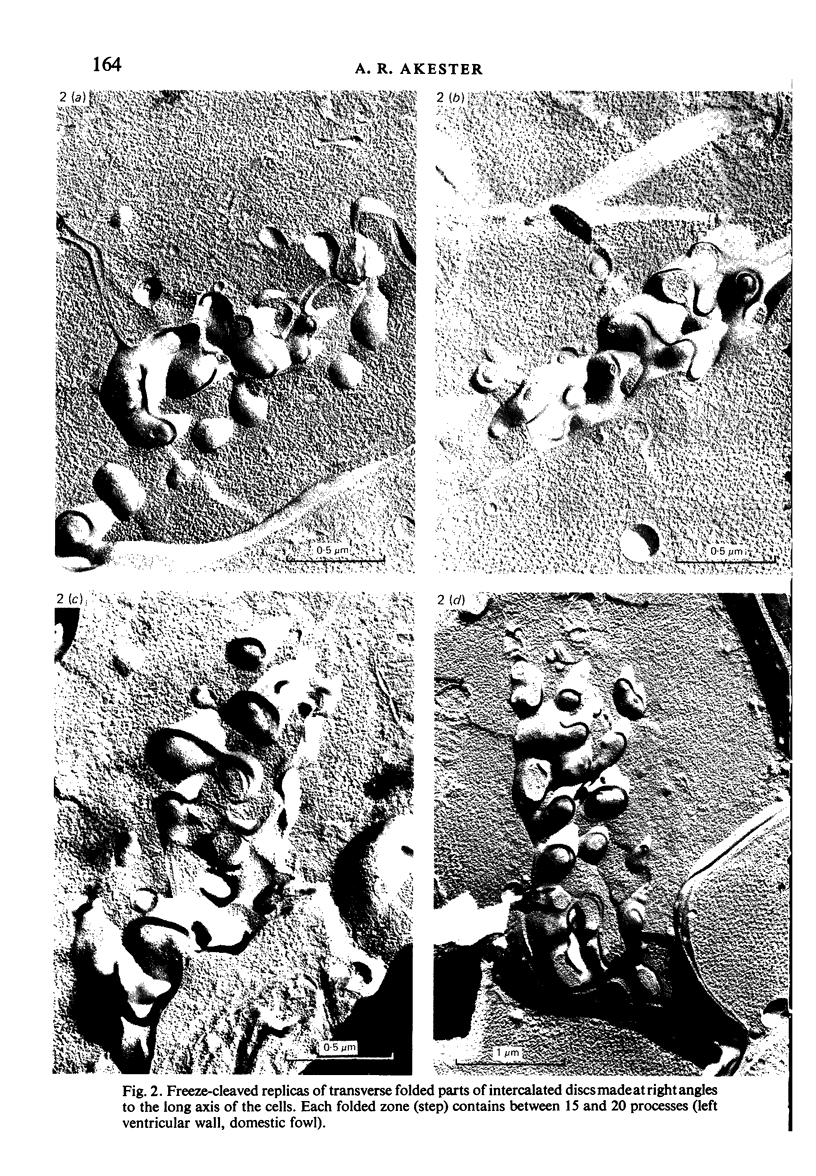
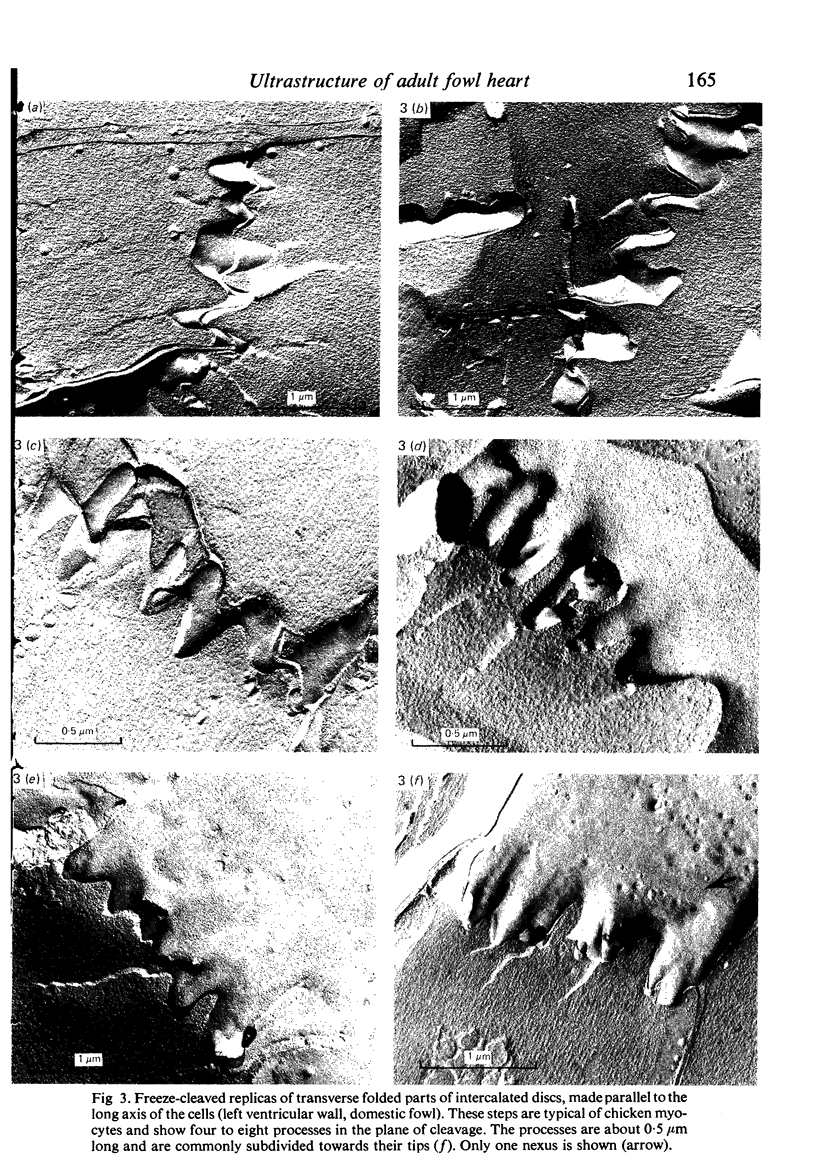
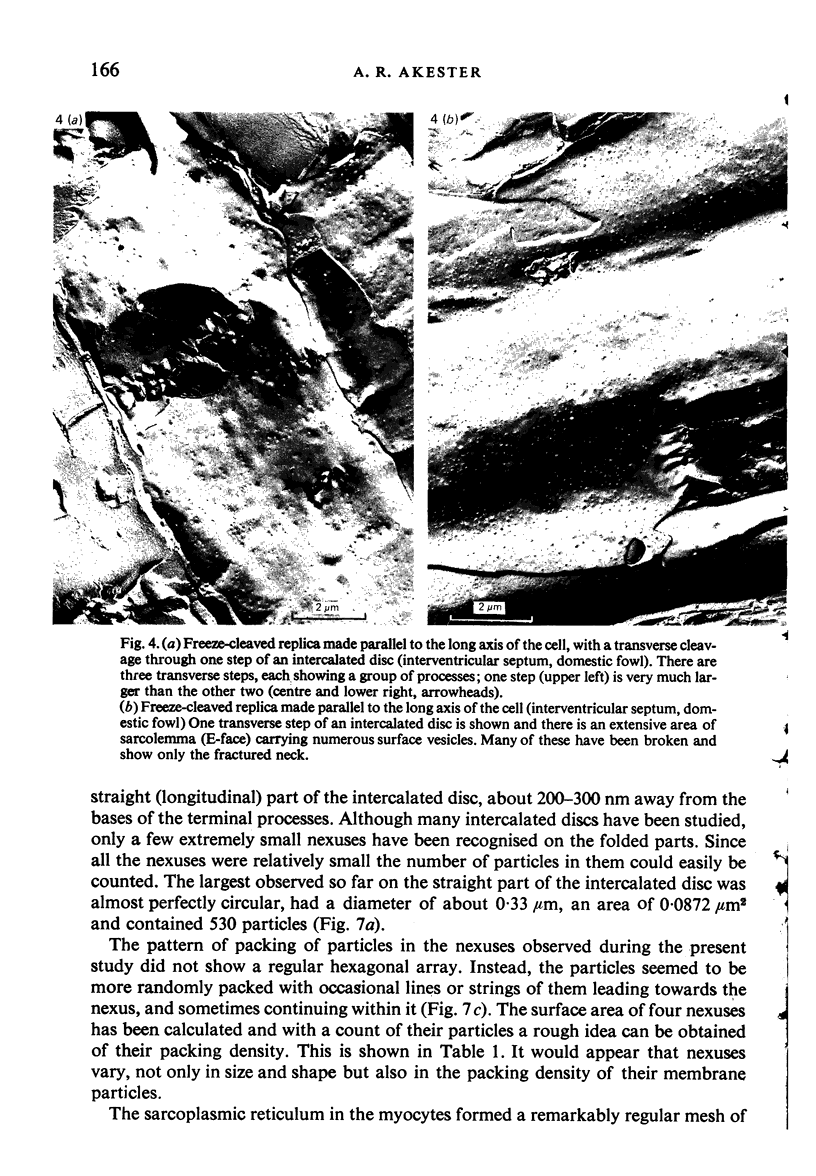
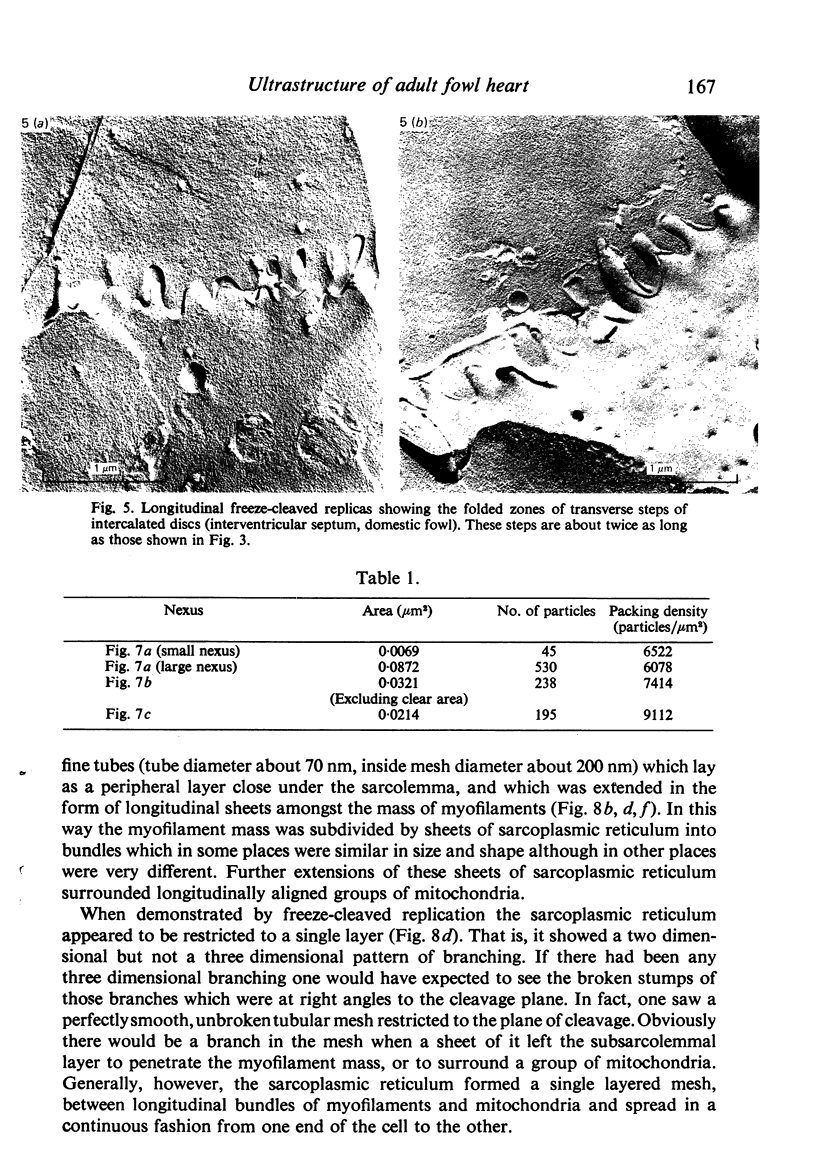
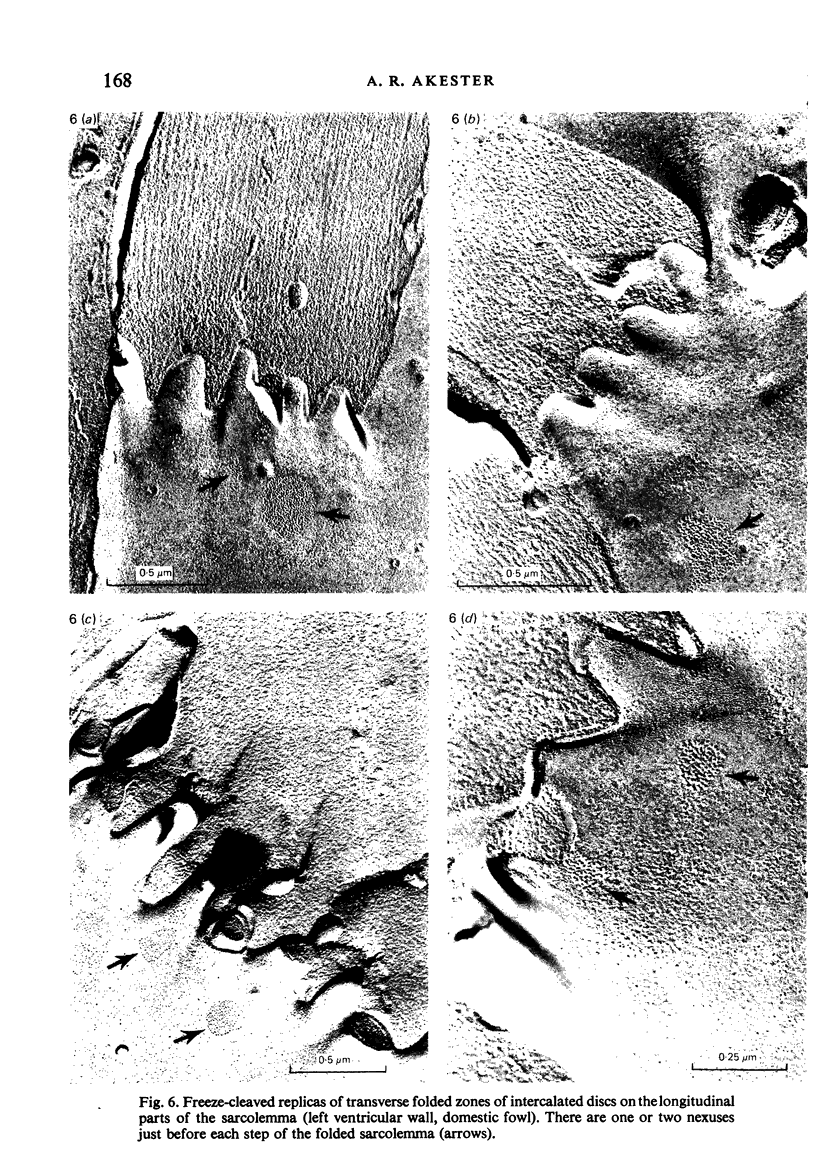
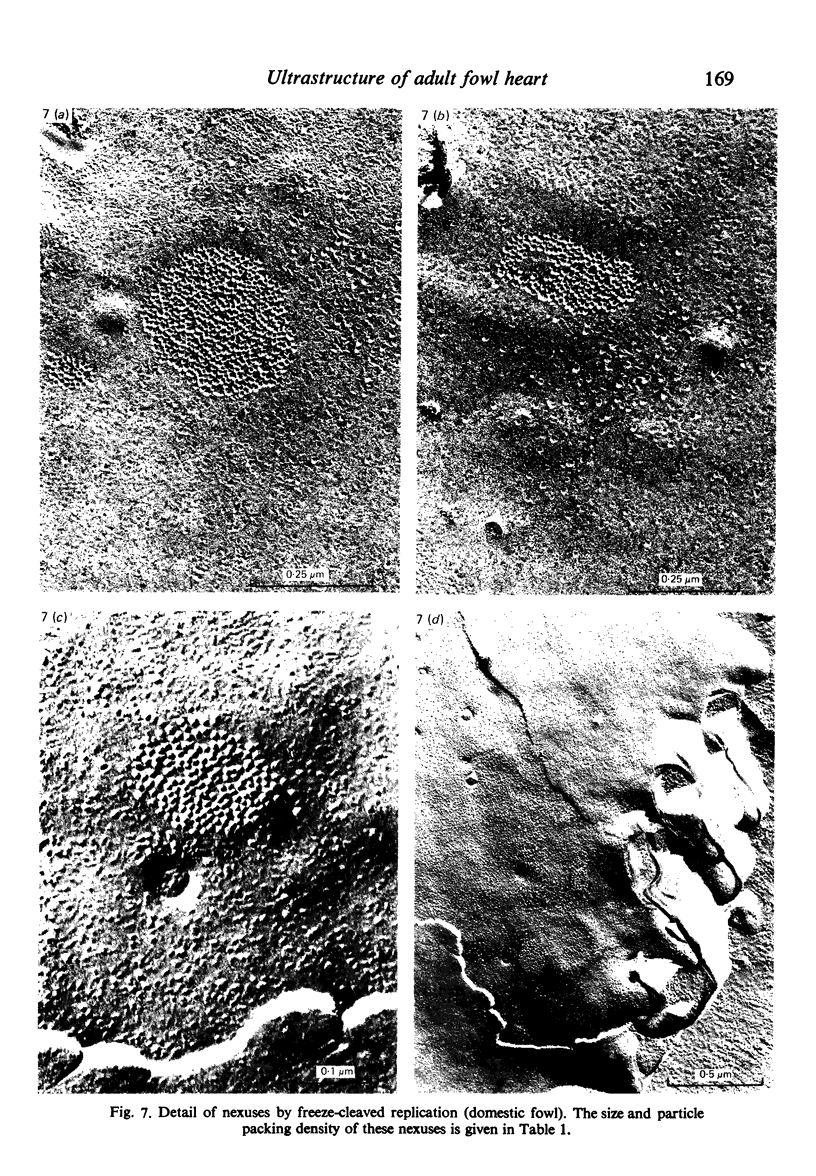
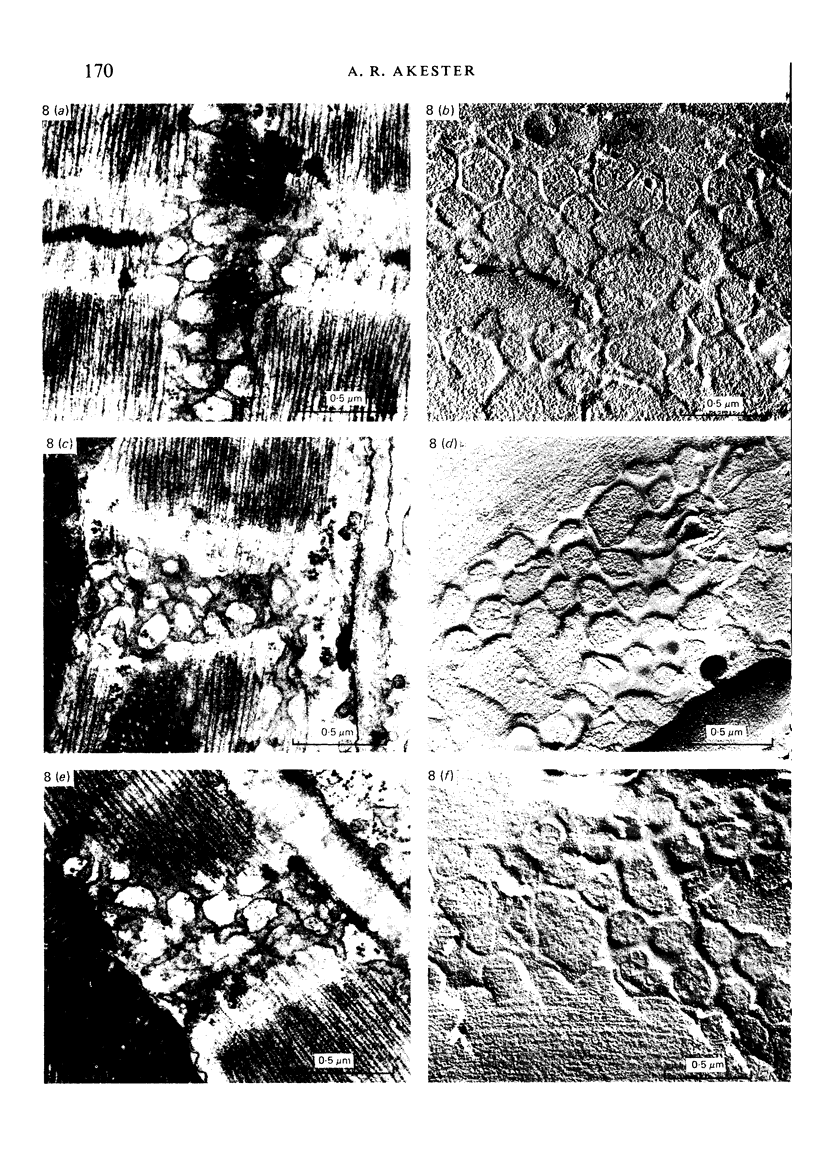
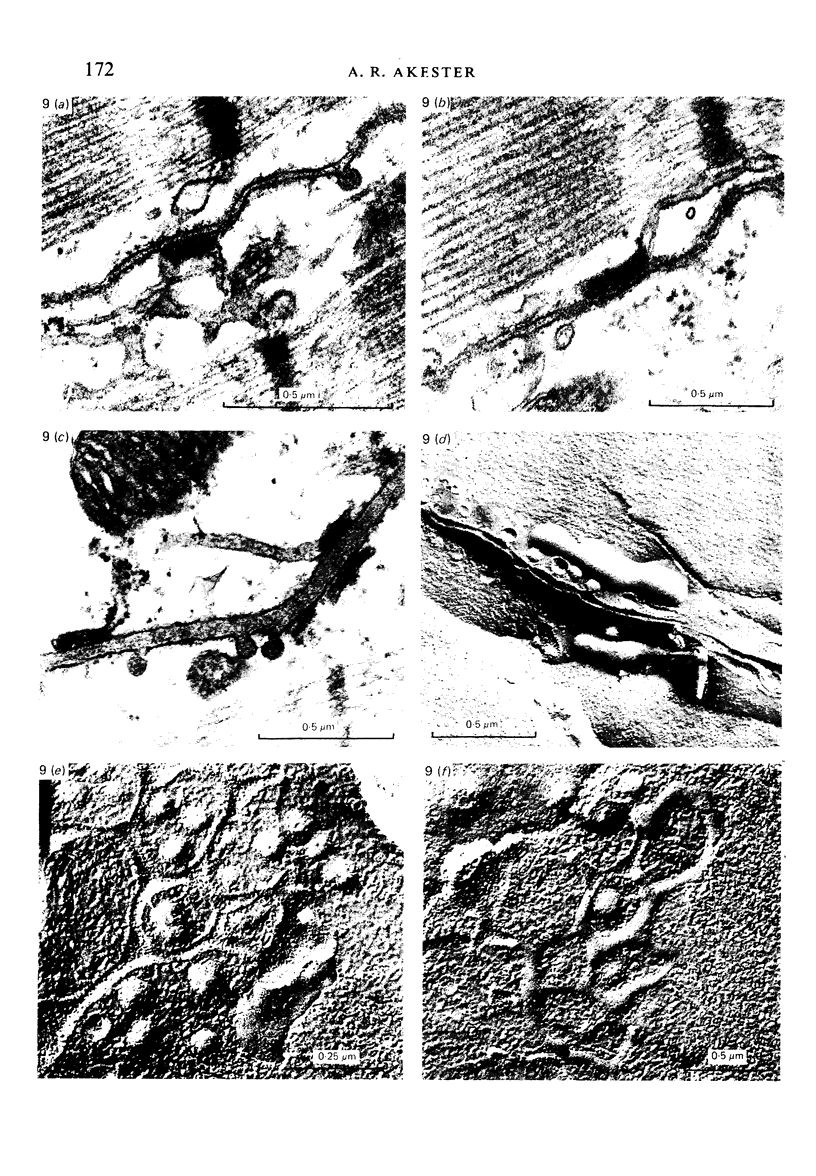
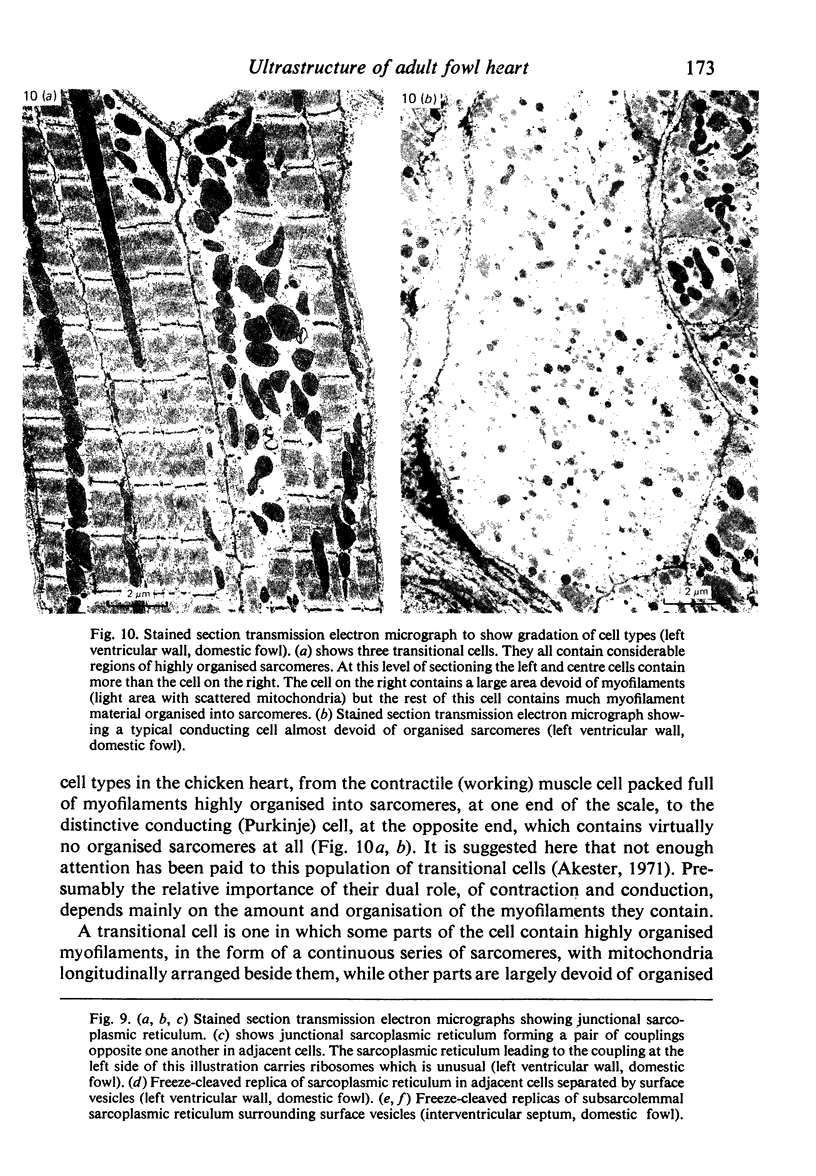
Intercalated discs usually consisted of three or four transverse steps of folded cell membrane connected by straight parts running longitudinally. The transverse folded parts contained a variable number of interlocking processes from contiguous cells. Large processes were frequently flanked by smaller ones. Nexuses appeared to be better developed on the straight parts of intercalated discs than on the folded parts. They varied in size, shape and particle-packing density. The largest nexus observed was almost perfectly circular. It covered an area of 0.087 micron2, contained 530 particles and had a particle-packing density of 6078 particles/micron2. The sarcoplasmic reticulum formed a uniform mesh of fine tubes which was distributed in sheets between bundles of myofilaments and longitudinally aligned mitochondria. Neither extended junctional sarcoplasmic reticulum nor fenestrations in the sarcoplasmic reticulum were observed. Peripheral couplings were numerous and widespread. They were not restricted to the sarcolemma in the regions of the Z-line, H- or I-bands. Transitional cells combine the characteristics of contractile and conducting cells in varying proportions. There is some speculation about the mechanism of nexus formation and the interconvertibility of contractile and conducting cells via the intermediary of the transitional cell.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARR L., DEWEY M. M., BERGER W. PROPAGATION OF ACTION POTENTIALS AND THE STRUCTURE OF THE NEXUS IN CARDIAC MUSCLE. J Gen Physiol. 1965 May;48:797–823. doi: 10.1085/jgp.48.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossen E. H., Sommer J. R., Waugh R. A. Comparative stereology of the mouse and finch left ventricle. Tissue Cell. 1978;10(4):773–784. doi: 10.1016/0040-8166(78)90062-9. [DOI] [PubMed] [Google Scholar]
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Carlson E. C., Grosso D. S., Romero S. A., Frangakis C. J., Byus C. V., Bressler R. Ultrastructural studies of metabolically active isolated adult rat heart myocytes. J Mol Cell Cardiol. 1978 May;10(5):449–459. doi: 10.1016/0022-2828(78)90366-8. [DOI] [PubMed] [Google Scholar]
- Jewett P. H., Leonard S. D., Sommer J. R. Chicken cardiac muscle: its elusive extended junctional sarcoplasmic reticulum and sarcoplasmic reticulum fenestrations. J Cell Biol. 1973 Feb;56(2):595–600. doi: 10.1083/jcb.56.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett P. H., Sommer J. R., Johnson E. A. Cardiac muscle. Its ultrastructure in the finch and hummingbird with special reference to the sarcoplasmic reticulum. J Cell Biol. 1971 Apr;49(1):50–65. doi: 10.1083/jcb.49.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A. M., Chacko S. Internal membrane junctions and unusual T-tubules in cultured chick cardiac muscle cells. J Mol Cell Cardiol. 1977 Jun;9(6):419–424. doi: 10.1016/s0022-2828(77)80023-0. [DOI] [PubMed] [Google Scholar]
- Mathur R., Shrivastava A. Cardiac conducting system of Gallus domesticus with special reference to the atrial bundles. Acta Morphol Neerl Scand. 1979 Aug;17(3):181–189. [PubMed] [Google Scholar]
- McNutt N. S. Ultrastructure of intercellular junctions in adult and developing cardiac muscle. Am J Cardiol. 1970 Feb;25(2):169–183. doi: 10.1016/0002-9149(70)90577-1. [DOI] [PubMed] [Google Scholar]
- Powell T., Steen E. M., Twist V. W., Woolf N. Surface characteristics of cells isolated from adult rat myocardium. J Mol Cell Cardiol. 1978 Mar;10(3):287–292. doi: 10.1016/0022-2828(78)90351-6. [DOI] [PubMed] [Google Scholar]
- Saetersdal T. S., Myklebust R., Berg Justesen N. P. Ultrastructural localization of calcium in the pigeon papillary muscle as demonstrated by cytochemical studies and x-ray microanalysis. Cell Tissue Res. 1974;155(1):57–74. doi: 10.1007/BF00220284. [DOI] [PubMed] [Google Scholar]
- Saettersdal T. S., Myklebust R. Ultrastructure of the pigeon papillary muscle with special reference to the sarcoplasmic reticulum. J Mol Cell Cardiol. 1975 Aug;7(8):543–551. doi: 10.1016/0022-2828(75)90113-3. [DOI] [PubMed] [Google Scholar]
- Scott T. M. The ultrastructure of ordinary and Purkinje cells of the fowl heart. J Anat. 1971 Nov;110(Pt 2):259–273. [PMC free article] [PubMed] [Google Scholar]
- Shibata Y., Yamamoto T. Freeze-fractrue studies of gap junctions in vertebrate cardiac muscle cells. J Ultrastruct Res. 1979 Apr;67(1):79–88. doi: 10.1016/s0022-5320(79)80020-9. [DOI] [PubMed] [Google Scholar]
- Sommer J. R., Johnson E. A. Cardiac muscle. A comparative ultrastructural study with special reference to frog and chicken hearts. Z Zellforsch Mikrosk Anat. 1969;98(3):437–468. [PubMed] [Google Scholar]
- Sommer J. R., Johnson E. A. Comparative ultrastructure of cardiac cell membrane specializations. A review. Am J Cardiol. 1970 Feb;25(2):184–194. doi: 10.1016/0002-9149(70)90578-3. [DOI] [PubMed] [Google Scholar]
- Sommer J. R., Waugh R. A. The ultrastructure of the mammalian cardiac muscle cell--with special emphasis on the tubular membrane systems. A review. Am J Pathol. 1976 Jan;82(1):192–232. [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N., Mayer G., Macdonald R. Velocity of propagation in vertebrate cardiac muscles as functions of tonicity and [K+]. Am J Physiol. 1970 Oct;219(4):952–963. doi: 10.1152/ajplegacy.1970.219.4.952. [DOI] [PubMed] [Google Scholar]
- Sperelakis N. Propagation mechanisms in heart. Annu Rev Physiol. 1979;41:441–457. doi: 10.1146/annurev.ph.41.030179.002301. [DOI] [PubMed] [Google Scholar]
- Tucker V. A. Respiratory physiology of house sparrows in relation to high-altitude flight. J Exp Biol. 1968 Feb;48(1):55–66. doi: 10.1242/jeb.48.1.55. [DOI] [PubMed] [Google Scholar]