Abstract
Purpose of review
Provide the most up-to-date information on the dynamic landscape of antibody–drug conjugates (ADCs) in gynecologic cancers. We discuss the latest research that supports the approved ADCs and outline the ongoing trials and preliminary results that may lead to ADC approvals in the future. Current gaps in knowledge and areas for future research are discussed.
Recent findings
ADCs are rapidly changing the landscape of gynecologic cancer care. Three ADCs are currently FDA approved and used routinely in clinical practice, with many more currently in clinical development. The most common ADC target is folate receptor alpha of which there are 8 different folate receptor targeting ADCs in development. Other targets under investigation include trophoblast cell surface antigen-2 (Trop-2), claudin-6 (CLDN6), cadherin-6 (CDH6), nectin-4, HER-2 and B7-H4. ADCs can cause new and unique adverse effects, including ocular toxicities and interstitial lung disease.
Summary
ADCs offer the opportunity for a more effective and personalized treatment approach for gynecologic cancer patients. Side effects must be closely monitored, and preventive measures must be followed to maximize benefit and minimize toxicity. A better understanding of the role of target proteins as biomarkers to predict response to ADCs will be critical for successful clinical implementation of ADCs and further research in this area is necessary.
Keywords: antibody–drug conjugates, gynecologic cancer
INTRODUCTION
Antibody drug conjugates (ADCs) combine the capabilities of targeted monoclonal antibodies with traditional cytotoxic agents. ADCs have three main components: a highly selective monoclonal antibody for a tumor-associated surface antigen, a potent cytotoxic agent to induce cell death after cellular internalization of the payload, and a linker that is stable while in circulation but releases the cytotoxic agent when in the target cells [1▪].
ADC approvals over the last year for gynecologic cancers include mirvetuximab soravtansine-gynx (MIRV) for the treatment of platinum resistant ovarian cancer (PROC) in March 2024 [2]. This was followed by the pan-cancer approval of trastuzumab-dertuxtecan (T-DXd) in April for any solid tumor that is HER2-positive, including endometrial (EC), cervical (CC), and ovarian cancer (OC) [3]. Tisotumab vedotin (TV) was also approved in April for recurrent or metastatic CC (r/mCC) following progression after chemotherapy [4].
In this review, we discuss the studies that support the approval of the currently approved ADCs and outline the ongoing trials that may lead to ADC approvals in the near future. Additionally, innovation leads to many more questions than answers, and we will discuss the current gaps in knowledge and areas for future research.
Box 1.
no caption available
OVERVIEW OF ANTIBODY–DRUG CONJUGATES
Monoclonal antibody and target antigen
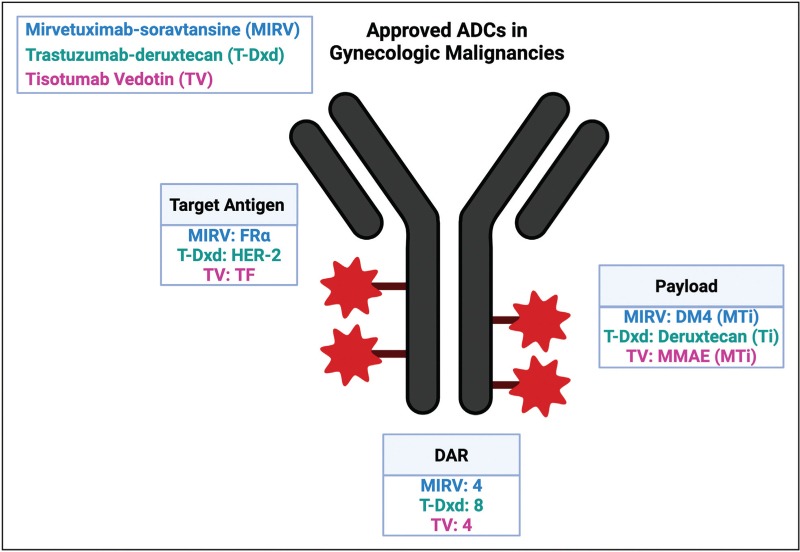
To deliver highly potent cytotoxic payloads to tumor cells, a monoclonal antibody (mAb) is needed that is specific to a target antigen present on the cancer cell surface. An ideal target would have sufficient density and homogeneity on tumor cells and minimal or no expression on normal cells to limit on target toxicity in normal cells. Most target antigens are tumor-associated and not tumor specific, and therefore have some expression on normal cells. Additionally, an optimal antigen should be on the cell surface and, when bound by the mAb, cause internalization of the ADC into a lysosomal-targeted pathway [5]. An ideal target has low level of removal of the antigen from the cell surface known as antigen shedding [5]. Other factors that impact the attractiveness of a molecule as a target for ADCs include the direct antitumor effect of the mAb itself, the function of the target antigen, and the downstream cellular changes induced after binding of the ADC to the target [6]. The ability of target antigen expression to predict response to an ADC is under active investigation. For the approved ADCs in gynecologic cancers, MIRV targets folate receptor alpha (FRα), TV targets tissue factor (TF) and T-DXd targets human-epidermal receptor-2 (HER-2). MIRV and T-DXd both require a companion diagnostic to confirm high antigen expression while TV does not.
Linker
To ensure that the cytotoxic payload is delivered to the tumor cell, linker stability is essential. An ideal linker should be stable in circulation and release the payload only once internalized into the tumor, as unstable linkers can increase off-target toxicity [7]. Linkers can be classified into cleavable and noncleavable. Most ADCs use cleavable linkers, which are cleaved based on pH level, or protease and glutathione levels in the tumor microenvironment. Less commonly, ADCs use noncleavable linkers which are covalently bonded and must be internalized into the cells and broken down by lysosomes to release the payload. Cleavable linkers may increase efficiency of payload release but have a higher risk of premature payload release. Premature release may lead to the bystander effect where the payload can diffuse into target antigen-negative cancer cells adjacent to the tumor site, potentially improving the efficacy of the drug 1[8]. Of the ADCs approved in gynecologic cancer, MIRV, TV, and T-Dxd all use cleavable linkers.
Cytotoxic payload
The cytotoxic payloads used in ADCs are 100–1000 times more potent than classic chemotherapy agents, and would be too toxic as anticancer drugs on their own [8]. The most common cytotoxic agents used are microtubule-targeting and DNA-damaging agents. Microtubule-targeting agents are cell-cycle specific and induce cell death in proliferating cells by arresting the cell cycle in G2/M phase. Alternatively, DNA-damaging agents act independent of the cell cycle and can target both proliferating and nonproliferating cells. MIRV and TV use microtubule inhibitors and T-Dxd uses a topoisomerase I inhibitor.
Drug–antibody ratio
ADC efficacy and toxicity can in part be explained by the DAR, which is the number of drug molecules attached to the antibody via a linker. A low DAR decreases ADC efficacy and decreases toxicity, while a higher DAR can enhance the efficacy, it can also cause ADC instability, reduce the half-life, and increase systemic toxicities. MIRV and TV have DARs of 4 : 1 whereas T-Dxd has a DAR of 8 : 1.
Figure 1 describes the ADCs currently approved for gynecologic cancers.
FIGURE 1.
Overview of antibody–drug conjugates approved in gynecologic malignancies. Created with Biorender.com. ADC, antibody–drug conjugates; DAR, drug antibody ratio; MTi, microtubule inhibitor; Ti, topoisomerase inhibitor; FRα, folate receptor alpha; CLDN6, claudin-6; HER-2, human epidermal growth factor receptor 2; DM4, N20-deacetyl-N20-(4-mer-capto-4-methyl-1-oxopentyl)-maytansin; MMAE, monomethyl auristatin E.
ANTIBODY–DRUG CONJUGATES IN OVARIAN CANCER
Mirvetuximab soravtansine-gynx for platinum resistant ovarian cancer
MIRV represents a first-in-class ADC designed to target folate receptor alpha (FRα) with a cleavable linker, DAR of 4 : 1 and a potent microtubule inhibitor (maytansinoid (DM4)) as its payload. About 86% of epithelial ovarian cancers constitutively express FRα [9▪]. FRα has scarce expression on normal tissues, including the choroid plexus, thyroid, salivary glands, breast, colon, bladder and lung [10]. FRα expression has been associated with resistance to chemotherapy and more aggressive tumors [11].
In the phase III MIRASOL study, patients with PROC who had high FRα expression by immunohistochemistry (IHC) with membrane staining intensity of at least 2+ (PS2+) among ≥75% of viable tumor cells, were randomized to MIRV or investigator's choice chemotherapy. MIRASOL demonstrated that patients treated with MIRV compared to chemotherapy had an objective response rate (ORR) of 42.3% vs. 15.9%, median progression free survival (mPFS) of 5.6 months vs. 3.9 months (P < 0.001) and median overall survival (mOS) of 16.5 months vs. 12.8 months with a hazard ratio (HR) of 0.67 [95% confidence interval (CI) 0.50–0.89], respectively [12▪▪]. Compared to chemotherapy, MIRV had lower grade 3 (Gr 3) adverse events (AEs) (42% vs. 54%) and lower hematologic toxicity.
In the phase III FORWARD I trial, patients were eligible if at least 50% of their tumor cells had any FRα membrane staining by IHC regardless of intensity [13▪▪]. In this study, progression-free survival (PFS) did not reach statistical significance. The FORWARD I data was re-analyzed using the PS2+ scoring, the same as the MIRASOL study, and 34% of patients were found to have had low folate receptor expression (<50% of tumor cells with 2+/3+ staining intensity). When re-analyzing the results using the PS2+ scoring system, patients with FRα high had improved PFS with a HR of 0.55 (P = 0.015) [14▪,15]. The differences in these two studies highlight the importance of identifying the right cut-off to create a reliable and replicable biomarker that can predict response to MIRV as well as other ADCs.
MIRV is being investigated in the platinum-resistant setting in combination with chemotherapy, bevacizumab and pembrolizumab in the FORWARD II trial. Preliminary results of 94 patients with PROC with FRα expression of ≥ 2+ intensity in at least ≥ 25% of tumor cells who received MIRV with bevacizumab, demonstrated an ORR of 44%, mPFS of 8.2 months and duration of response (DOR) of 9.7 months [16]. More data are needed to better understand if the addition of bevacizumab to MIRV will lead to a meaningful improvement on clinical activity compared to MIRV alone. The GLORIOSA study is a Phase III study assessing MIRV and bevacizumab as maintenance therapy in platinum sensitive patients [17]. Only preliminary data has been reported on 14 patients treated with MIRV and pembrolizumab in PROC, which demonstrated an ORR of 43%, median DOR of 6.9 months and mPFS of 5.2 months, therefore further study is necessary and in progress [18].
Mirvetuximab soravtansine-gynx for platinum sensitive ovarian cancers
Although MIRV is currently Food and Drug Administration (FDA) approved in the platinum-resistant setting, its role in platinum sensitive recurrent disease is currently being explored in a number of clinical trials, including the FORWARD II, PICCOLO, GLORIOSA, and IMGN853-0420 trials. Preliminary results from the FORWARD II trial, which evaluated MIRV with carboplatin in 18 patients with low-high FRα expression, demonstrated an ORR of 71% and mPFS of 15 months. Patients with high FRα expressing PSOC (n = 16) who were treated with MIRV and bevacizumab achieved an ORR of 69% and mPFS of 13.3 months [19▪]. Additionally, the phase 1b study of MIRV, carboplatin and bevacizumab followed by MIRV and bevacizumab maintenance (n = 41), resulted in a PFS of 12 months, ORR of 81% and DOR of 10.7 months [20]. However, adverse events were significant including diarrhea 83% (Gr 3/4 10%), nausea 72% (2%), fatigue 76% (5%), thrombocytopenia 71% (51%), blurred vision 68% (0%), neutropenia 51% (39%), and hypertension 32% (10%). Although the ORR and PFS outcomes are encouraging, the observed toxicity profile suggests further data are needed to validate these findings and better understand the therapeutic potential of this combination regimen.
The PICCOLO study, a phase Ib/II study evaluating MIRV in patients with high FRα expressing (PS2+ staining intensity in ≥ 75% of tumor cells) PSOC with at least two prior lines of platinum-containing therapies, enrolled 79 patients and demonstrated an ORR of 51.9% with 6 complete responses and mDOR of 8.3 months [21▪]. This study supports use of MIRV after two lines of platinum therapies in the platinum sensitive setting, particularly in patients that progressed on a preceding maintenance therapy with a PARP inhibitor, where recent data suggest lower efficacy of another line of platinum-based chemotherapy [22].
Other antibody–drug conjugates targeting folate receptor alpha
Luveltamab tazevibulin (STRO-002) is an ADC targeting FRα with a microtubule payload (hemiasterlin) and DAR of 4 that is currently under clinical investigation. The preliminary results of the phase I trial, STRO-002-GM1, were presented at the American Society of Clinical Oncology (ASCO) 2023 [23]. Luveltamab was tested in patients with both PROC and PSOC and with a tumor proportion score (TPS) of FRα expression of > 25% (n = 35) at a dose of 4.3 mg/kg and 5.2 mg/kg. This study resulted in an ORR of 31.3% and 43.8%, mDOR of 13 months and 5.4 months, and mPFS of 6.1 months and 6.6 months, respectively. The incidence of Gr 3/4 AEs were higher in the 5.2 mg/kg dose, including GR 3/4 neutropenia (65% vs. 76%), and dose reductions were required in 95% of patients at 5.2 mg/kg dose.
Following this trial, the phase II/III trial REFRaME-01 was designed to randomize patients with PROC with TPS of FRα expression ≥25% to luveltamab at doses of 4.3 mg/kg or 5.2 mg/kg with prophylactic G-CSF for 2 cycles followed by 4.3 mg/kg or investigator's choice chemotherapy [24]. As of April 30, 2024 the planned 50 patients in the dose-optimization (part 1) were enrolled and part 2 has been initiated [25].
Farletuzumab ecteribulin (MORAb-202) is also an ADC targeting FRα with a microtubule inhibitor payload (eribulin mesylate) and a DAR of 4 that is under clinical investigation. The phase I dose escalation trial of MORab-202 in solid tumors included 12 patients with ovarian cancer and resulted in an ORR of 50% [26▪]. Subsequently the expansion portion of the phase I trial in PROC was conducted at doses of 0.9 mg/kg and 1.2 mg/kg and found an ORR of 25% and 52.4%, disease control rate (DCR) of 66.7% and 95.2%, and mPFS of 6.7 and 8.2 months, respectively. The most significant AE was interstitial lung disease (ILD), occurring in 37.5% of patients receiving a dose of 0.9 mg/kg, and 66.7% in patients receiving 1.2 mg/kg, with only one case of Gr 3 ILD [27]. With these results a phase II trial is currently recruiting for MORaB-202 vs. investigators choice chemotherapy at a dose of 0.68 mg/kg (25 mg/m2), which is slightly lower than previous trials due to the high incidence of ILD [28].
Other FRα targeted ADCs in phase I clinical development include BAT8006 which was evaluated in 52 patients with PROC and had an ORR of 41.7%, DCR of 86.1%, and in 12 patients that were FRα high had an ORR of 50% [29]. In this study, 57.7% of patients experienced Gr 3 toxicities. Rinatabart sesutecan (PRO1184) is being studied in advanced ovarian and endometrial cancers irrespective of FRα expression and was granted FDA fast track designation after preliminary results showed tolerable safety profile and promising initial responses, with updated results showing an ORR of 35% in part A, and 50% (6/12) in part B at the 120 mg/m2 dose [30▪,31]. The preliminary results for AZD5335, another FRα targeted ADC, were presented at European Society for Medical Oncology (ESMO) 2024 from the FONTANA study. This study did not select for FRα status, but reported that in high FRα expressers 5 out of 8 (62%) had objective responses and 4 out of 5 in the top 3 dose levels [32▪]. Other FRα targeting ADCs are also in phase I trials including TAK-85, IMGN151, and LY4170156, however preliminary results are still pending.
Other antibody–drug conjugates in clinical development for ovarian cancer
Raludotatug deruxtecan (R-DXd) is an ADC targeting cadherin-6 (CDH6) conjugated to a topoisomerase inhibitor. In the phase I trial, 42 patients with heavily pretreated PROC demonstrated an ORR of 38%, median DOR of 18.5 weeks (weeks) and Gr 3 treatment emergent adverse events (TEAEs) of 50% [33,34▪]. This drug is now recruiting in a phase II/III REJOICE-Ovairan01 trial. TORL-1-23 is an ADC targeting claudin-6 (CLDN6) conjugated to a microtubule inhibitor (MMAE). Initial results from the phase 1 trial demonstrated an ORR of 67% (4/6) at the 2.4 mg/kg dose and 50% (6/12) at the 3.0 mg/kg dose with 26 patients still on treatment at >100 weeks [35,36▪]. The most common Gr 3 AE was neutropenia (23%) [35,36▪].
Datopotamab deruxtecan a trophoblast cell surface antigen 2 (Trop-2) ADC was studied in TROPION-PanTumor03 in 35 patients with PROC and PSOC unselected for Trop-2 expression, and resulted in ORR of 42.9%, 1 complete response, and DCR of 91.4% [37]. Gr 3 treatment-related adverse events (TRAEs) were seen in 45.8% of patients and one Gr 3 ILD. A phase II trial of Sacituzumab tirumotecan, another trop-2 ADC, in 40 patients with predominantly PROC, demonstrated an ORR of 40%, DCR of 75% and mPFS of 6.0 months. In thus study, Trop-2 IHC H-score of >200 was compared to <200 and the ORR was 61.5% and 27.3%, respectively [38▪]. Gr 3 TRAEs were experienced among 67.5% of patients with OC, most commonly neutropenic and anemia. RC88 is an ADC targeting mesothelin (MSLN), and was tested in a phase I/II trial in 60 patients with heavily pretreated mostly PROC with 2+ or 3+ MSLN expression [39]. In 43 evaluable patients, the ORR was 37.2% and the FDA subsequently granted this drug fast track designation for PROC.
Other ADCs under Investigation for Ovarian Cancer are outlined in Table 1.
Table 1.
ADCs in clinical development for gynecologic malignancies
| ADC | Target | Payload and DAR | Company | Status | Key trials |
| Pan tumor | |||||
| Trastruzumab deruxtecan | HER2 | Deruxtecan (Ti) 8 |
AstraZeneca | FDA approved – HER2 3+ tumors | Phase II: DESTINY-PanTumor02 [40▪▪] |
| IBI354 | HER2 | Camptothecin derivative (Ti) 8 |
Innovent Biologics | Phase I/II ongoing | Phase I/II: NCT05636215 |
| Ovarian | |||||
| Mirvetuximab soravtansine | FRα | DM4 (MTi) 4 |
AbbVie/ ImmunoGen | FDA approved- PROC, ongoing trials for PSOC | Phase III: MIRASOL [12▪▪], FORWARD I [13▪▪] Ongoing: FORDWARD II, PICCOLO GLORIOSA, IMGN853-0420 |
| Farletuzumab ecteribulin (MORAb-202) | FRα | Ecteribulin (MTi) 4 |
Bristol-Myers Squibb | Phase II ongoing | Phase I: First in human (NCT04300556) [28], Expansion PROC [27] Phase II: NCT05613088 |
| Levultemab tazevibulin (STRO-002) | FRα | Hemasterlin (MTi) 4 |
Sutro Biopharma | FDA Fast track, Phase III trial recruiting | Phase I: STRO-002-GM1 [23] Phase II/III trial REFRaME-01 [24] |
| Rinatabart sesutecan (PRO1184) | FRα | Exatecan (Ti) 8 |
Profound Bio | FDA fast track, Phase I/II ongoing | Phase I/II: NCT05579366 |
| BAT8006 | FRα | NR (Ti) 8 |
Bio-Thera Solutions | Phase I ongoing China Only | Phase I: NCT05378737 |
| IMGN151 | FRα | DM21 (MTi) 3.5 |
Immunogen | Phase I ongoing | Phase I: NCT05527184 |
| AZD5335 | FRα | TOP1i (Ti) 8 |
AstraZeneca | Phase I/II ongoing | Phase I/II: NCT05797168 |
| LY4170156 | FRα | Exatecan (Ti) 8 |
Eli Lilly and Company | Phase I ongoing | Phase I: NCT06400472 |
| DB-1305 | Trop-2 | P1021 (Ti) 4 |
DualityBio Inc. | FDA Fast Track | Phase I/II: NCT05438329 |
| Sacituzimab govitecan | Trop-2 | SN-38 (Ti) 7 |
Gilead Sciences | Phase II ongoing | Phase II: NCT04251416 |
| Datopotomab deruxtecan | Trop-2 | Deruxtecan (Ti) 4 |
AztraZeneca | Phase II ongoing | Phase II: NCT05489211 (TROPION-PanTumor03) |
| Sacituzumab tirumotecan (MK-2870) |
Trop-2 | Belotecan-derivative (Ti) 7.4 |
Merck Sharp & Dohme | Phase I/II ongoing | Phase I/II: NCT04152499 |
| TORL-1-23 | CLDN6 | MMAE (MTi) 4 |
TORL Biotherapeutics | Phase I ongoing | Phase I: NCT05103683 |
| Raludotatug deruxtecan | CDH6 | Deruxtecan (Ti) 8 |
Daiichi Sankyo | Phase II/III ongoing | Phase I: NCT04707248 Phase II/III: NCT06161025 |
| CUSP06 | CDH6 | Exatecan (Ti) 8 |
OnCusp Therapeutics | Phase I ongoing | Phase I: NCT06234423 |
| LY4101174 | Nectin-4 | Exatecan (Ti) 8 |
Eli Lilly and Company | Phase I ongoing | Phase I: NCT06400472 |
| TUB-040 | NaPi2b | Exatecan (Ti) 8 |
Tubulis GmbH | FDA fast track Phase I/II ongoing |
Phase I/II: NCT06303505 (NAPISTAR 1-01) |
| HS-20089 | B7-H4 | (Ti) 6 |
Hansoh BioMedical R&D | Phase II ongoing China only | Phase I: NCT05194072 Phase II: NCT06014190 |
| AZD8205 | B7-H4 | TOP1i (Ti) 8 |
AztraZeneca | Phase I/II ongoing | Phase I/II: NCT05123482 |
| RC88 | MSLN | MMAE (Ti) 4 |
RemeGen Co. | FDA fast track designation for PROC | Phase I/II: NCT04175847 Phase II: NCT06173037 |
| Endometrial | |||||
| Trastuzumab duocarmazine (SYD985) | HER2 | Duocarmycin (AA) 3 |
Byondis B.V. | Phase II ongoing | Phase II: NCT04205630 |
| DB-1303 | HER2 | P1003 (Ti) 8 |
DualityBio | FDA breakthrough designation | Phase I/II: NCT05150691 |
| IMGN151 | FRα | DM21 (MTi) 3.5 |
Immunogen | Phase I ongoing | Phase I: NCT05527184 |
| LY4170156 | FRα | Exatecan (Ti) 8 |
Eli Lilly and Company | Phase I ongoing | Phase I: NCT06400472 |
| Sacituzimab govitecan | Trop-2 | SN-38 (Ti) 7 |
Gilead Sciences | Phase II ongoing | Phase II: NCT04251416 |
| Sacituzumab tirumotecan (MK-2870) |
Trop-2 | Belotecan-derivative (Ti) 7.4 |
Merck Sharp & Dohme | Phase III recruiting | Phase III: NCT06132958 |
| Datopotomab deruxtecan | Trop-2 | Deruxtecan (Ti) 4 |
AztraZeneca | Phase II ongoing | Phase II: NCT05489211 (TROPION-PanTumor03) |
| AZD8205 | B7-H4 | TOP1i (Ti) 8 |
Aztrazeneca | Phase I/II ongoing | Phase I/II: NCT05123482 |
| HS-20089 | B7-H4 | (Ti) 6 |
Hansoh BioMedical R&D | Phase II ongoing China only | Phase I: NCT05194072 Phase II: NCT06014190 |
| SGN-B7H4V | B7-H4 | MMAE (MTi) 4 |
Seagen Inc. | Phase I ongoing | Phase I: NCT05194072 |
| LY4101174 | Nectin-4 | Exatecan (Ti) 8 |
Eli Lilly and Company | Phase I ongoing | Phase I: NCT06400472 |
| Cervical | |||||
| Tisotumab vedotin | TF | MMAE (MTi) 4 |
Seagen Inc. | FDA approved, 2L r/m CC | Phase II: InnovaTV 204 trial [48▪] Phase III: InnovaTV301 [49▪▪,50▪] Ongoing trials: phase 1b/II: innovaTV 205 [51] |
| LY4101174 | Nectin-4 | Exatecan (Ti) | Eli Lilly and Company | Phase I ongoing | Phase I: NCT06400472 |
| Sacituzimab govitecan | Trop-2 | SN-38 (Ti) 7 |
Gilead Sciences | Phase II ongoing | Phase II: NCT05838521 |
| Sacituzumab tirumotecan (MK-2870) |
Trop-2 | Belotecan-derivative (Ti) 7.4 |
Merck Sharp & Dohme | Phase III recruiting | Phase II: NCT05642780 Phase III: NCT06459180 |
| RC88 | MSLN | MMAE (Ti) 4 |
RemeGen Co. | Phase I/II ongoing | Phase I/II: NCT04175847 |
AA, alkylating agent; ADC, antibody–drug conjugate; CDH6, cadherin-6; CLDN6, claudin-6; DAR, drug-antibody ratio; DM4, N20-deacetyl-N20-(4-mer-capto-4-methyl-1-oxopentyl)-maytansin; FDA, Food and Drug Administration; FRα, folate receptor alpha; MMAE, monomethyl auristatin E; MSLN, mesothelin; MTi, microtubule inhibitor; NR, not reported; PROC, platinum-resistant ovarian cancer; PSOC, platinum-sensitive ovarian cancer; TF, tissue factor; Ti, topoisomerase inhibitor; Trop-2, trophoblast cell surface antigen-2.
PanTumor ANTIBODY–DRUG CONJUGATES
Trastuzumab deruxtecan (T-DXd) is a HER-2 targeting ADC with a topoisomerase I inhibitor payload. The FDA has approved T-Dxd for the treatment of any HER2 high (IHC 3+) tumors while the National Comprehensive Cancer Network (NCCN) guidelines allow for IHC 2+ or 3+. These approvals were based on the DESTINY-PanTumor02 trial which included patients with HER2 IHC 2+/3+ cervical, endometrial and ovarian cancers, averaging about 40 patients in each cohort [40▪▪]. For endometrial, cervical and ovarian the ORR in IHC 3+ was 84.6%, 75.0%, 63.6% and in IHC 2+ 47.1%, 40.0% and 36.8%, respectively. In terms of safety, in the gynecologic cohort nausea was the most common AE occurring in 68.4% of patients, similar to highly emetogenic chemotherapy agents. Gr 3 AEs occurred in 45% of patients, the most common was neutropenia (10.9%) and pneumonitis (10.8%), with one ultimately leading to death.
A different HER-2 targeted ADC, IBI354, is also being tested across HER2 IHC 1+, 2+ or 3+ OC, CC, and EC. Phase I results of 89 patients with OC, 26 CC, and 14 EC resulted in an ORR of 39.5%, DCR of 83.1% and Gr 3 TRAEs occurred in 16% of patients [41].
Antibody–drug conjugates in clinical development for endometrial cancer
DB-1303, a HER-2 targeting ADC with a topoisomerase payload, was granted breakthrough designation after Phase I data demonstrate an ORR of 58.8% and DCR of 94.1% in 17 evaluable patients in patients with both HER2-positive and HER-2 low endometrial cancer [42]. A phase I trial of Trastuzumab duocarmazine (SYD985), another HER-2 targeted ADC, enrolled 61 patients with HER-2 expressing (1+/2+/3+) endometrial cancer and demonstrated an ORR of 32.8%, mPFS of 5.6 months, and 18% of patients had serious AEs [43].
Sacituzumab govitecan (SG), an ADC targeting Trop-2, was initially studied in a Phase I/II IMMU-132-01 basket trial, which included 18 patients with endometrial cancer, and resulted in an ORR of 22.2% and CBR of 44.4% [44]. A phase II trial of SG for patients with recurrent endometrial carcinoma with Trop-2 overexpression (at least 2+ staining) revealed an ORR of 35% and DCR of 85% and will now expand to stage 2 of this trial [45].
Datopotamab deruxtecan another Trop-2 ADC was studied in TROPION-PanTumor03 trial which included 40 patients with EC who demonstrated an ORR of 27.5%, with 1 complete response and PFS of 6.3 months [37]. Gr 3 or higher TRAE were present in 42.5% of patients and only one patients with Gr 3 ILD. A phase II trial of Sacituzumab tirumotecan, a separate trop-2 ADC, included 40 patients with advanced EC, revealed an ORR of 34.1%, DCR of 75% and mPFS of 5.7 months. The patients were stratified by Trop-2 IHC H-score >200 compared to <200 and the ORR was 41.7% and 35.7%, respectively [38▪]. Gr 3 TRAEs were experienced by 72% of EC patients, most commonly neutropenic and anemia. The phase III trial is ongoing.
ANTIBODY–DRUG CONJUGATES IN CERVICAL CANCER
Tisotumab vedotin
Tisotumab vedotin (TV) is an ADC that targets tissue factor (TF) with a cleavable linker to a microtubule-disrupting agent, monomethyl auristatin E (MMAE). TF is highly expressed in up to 95% of cervical cancers [46,47]. TF plays a known role in hemostasis and thrombosis as well as enhancing oncogenesis and hematogenous metastases [47]. TV is approved for recurrent or metastatic cervical cancer (r/mCC) after first line chemotherapy. It was granted accelerated approval after promising phase II results in the innovaTV 204 trial. In this trial, 101 patients treated with TV demonstrated an ORR of 24%, DOR of 8.3 months and only 28% had Gr 3 or higher AEs [48▪]. It was granted full approval after the phase III trial, innovaTV301, where TV vs. investigators choice chemotherapy showed a significant improvement in overall survival of 11.5 months compared to 9.5 months (HR 0.70, 95% CI: 0.54–0.89) [49▪▪]. In general, TV had lower Gr 3 AEs than traditional chemotherapy (29.2% vs. 45.2%) [50▪]. However, TV had higher rates of ocular toxicities (50.4%), neuropathy (35.6%), and bleeding events (27.6%), most commonly epistaxis. Keratitis occurred in 15.6% of patients. Ocular toxicities of TV require special mitigation strategies outlined in the toxicity section below.
TV has been studied in combination with either bevacizumab, pembrolizumab or carboplatin in a phase 1b/II innovaTV 205 for r/mCC. In the first line (1L) setting, TV + carboplatin (n = 33) and TV + pembrolizumab (n = 32), demonstrated an ORR of 54.5% and 40.6%, DOR of 8.6 months and not reached, and a PFS of 6.9 months and 5.3 months, respectively [51]. In the first-line setting, although the combination of TV and carboplatin showed a higher response rate, patients who respond to TV and pembrolizumab tend to experience longer durations of response. Additional follow-up is required to confirm these outcomes. In second- or third-line setting, TV+ pembrolizumab resulted in an ORR of 35.3%, DOR of 14.1 months, PFS of 5.6 months and a mOS of 15.3 months [51]. The Gr 3 AE rate of TV + carboplatin and TV + pembrolizumab were, 78.8% and 66.7%, the most common of which being cytopenias, diarrhea, and keratitis (6.1%). Phase III trials are needed to understand the role of these combinations upfront vs. TV as a second- or third-line agent.
Antibody–drug conjugates in development for cervical cancer
A phase II trial is evaluating Disitamab vedotin, a HER2 targeted ADC, for patients with r/mCC with HER2+ expression of 1+ or greater who failed at least one line of platinum therapy. Preliminary results of 22 patients demonstrated an ORR of 31.8%, mDOR of 5.5 months, DCR of 86.4 months and mPFS of 4.4 months [52]. A phase II trial of Sacituzumab tirumotecan, a Trop-2 ADC, in combination with pembrolizumab in 38 patients with r/mCC revealed an ORR of 57.9% with 3 complete responses, and 6-month DOR was 82% [53▪]. In this study 47% of patients experienced grade 3 or high TRAEs, most commonly neutropenia and anemia. Ongoing trials are evaluating Sacituzumab govetecan, another Trop-2 ADC, for patients with CC. RC88 is an ADC targeting mesothelin (MSLN), and was tested in a phase I/II trial in 17 patients with r/mCC and had an ORR of 35.3% [39].
TOXICITY PROFILE OF ANTIBODY–DRUG CONJUGATES
Although each ADC has unique AEs, studies have shown that the side effects are mostly due to the linker/payload, with a smaller effect due to target expression on healthy tissue. ADCs with similar linker/payloads have similar toxicities due to poor linker stability and direct toxic effects of the cytotoxic payload [54]. In a review of 169 ADC clinical trials, 91.2% of patients had TRAEs, the most common including lymphopenia (53%), nausea (44%), neutropenia (43.7%), blurred vision (40%), and peripheral neuropathy (9.6%) [55▪▪]. The most common grade 3 or higher AEs were neutropenia, hypoesthesia, thrombocytopenia and lymphopenia. The management of AEs are similar to traditional chemotherapy and include premedications for nausea, growth factor supplementation for neutropenia, dose reductions, and close monitoring of blood counts with transfusions as needed.
Ocular toxicities of antibody–drug conjugates
Ocular toxicities occur with both TV and MIRV [56]. Not only can the microtubule inhibitor payload cause off target ocular effects, but TF is expressed in the retina and FRα in the choroid plexus. In the phase II trial, innovaTV204, 53% of patients treated with TV had ocular toxicities, mostly low grade including conjunctivitis, dry eyes and keratitis, and 2% had Gr 3 ulcerative keratitis [48▪]. In the FORWARD I and SORAYA trials, 40% of patients treated with MIRV developed blurry vision most commonly during cycle 2 and 2.5–5.7% developed Gr 3 or higher blurred vision [13▪▪,57]. For both MIRV and TV, ∼20% of patients required dose reductions due to ocular toxicities.
Mitigation strategies can improve the rates of ocular toxicities and have been well described [58▪]. This includes preinfusion ophthalmic exam and use of corticosteroid and vasoconstrictor eye drops [59▪]. Cold packs to keep the eye area cold can also be used for TV only. Lubricating eye drops should be used throughout treatment and contact lens should be avoided.
Interstitial lung disease from antibody–drug conjugates
Interstitial lung disease (ILD) is an umbrella term used for a range of sequelae including pneumonitis that can progress to fibrosis. In DESTINY-PanTumor02, pneumonitis occurred in 10.8% of patients on T-Dxd with gynecologic cancers, mostly Gr 1–2 (10.0%) and one fatal event (0.8%) [40▪▪]. In the pooled safety analysis for T-DXd across multiple tumor types, the median time to first onset of ILD was 5.4 months (range <0.1–46.8 months) and the overall incidence was around 15%, (Gr 1–2 11.9%, Gr 3–4 1.3%, Gr 5 2.2%) [60]. In the FORWARD I study, MIRV was associated with noninfectious pneumonitis in only 2.9% of patients (Gr 1–3) [13▪▪].
In order to mitigate pneumonitis, Tarantino et. Al published the Five “S” Rules to detect and manage T-DXd related ILD [61▪▪]. First, Screen with history and physical using high resolution CT scan as a baseline and pulse oximetry. Secondly, Scans should be done at least every 12 weeks. Thirdly, Synergy, meaning working together with the patient to understand when to seek medical attention and working with a multidisciplinary team including pulmonologists and radiologists to detect early signs. Fourth, Suspend treatment as soon as ILD is suspected even Gr 1 and permanently if Gr 2 or worse. Lastly, Steroids can be initiated as soon as ILD is suspected (0.5 mg/kg/day of prednisone if Gr 1), and at higher doses if symptomatic Gr 2 or worse (1 mg/kg/day if Gr 2 or higher).
CURRENT GAPS IN KNOWLEDGE
Despite significant progress in the development of novel ADCs for gynecologic cancers, many critical questions remain unanswered, requiring further research to optimize their use. One key area is the sequencing of ADCs, particularly as the expression of target antigens may shift in response to prior therapies, and ADCs with similar payloads may confer cross-resistance. Additionally, the identification of reliable biomarkers to predict treatment efficacy or resistance is essential. Another challenge is the dynamic nature of ADC target antigen expression. Particularly, we need to better understand the impact of tissue processing via formalin fixation on antigen detection levels, need to confirm the consistency of expression between tissue obtained at diagnosis and at the time of recurrence, and study whether intra-tumoral or intra-patient heterogeneity exists.
CONCLUSION
ADCs are an exciting new class of cancer therapeutics that are rapidly expanding and changing the treatment landscape of gynecologic malignancies. This review summarizes the most up to date information on the current ADCs in clinical practice and those in development for the treatment of gynecologic cancers. As ADCs expand, it is important to fully understand not only the efficacy and impact on outcomes, but also their unique side effect profiles, ways to mitigate them and the impact on a patient's quality of life.
Acknowledgements
We extend our gratitude to the patients who have participated, and continue to participate, in these clinical trials, as well as to their families. Their contributions are invaluable in advancing our ability to better treat gynecologic malignancies.
Ethics approval and consent to participate: No consent was needed for this review article.
Consent for publication: Yes.
Availability of data and material: N/A.
Authors’ contributions: J.S., B.K., G.K., N.H. – manuscript preparation and analysis.
Financial support and sponsorship
This research was funded by the National Institute of Health T32 fellowship titled Patient-Centered Outcomes Research Training in Urologic and Gynecologic Cancers (PCORT UroGynCan) (T32CA251072).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Karpel HC, Powell SS, Pothuri B. Antibody–drug conjugates in gynecologic cancer. American Society of Clinical Oncology Educational Book. 2023 May 25 [cited 2024 Jun 5]. Available at: https://ascopubs.org/doi/10.1200/EDBK_390772. [DOI] [PubMed] [Google Scholar]; A previous review article highlighting the ADCs that were in development in 2023 in comparison to the current review underscoring significant progress in ADC development over the last two years.
- 2.Center for Drug Evaluation and Research and. FDA grants accelerated approval to mirvetuximab soravtansine-gynx for FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or peritoneal cancer. FDA. 2022 Nov 14 [cited 2024 Apr 2]. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mirvetuximab-soravtansine-gynx-fra-positive-platinum-resistant. [Google Scholar]
- 3.Center for Drug Evaluation and Research and. FDA D.I.S.C.O. Burst Edition: FDA approval of Enhertu (fam-trastuzumab deruxtecan-nxki) for unresectable or metastatic HER2-positive solid tumors. FDA [Internet]. 2024 Apr 26 [cited 2024 Jun 5]. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-enhertu-fam-trastuzumab-deruxtecan-nxki-unresectable-or. [Google Scholar]
- 4.Center for Drug Evaluation and Research and. FDA approves tisotumab vedotin-tftv for recurrent or metastatic cervical cancer. FDA [Internet]. 2024 Apr 29 [cited 2024 Jun 5]. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisotumab-vedotin-tftv-recurrent-or-metastatic-cervical-cancer. [Google Scholar]
- 5.Esapa B, Jiang J, Cheung A, et al. Target antigen attributes and their contributions to clinically approved antibody–drug conjugates (ADCs) in haematopoietic and solid cancers. Cancers (Basel) 2023; 15:1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathur R, Weiner GJ. Picking the optimal target for antibody–drug conjugates. Am Soc Clin Oncol Educ Book 2013; (33):e103–e107. [DOI] [PubMed] [Google Scholar]
- 7.Sheyi R, de la Torre BG, Albericio F. Linkers: an assurance for controlled delivery of antibody–drug conjugate. Pharmaceutics 2022; 14:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baah S, Laws M, Rahman KM. Antibody–drug conjugates—a tutorial review. Molecules 2021; 26:2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪.Scaranti M, Cojocaru E, Banerjee S, Banerji U. Exploiting the folate receptor ( in oncology. Nat Rev Clin Oncol 2020; 17:349–359. [DOI] [PubMed] [Google Scholar]; Comprehensive overview of folate receptor alpha as an ADC target antigen.
- 10.Gonzalez-Ochoa E, Veneziani AC, Oza AM. Mirvetuximab soravtansine in platinum-resistant ovarian cancer. Clin Med Insights Oncol 2023; 17:11795549231187264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogani G, Coleman RL, Vergote I, et al. Mirvetuximab soravtansine-gynx: first antibody/antigen–drug conjugate (ADC) in advanced or recurrent ovarian cancer. Int J Gynecol Cancer 2024; 34:469–477. [DOI] [PubMed] [Google Scholar]
- 12▪▪.Moore Kathleen N, Angelergues Antoine, Konecny Gottfried E, et al. Mirvetuximab soravtansine in FRα-positive, platinum-resistant ovarian cancer. N Engl J Med 2023; 389:2162–2174. [DOI] [PubMed] [Google Scholar]; Phase III MIRASOL study which showed an overall survival and progression free survival benefit in patients that had high FRα expression.
- 13▪▪.Moore KN, Oza AM, Colombo N, et al. Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: primary analysis of FORWARD I. Ann Oncol 2021; 32:757–765. [DOI] [PubMed] [Google Scholar]; Phase III FORWARD-I trial which did not meet its primary PFS endpoint since this included patients that had at least 50% of tumor cells had any FRα membrane staining.
- 14▪.Moore KN, Oza AM, Colombo N, et al. FORWARD I (GOG 3011): a phase III study of mirvetuximab soravtansine, a folate receptor alpha (FRa)-targeting antibody–drug conjugate, versus chemotherapy in patients with platinum-resistant ovarian cancer. [Google Scholar]; Presentation that highlights the Folate receptor alpha scoring differences between FORWARD I and MIRASOL highlighting the inclusion of low folate receptor expressors in the FORWARD I trial.
- 15.Targeted Oncology. 2019 [cited 2024 Sep 12]. Expert discusses the safety and efficacy of mirvetuximab soravtansine in ovarian cancer following FORWARD I findings. Available at: https://www.targetedonc.com/view/expert-discusses-the-safety-and-efficacy-of-mirvetuximab-soravtansine-in-ovarian-cancer-following-forward-i-findings. [Google Scholar]
- 16.Gilbert L, Oaknin A, Matulonis UA, et al. Safety and efficacy of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody–drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol Oncol 2023; 170:241–247. [DOI] [PubMed] [Google Scholar]
- 17.O’Malley DM, Myers TKN, Zamagni C, et al. GLORIOSA: a randomized, open-label, phase 3 study of mirvetuximab soravtansine with bevacizumab vs. bevacizumab as maintenance in platinum-sensitive ovarian, fallopian tube, or primary peritoneal cancer. JCO 2023; 41: (Suppl): TPS5622–TPS5622. [Google Scholar]
- 18.Matulonis UA, Moore KN, Martin LP, et al. Mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody–drug conjugate (ADC), with pembrolizumab in platinum-resistant ovarian cancer (PROC): initial results of an expansion cohort from FORWARD II, a phase Ib study. Ann Oncol 2018; 29:viii339. [Google Scholar]
- 19▪.O’Malley DM, Oaknin A, Matulonis UA, et al. Mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody–drug conjugate (ADC), in combination with bevacizumab in patients (pts) with platinum-agnostic ovarian cancer: final analysis. JCO 2021; 39: (Suppl): 5504–15504. [Google Scholar]; Preliminary results of the FORWARD II trial of MIRV in PSOC.
- 20.Richardson DL, Moore KN, Vergote I, et al. Phase 1b study of mirvetuximab soravtansine, a folate receptor alpha (FRα)–targeting antibody–drug conjugate, in combination with carboplatin and bevacizumab in patients with platinum-sensitive ovarian cancer. Gynecol Oncol 2024; 185:186–193. [DOI] [PubMed] [Google Scholar]
- 21▪.Secord AA, Corr BR, Lewin S, Diver E. Mirvetuximab soravtansine (MIRV) in recurrent platinum-sensitive ovarian cancer (PSOC) with high folate receptor-alpha (FRα) expression: Results from the PICCOLO trial. Presented at: 2024 ESMO Congress; September 13–17, 2024; Barcelona, Spain. Abstract 718MO. [Google Scholar]; Preliminary results of the PICCOLO trial of MIRV in PSOC.
- 22.MacAulay Vacheresse G, Sabri E, Domingo S, Le T. Response to subsequent platinum-based chemotherapy post PARP inhibitor in recurrent epithelial ovarian cancer. JCO 2023; 41: (Suppl): 5578–15578. [Google Scholar]
- 23.Oaknin A, Fariñas-Madrid L, García-Duran C, et al. Luveltamab tazevibulin (STRO-002), an antifolate receptor alpha (FolRα) antibody drug conjugate (ADC), safety and efficacy in a broad distribution of FolRα expression in patients with recurrent epithelial ovarian cancer (OC): Update of STRO-002-GM1 phase 1 dose expansion cohort. JCO 2023; 41: (Suppl): 5508–15508. [Google Scholar]
- 24.Oaknin A, Lee JY, Cibula D, et al. Efficacy and safety of luveltamab tazevibulin vs investigator's choice of chemotherapy in patients with recurrent platinum-resistant ovarian cancer (PROC) expressing folate receptor alpha (FRα): The REFRaME-01 (GOG-3086, ENGOT-79ov, and APGOT-OV9) phase 2/3 study. JCO 2024; 42: (Suppl): TS5637–TS5637. [Google Scholar]
- 25.Sutro Biopharma announces initiation of randomized portion (Part 2) of REFRαME-O1 Trial. Sutro Biopharma, Inc. [cited 2024 Jun 19]. Available at: https://www.sutrobio.com/sutro-biopharma-announces-initiation-of-randomized-portion-part-2-of-refr%ce%b1me-o1-trial/. [Google Scholar]
- 26▪.Shimizu T, Fujiwara Y, Yonemori K, et al. First-in-human Phase 1 study of MORAb-202, an antibody–drug conjugate comprising farletuzumab linked to eribulin mesylate, in patients with folate receptor-α-positive advanced solid tumors. Clin Cancer Res 2021; 27:3905–3915. [DOI] [PubMed] [Google Scholar]; Phase I results of Farletuzumab ecteribulin.
- 27.Nishio S, Yunokawa M, Matsumoto K, et al. Safety and efficacy of MORAb-202 in patients (pts) with platinum-resistant ovarian cancer (PROC): results from the expansion part of a phase 1 trial. JCO 2022; 40: (Suppl): 5513–15513. [Google Scholar]
- 28.Bristol-Myers Squibb. A Phase 2 open-label randomized study of farletuzumab ecteribulin (MORAb-202), a folate receptor alpha-targeting antibody–drug conjugate, versus investigator's choice chemotherapy in women with platinum-resistant high-grade serous (HGS) ovarian, primary peritoneal, or fallopian tube cancer. clinicaltrials.gov; 2024 May [cited 2023 Dec 31]. Report No.: NCT05613088. Available at: https://clinicaltrials.gov/study/NCT05613088. [Google Scholar]
- 29.Jia H, Zhang S, Sun Y, et al. Phase 1 study of BAT8006, a folate receptor α antibody drug conjugate with strong bystander effect, in subjects with advanced solid tumors. JCO 2024; 42: (Suppl): 5550–15550. [Google Scholar]
- 30▪.Call JA, Orr DW, Anderson IC, et al. Phase 1/2 study of PRO1184, a novel folate receptor alpha-directed antibody–drug conjugate, in patients with locally advanced and/or metastatic solid tumors. JCO 2023; 41: (Suppl): TS3157–TS3157. [Google Scholar]; Phase I/II results of Rinatabart sesutecan.
- 31.Lee EK, Yeku O, Winer I, Hamilton EP. A phase I/II study of rinatabart sesutecan (Rina-S) in patients with advanced ovarian or endometrial cancer. Presented at: 2024 ESMO Congress; September 13–17, 2024; Barcelona, Spain Abstract 719MO. [Google Scholar]
- 32▪.Shapira-Frommer R, Sudo K, Mileshkin L, et al. Initial results from a first-in-human study of AZD5335, a folate receptor α (FRα)-targeted antibody–drug conjugate, in patients (pts) with platinum-resistant recurrent ovarian cancer (PRROC). Presented at: 2024 ESMO Congress; September 13–17, 2024; Barcelona, Spain. Abstract 754P. [Google Scholar]; Updated results of ADC5335 presented at ESMO.
- 33.Moore KN, Philipovskiy A, Harano K, et al. 745MO Raludotatug deruxtecan (R-DXd; DS-6000) monotherapy in patients with previously treated ovarian cancer (OVC): Subgroup analysis of a first-in-human phase I study. Ann Oncol 2023; 34:S510. [Google Scholar]
- 34▪.Moore Kathleen N, Philipovskiy A, Harano K, Rini BI. Raludotatug deruxtecan (R-DXd; DS-6000) monotherapy in patients with previously treated ovarian cancer (OVC): subgroup analysis of a first-in-human phase I study. Presented at: 2024 ESMO Congress; September 13–17, 2024; Barcelona, Spain. Abstract 745MO. [Google Scholar]; Updated results of Raludotatug deruxtecan presented at ESMO.
- 35.Konecny GE, Wahner Hendrickson AE, Winterhoff B, et al. Initial results of dose finding in a first-in-human phase 1 study of a novel Claudin 6 (CLDN6) targeted antibody drug conjugate (ADC) TORL-1-23 in patients with advanced solid tumors. JCO 2023; 41: (Suppl): 3082–13082. [Google Scholar]
- 36▪.Konecny GE, Hendrickson AW, Winterhoff B, et al. Phase I, two-part, multicenter first-in-human (FIH) study of TORL-1-23, a novel Claudin 6 (CLDN6) targeting antibody drug conjugate (ADC) in patients with advanced solid tumors. Presented at: 2024 ESMO Congress. 2024 Sep 13. [Google Scholar]; Updated results of TORL-1-23 presented at ESMO 2024.
- 37.Oaknin A, Ang JE, Rha SY, et al. Datopotamab deruxtecan (Dato-DXd) in patients with endometrial (EC) or ovarian cancer (OC): Results from the phase II TROPION-PanTumor03 study. Presented at: 2024 ESMO Congress; September 13–17, 2024; Barcelona, Spain Abstract 714MO. [Google Scholar]
- 38▪.Wang D, Wang K, An R, Yu G. 715MO – safety and efficacy of sacituzumab tirumotecan (sac-TMT) in patients (pts) with previously treated advanced endometrial carcinoma (EC) and ovarian cancer (OC) from a phase II study. Presented at: 2024 ESMO Congress; September 13–17, 2024; Barcelona, Spain Abstract 715M0. [Google Scholar]; Updated results of Sacituzumab tirumotecan presented at ESMO 2024.
- 39.Liu Y, Li G, Yang R, et al. The efficacy and safety of RC88 in patients with ovarian cancer, nonsquamous-nonsmall-cell lung-carcinoma and cervical cancer: Results from a first-in-human phase 1/2 study. JCO 2024; 42: (Suppl): 5551–15551. [Google Scholar]
- 40▪▪.Meric-Bernstam F, Makker V, Oaknin A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 Phase II Trial. J Clin Oncol 2024; 42:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]; Phase II trial DESTINY-PanTumor02 that led to the pan-cancer approval of trastuzumab deruxtecan in HER2 2+/3+ tumors particularly showing promising results in ovarian and endometrial cancer.
- 41.Shu J, Zhu T, Huang Y, Xu Q. IBI354 (anti-HER2 antibody–drug conjugate [ADC]) in patients (pts) with advanced gynecological cancers (Gynecol C): results from a phase I study. Presented at: 2024 ESMO Congress; September 13–17, 2024; Barcelona, Spain Abstract 720M0. [Google Scholar]
- 42.Moore K, Makker V, Yeku O, et al. #430 DB-1303, a HER2-targeting ADC, for patients with advanced/metastatic endometrial cancer: preliminary clinical results from an ongoing phase 1/2a trial (NCT05150691). Int J Gynecol Cancer. 2023;33(Suppl 3). Available at: https://ijgc.bmj.com/content/33/Suppl_3/A9. [Google Scholar]
- 43.Study results | SYD985 in patients with HER2-expressing recurrent, advanced or metastatic endometrial carcinoma. ClinicalTrials.gov. [cited 2024 Jun 25]. Available at: https://clinicaltrials.gov/study/NCT04205630?cond=endometrial&term=antibody%20drug%20conjugate&tab=results&rank=7. [Google Scholar]
- 44.Bardia A, Messersmith WA, Kio EA, et al. Sacituzumab govitecan, a Trop-2-directed antibody–drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol 2021; 32:746–756. [DOI] [PubMed] [Google Scholar]
- 45.Santin A, McNamara B, Siegel ER, et al. Preliminary results of a phase II trial with sacituzumab govitecan-hziy in patients with recurrent endometrial carcinoma overexpressing Trop-2. JCO 2023; 41: (Suppl): 5599–15599. [Google Scholar]
- 46.Cocco E, Varughese J, Buza N, et al. Expression of tissue factor in adenocarcinoma and squamous cell carcinoma of the uterine cervix: implications for immunotherapy with hI-con1, a factor VII-IgGFc chimeric protein targeting tissue factor. BMC Cancer 2011; 11:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao X, Cheng C, Gou J, et al. Expression of tissue factor in human cervical carcinoma tissue. Exp Ther Med 2018; 16:4075–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48▪.Coleman RL, Lorusso D, Gennigens C, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2021; 22:609–619. [DOI] [PubMed] [Google Scholar]; Phase II trial that led to accelerated approval of TV for r/mCC.
- 49▪▪.Vergote IB, Martin AG, Fujiwara K, et al. LBA9 innovaTV 301/ENGOT-cx12/GOG-3057: A global, randomized, open-label, phase III study of tisotumab vedotin vs investigator's choice of chemotherapy in 2L or 3L recurrent or metastatic cervical cancer. Ann Oncol 2023; 34:S1276–S1277. [Google Scholar]; Phase III trial that led to the full approval of tisotumab vedotin for recurrent or metastatic cervical cancer after first line chemotherapy.
- 50▪.Manso L, Vergote I, Fujiwara K, et al. Tisotumab vedotin in 2L/3L recurrent or metastatic cervical cancer: Subsequent therapy data from ENGOT-cx12/GOG-3057/innovaTV 301. JCO 2024; 42: (Suppl): 5531–15531. [Google Scholar]; Follow up safety and efficacy data from the phase III trial.
- 51.Vergote I, Van Nieuwenhuysen E, O’Cearbhaill RE, et al. Tisotumab vedotin in combination with carboplatin, pembrolizumab, or bevacizumab in recurrent or metastatic cervical cancer: results from the innovaTV 205/GOG-3024/ENGOT-cx8 Study. JCO 2023; 41:5536–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan G, Li G, Li Q, et al. Evaluation of the effectiveness and safety of disitamab vedotin in HER2-expressing 2L recurrent or metastatic cervical cancer (r/mCC): Interim results of RC48-C018. JCO 2024; 42: (Suppl): 5528–15528. [Google Scholar]
- 53▪.Wang J, An R, Huang Y, Zhang J. Efficacy and safety of sacituzumab tirumotecan (sac-TMT) plus pembrolizumab in patients with recurrent or metastatic cervical cancer. Presented at: 2024 ESMO Congress; September 13–17, 2024; Barcelona, Spain Abstract 716MO. [Google Scholar]; Preliminary results of Sacituzumab tirumotecan presented at ESMO 2024.
- 54.Nguyen TD, Bordeau BM, Balthasar JP. Mechanisms of ADC toxicity and strategies to increase ADC tolerability. Cancers (Basel) 2023; 15:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55▪▪.Zhu Y, Liu K, Wang K, Zhu H. Treatment-related adverse events of antibody–drug conjugates in clinical trials: a systematic review and meta-analysis. Cancer 2023; 129:283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]; Large meta-analysis outlining the treatment-related adverse effects reported in clinical trials for ADCs.
- 56.Richardson DL. Ocular toxicity and mitigation strategies for antibody drug conjugates in gynecologic oncology. Gynecol Oncol Rep 2023; 46:101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA Study. JCO 2023; 41:2436–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58▪.Hendershot A, Slabaugh M, Riaz KM, et al. Strategies for prevention and management of ocular events occurring with mirvetuximab soravtansine. Gynecol Oncol Rep 2023; 47:101155. [DOI] [PMC free article] [PubMed] [Google Scholar]; Practical strategies for prevention and management of ocular toxicities from mirvetuximab.
- 59▪.Kim SK, Ursell P, Coleman RL, et al. Mitigation and management strategies for ocular events associated with tisotumab vedotin. Gynecol Oncol 2022; 165:385–392. [DOI] [PubMed] [Google Scholar]; Practical strategies for prevention and management of ocular toxicities from tisotumab vedotin.
- 60.Powell CA, Modi S, Iwata H, et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open 2022; 7:100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61▪▪.Tarantino P, Tolaney SM, Detecting. Managing T-DXd–related interstitial lung disease: the five “S” rules. JCO Oncol Pract 2023; 19:526–527. [DOI] [PubMed] [Google Scholar]; Practical strategies for prevention and management of T-Dxd associated ILD.